Abstract
Background
Unipolar major depressive disorder (MDD) is characterized by impaired cognitive control in affective contexts, but the potential for psychotherapy to affect the neural correlates of these functions has not been evaluated.
Method
Twelve adults with and 15 adults without MDD participated in two identical functional magnetic resonance imaging (fMRI) scans that utilized a task requiring cognitive control in both sad and neutral contexts. Between scans, MDD outpatients received Behavioral Activation Therapy for Depression, a psychotherapy modality designed to increase engagement with positive stimuli and reduce avoidance behaviors.
Results
Seventy-five percent of adults with MDD were treatment responders, achieving post-treatment Hamilton Rating Scale for Depression score of six or below. Consistent with predictions, psychotherapy resulted in decreased activation in response to cognitive control stimuli presented within a sad context in prefrontal structures, including the paracingulate gyrus, the right orbital frontal cortex, and the right frontal pole. Furthermore, the magnitude of pretreatment activation in the paracingulate gyrus cluster responsive to psychotherapy predicted the magnitude of depressive symptom change after psychotherapy.
Limitations
Replication with larger samples is needed, as are follow-up studies that involve placebo control groups, wait-list control groups, and alternative forms of antidepressant intervention.
Conclusions
Behavioral Activation Therapy for Depression improves depressive symptoms and concomitantly influences brain systems mediating cognitive control in affective contexts.
Keywords: Unipolar Depression, Target Detection, Cognitive Control, Functional Magnetic Resonance Imaging, Psychotherapy, Prefrontal Cortex, Paracingulate Gyrus
Introduction
Major depressive disorder (MDD) is characterized by anomalous processing of sad stimuli (American Psychiatric Association 1994; Peeters et al. 2003; Rottenberg 2005) as well as by deficits in cognitive control (McDermott & Ebmeier 2009; Veiel 1997; Zakzanis, Leach & Kaplan 1998). Recently, there has been new interest in the interactive effects of these deficits: that is, how does anomalous processing of sad stimuli adversely affect goal-directed behavior and cognitions in MDD (Clark, Chamberlain & Sahakian 2009)? This question has direct clinical relevance, given that the real-world impact of poorly modulated emotional responses in MDD extends beyond responses to affective events themselves to other domains of cognitive processes that are critical for effective functioning.
Unipolar MDD is characterized by deficits in cognitive control and hyper-responsivity to sad events that have been linked to prefrontal and limbic dysfunction, respectively (Clark, Chamberlain & Sahakian 2009; Fales et al. 2008; Grimm et al. 2008; Halari et al. 2009; Holmes & Pizzagalli 2008). For example, there is a wealth of evidence indicating dorsolateral and ventrolateral prefrontal cortical dysfunction in MDD that mediates impaired cognitive control (Brody, Barsom et al. 2001; Rogers et al. 2004). Additionally, there is evidence of limbic hyper-reactivity to sad stimuli in MDD that has been proposed to mediate the sad mood that is characteristic of the disorder (Price & Drevets 2009; Yurgelun-Todd, Sava & Dahlgren 2007). Most relevant in the present context, MDD is characterized not only by heightened responses to sad events (Cohen et al. 2005; Segal et al. 2006) but also by prolonged reactions to such events (Cohen et al. 2008; Goplerud & Depue 1985; Peeters et al. 2003), suggesting that the neurocognitive consequences of hyper-responsivity to sad stimuli in MDD may impact events that follow the presentation of such stimuli.
Relatively little research to date has examined the interactive effects of impaired emotional responsivity and cognitive control in MDD. In a recent functional magnetic resonance imaging (fMRI) study that used an oddball target detection task, our research group demonstrated that prefrontal cortical responses to cognitive control stimuli in MDD were moderated by the affective context (i.e., sad or neutral) within which cognitive control stimuli were presented (Dichter, Felder & Smoski 2009). Specifically, relative to a nondepressed control sample, MDD outpatients demonstrated prefrontal hypoactivation in response to target events presented in a neutral context. However, when target events were presented in a sad context, the MDD group demonstrated relative prefrontal hyperactivation in a number of regions, including the mid-, inferior, and orbito-frontal gyri and the anterior cingulate cortex. Thus, the pattern of prefrontal activation was contingent on the affective context of the task.
The purpose of the present investigation was to evaluate the effects of psychotherapy on prefrontal function recruited during the same cognitive control task described above (Dichter, Felder & Smoski 2009). A small handful of studies has investigated the effects of psychopharmacologic intervention on the neural correlates of limbic and prefrontal dysfunction in MDD: Fales and colleagues (2009) reported that outpatients with MDD demonstrated increased dorsolateral prefrontal cortex activity to fear stimuli after escitalopram treatment, and a number of studies have found that antidepressant treatment appears to attenuate limbic hyper-reactivity in MDD (Fu et al. 2004; Langenecker et al. 2007; Norbury et al. 2007). There are only two studies to date to assess the effects of antidepressant treatment on the neural correlates of cognitive-control in affective contexts in MDD: Miskowiak et al (2009) reported that erythropoietin treatment for MDD decreased ventromedial prefrontal activation to unpleasant pictures, but not to pleasant or neutral pictures, presented within a cognitive control task. Benedetti et al (2009) reported that venlafaxine in combination with light therapy treatment for MDD produced decreased dorsolateral prefrontal cortex activity to negative stimuli presented within the context of an emotional go-no-go task. Neither study, however, evaluated the effects of psychotherapy treatment on the neural correlates of cognitive-control in affective contexts in MDD.
In the present study, outpatients with MDD completed a mixed block/event target detection task using functional magnetic resonance imaging (fMRI) before and after treatment with Brief Behavioral Activation Therapy for Depression. This task allows for an examination of brain activation to events requiring cognitive control presented within both sad and neutral blocks. An oddball target detection task was employed because this task has been shown to robustly recruit prefrontal brain regions in nonclinical contexts (Fichtenholtz et al. 2004; Yamasaki, LaBar & McCarthy 2002). Changes in regional brain activation were compared to changes observed in a matched nondepressed control group scanned twice using the same task. Based on the central findings of Dichter et al (2009) that MDD is characterized by relative prefrontal hyperactivation to targets embedded within sad (but not neutral) blocks, as well as evidence of decreased prefrontal activity after antidepressant treatment to cognitive control stimuli presented in sad contexts (Benedetti et al. 2009; Miskowiak et al. 2009), we hypothesized that effective psychotherapy would induce reductions in prefrontal activation to targets in sad contexts relative to targets within neutral contexts. A secondary aim was to identify, in an exploratory manner, baseline fMRI predictors of response to treatment.
Method
Participants
Inclusion/exclusion criteria and Time 1 (i.e., pretreatment) fMRI results of have been reported previously (Dichter, Felder & Smoski 2009), as have sample characteristics and treatment outcomes from this sample (Dichter et al. 2009). All participants received a Structured Clinical Interview for DSM-IV (SCID; First et al. 1996) to confirm Axis I inclusion/exclusion criteria. At the time of consent, participants in the MDD group met DSM-IV criteria for a current episode of Major Depressive Disorder, no other current Axis I disorder other than dysthymia, and scored 15 or above on the Hamilton Rating Scale for Depression (HAM-D, Hamilton 1960). One MDD participant met criteria for concurrent dysthymia. Participants in the control group scored 6 or lower on the HAM-D, and did not meet criteria for a current Axis I disorder or current/lifetime episode of mood or anxiety disorder. One control participant and two MDD participants met criteria for past substance dependence; all were in remission for at least one year. One MDD participant reported a history of PTSD, but no longer met diagnostic criteria for PTSD. Among MDD participants, four were in their first depressive episode, three reported 2–3 lifetime episodes, and five reported 4 or more lifetime episodes. Four reported current episode durations of 6 months or less, four durations of 7–12 months, and four current episodes of more than one year (range: 2.5 – 22 years). Two MDD participants had prior single hospitalizations.
Participant exclusion criteria for both groups included: 1) coexisting bipolar or psychotic disorder, 2) comorbid current Axis I diagnosis including substance dependence, 3) active suicidal ideation, 4) evidence of organicity, 5) estimate verbal IQ below 70 (as indicated by North American Adult Reading Test verbal IQ (NAART), 6) magnetic resonance imaging contraindicated (e.g., metal in body), 7) history of neurological injury or disease, 8) current use of psychoactive medications including antidepressants, and 9) current pregnancy. Medical comorbidities were not assessed.
After a complete description of the study to participants, written informed consent was obtained. Participants were paid $45 for each imaging session. Sixteen depressed (9 females) and 15 nondepressed (9 females) participants enrolled in the study. One depressed female withdrew after her initial interview. Not included in the MRI analyses are the data from one depressed female who had frank abnormalities in brain anatomy. Two depressed participants did not return for psychotherapy sessions after the first imaging session. Thus, the final sample was 12 depressed (6 females, average age 39.0 ± 10.4 years) and 15 nondepressed (9 females, average age 30.8 ± 9.6 years) participants. Groups did not differ in age [MDD mean(SD)= 34.8(14.3) years, range = 23–53; nondepressed mean(SD)= 30.8 (9.7) years, range = 21–43], estimated verbal IQ (Blair & Spreen 1989) (MDD=112.8, nondepressed =117.7), smoking status (all nondepressed participants were non-smokers, all but two depressed participants were non-smokers), the number of days between scans [MDD mean(SD)=102.2 (15.4) days; nondepressed mean(SD)=102.5 (10.1) days], p’s > .05, or gender distribution, χ2 (1) = .99 p >0.32, but differed in socioeconomic status (Hollingshead 1975) [MDD mean(SD)= 36.8 (12.0); nondepressed mean(SD)= 45.8 (2.4)].
Brief Behavioral Activation Therapy for Depression (BATD)
MDD outpatients received an average of 11.4 (SD=2.0; range: 8–14) weekly sessions of Brief Behavioral Activation Therapy for Depression (BATD). Additional sessions (up to a total of 15 sessions; average of 1.4 per participant) were subsequently offered to help participants consolidate therapeutic gains and transition to follow-up care, as necessary. Early responders were given the option to end therapy after eight sessions and non-responders received the maximum number of sessions before being referred for additional treatment. BATD is a structured and validated psychotherapy designed to increase engagement with rewarding behaviors and reduce avoidance behaviors (Hopko et al. 2003). Patients are encouraged to expose themselves to reinforcing situations and to inhibit the behavioral withdrawal often characteristic of MDD (Jacobson et al. 1996). Behavioral activation interventions were recently shown to be as effective as cognitive behavioral therapy or paroxetine in reducing depressive symptoms in a large-scale clinical trial (Dimidjian et al. 2006).
fMRI Task
Participants completed five functional imaging runs (see Figure 1). Each run consisted of a 5′38″ mixed block and event-related target detection task during which a rare target stimulus (i.e., a bullseye) was presented embedded within alternating blocks of sad and neutral pictures. Runs began and ended with a neutral block. Stimuli were presented for 1000 ms and with a 2000 ms stimulus onset asynchrony. Blocks were 30 seconds long and consisted of two target stimuli embedded within 13 nontarget emotional pictures. All target events were separated by a minimum of 12 seconds, and targets were not presented within the first six seconds of each block. In this forced-choice reaction time paradigm, participants were instructed to respond via right-hand button press to every stimulus as quickly and accurately as possible by pressing one button for all non-target images and an alternate button for all targets. In this manner, motor activity related to making button-presses was incorporated into the task baseline.
Figure 1.
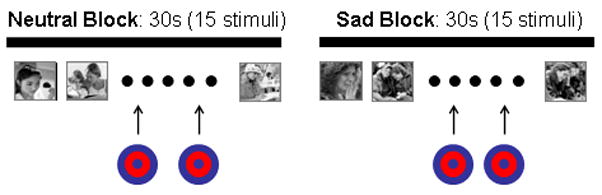
The fMRI task was a mixed block/event design with alternating 30-second blocks of neutral and sad images. Target events were embedded within the neutral and sad blocks.
Stimuli
Stimuli were identical to those developed by Wang and colleagues (2005) specifically for studies of MDD, and have been described in Dichter et al (2009). Given that sadness is a diagnostic feature of MDD, images were chosen to elicit that particular emotion. An insufficient number of images from the more commonly used International Affective Picture System (Lang, Bradley & Cuthbert 2005) elicit sadness (Mikels et al. 2005), and thus an image set was employed that was designed to assess responses to sad stimuli (Wang et al. 2005). In a subsequent study by Wang and colleagues, both controls and MDD participants again rated the images as sad, with MDD participants more likely to rate the images as “very sad” than controls (Wang et al. 2008).
This grayscale stimulus set contains 56 sad and 54 neutral images. Sad images were those that elicited average sadness ratings of 2 or higher on a 3-point sadness intensity scale (1 = not sad/unsure, 2 = mildly sad, 3 = sad) from a nonclinical validation sample. The sad pictures contained scenes of humans crying or portrayed sad facial expressions. The neutral images were matched as closely as possible to the final pool of sad pictures for presence and number of human figures in the image, postural features, gaze direction, and gender. The initial fMRI validation study employing these images confirmed robust amygdala activation in response to these sad images (Wang et al. 2005). Stimuli were presented using CIGAL presentation software (Voyvodic 1999) and displayed to the participants through magnet-compatible goggles (Resonance Technology, Inc., Northridge CA).
After the fMRI session, pictures were presented again outside of the scanner and participants rated each with respect to pleasure and arousal using the Self Assessment Manikin (Bradley & Lang 1994), a 9-point Likert non-verbal pictorial assessment technique.
Imaging
Head motion was analyzed by center of mass measurements in three orthogonal planes, and imaging epochs with mean intensities greater than three standard deviations of the average intensity in a run were excluded from analyses. Only epochs during which participants gave a correct response were included in analyses.
Scanning was performed on a General Electric 4T LX NVi MRI scanner system equipped with 41 mT/m gradients (General Electric, Waukesha, Wisconsin, USA). A quadrature birdcage radio frequency (RF) head coil was used for transmit and receive. The participant’s head was immobilized using blocks of foam. Sixty-eight high resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 12 ms; TE = 5.4 ms; FOV = 24 cm; image matrix = 256 × 192; voxel size = 0.9375 × 0.9375 × 1.9 mm; 72 oblique axial slices; δ = 20°) and used for coregistration with the functional data. Structural images were aligned in a near axial plane defined by the anterior and posterior commissures. Whole brain functional images were acquired using an echoplanar pulse sequence sensitive to blood oxygenation level dependent (BOLD) contrast (TR, 2000 ms; TE, 25 ms; FOV, 24 cm; image matrix = 64°; δ = 60°; voxel size, 3.75 × 3.75 × 3.8 mm; 34 axial slices). The functional images were aligned similarly to the structural images. A semi-automated high-order shimming program ensured global field homogeneity.
Functional data were preprocessed using FSL version 4.0.2 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U.K.). Timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing was applied in the following steps: (i) brain extraction for non-brain removal (Smith et al. 2004), (ii) motion correction using MCFLIRT (Smith 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor, and (v) high-pass filtering (Jenkinson, Mark et al. 2002). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool (Jenkinson, Mark et al. 2002; Smith et al. 2004). Voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (Jenkinson, M. & Smith 2001).
Onset times of events were used to model a signal response containing a regressor for each response type, which was convolved with a double-γ function to model the hemodynamic response. Model fitting generated whole brain images of parameter estimates and variances, representing average signal change from baseline. Group-wise activation and deactivation images were calculated by a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FILM 1+2, Woolrich et al. 2001) with an uncorrected threshold of Z>2.3 (Beckmann, Jenkinson & Smith 2003). Activation localizations were based on Harvard-Oxford cortical and subcortical structural probabilistic atlases as implemented in FSLView v3.0.
Results
Psychotherapy Outcomes
As reported previously (Dichter et al. 2009), average HAM-D scores in the MDD group changed from 23.8 (2.3) to 8.7 (9.4), p<.0001, and BDI scores changed from 27.1 (SD=5.1) to 11.6 (SD=8.6), p<.0001. 75% (9/12) of participants were responders, defined as Time 2 HAM-D scores of six or below, and 83% (10/12) of participants were partial responders, defined as Time 2 HAM-D scores of 10 or below.
Behavioral Performance
The top of Figure 2 illustrates average accuracy and latency to target events for each group and timepoint, subdivided by affective block type (i.e., sad or neutral blocks). A 2 (Group: Depressed, Nondepressed) × 2 (Block Type: Sad, Neutral) × 2 (Time1, Time 2) repeated measure MANOVA conducted separately on accuracy and latency of responses to target events revealed no significant main effects or interactions, p’s>.10.
Figure 2.
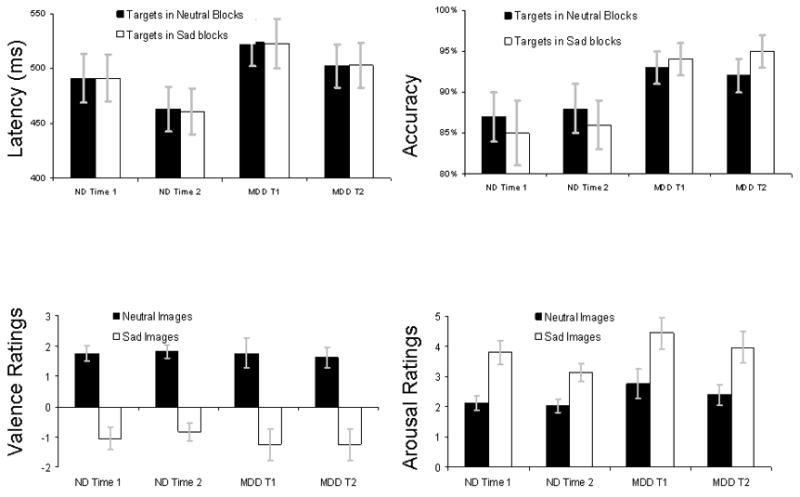
Top Left: In-scanner latency (in ms) for target events. Top Right: In-scanner accuracy (percent correct) for target events. Bottom Left: Mean valence ratings of neutral and sad images. The range of ratings was: −4 (extremely unpleasant) to +4 (extremely pleasant). Bottom Right: Mean arousal ratings of neutral and sad images. The range of ratings was: 1 (not at all aroused) to 9 (extremely aroused). Errors bars represent group standard errors of the mean
Self-report Responses to Pictures
The bottom of Figure 2 illustrates average ratings of valence and arousal for each group and timepoint, subdivided by affective block type (i.e., sad or neutral blocks). A 2 (Group: Depressed, Nondepressed) × 2 (Block Type: Sad, Neutral) × 2 (Time1, Time 2) repeated measure MANOVA conducted separately on valence and arousal ratings revealed, not surprisingly, main effects of Valence, multivariate F’s >14, p’s < .001, but no other significant main effects or interactions, p’s>.05.
Imaging Data
The contrast of interest in the present study was [Targets within Sad Blocks] minus [Targets within Neutral Blocks]. This contrast isolates neurocognitive processes required to respond to a stimulus requiring cognitive control (i.e., the target event) in a sad context while controlling for neurocognitive processes required to respond to the same stimulus in a neutral context. In other words, this contrast allows for an examination of the moderating effects of affective context on the neural correlates of cognitive control.
The effects of psychotherapy were evaluated via a 2 (Group: MDD, nondepressed) × 2 (Time: Time 1, Time 2) interaction test on the contrast of interest described above. Because voxels corresponding to significant interactions may reflect increased, decreased, or unchanged signal intensity in the MDD group relative to change in signal intensity in the nondepressed group, whole-brain analyses were followed by two-tailed within-groups t-tests (α=.05) of changes in signal intensity in voxels identified by Group × Time interaction tests. In this manner, statistical tests of fMRI change due to psychotherapy were restricted to voxels with significant interaction terms. This approach allows for a reduction of the number of post-hoc statistical tests performed.
The left side of Figure 3 depicts voxels with significant 2 (Group: MDD, nondepressed) × 2 (Time: Time 1, Time 2) interaction terms, and the right side of Figure 3 illustrates average signal intensity in the paracingulate gyrus cluster depicted on the left side of Figure 3 for both groups and timepoints in response to the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast. The bar graph illustrates that, at Time 1, the MDD group recruited this cluster to a significantly greater degree than did the control group (p<.05). However, at Time 2, this pattern was reversed, and the MDD group recruited this cluster to a lesser degree than did the control group (p<.05). Further, the MDD group demonstrated a significant decrease in activation in this cluster after psychotherapy (p<.05).
Figure 3.
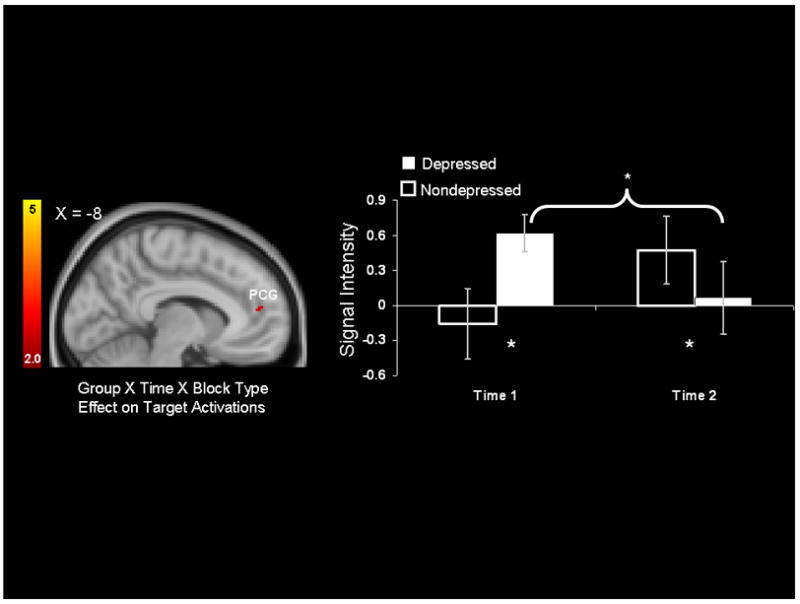
Left: Significant Group (Depressed, Nondepressed) × Time (Time 1, Time 2) interaction for the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast. The x-coordinate is in MNI space. Right: Signal intensity (in t statistic units) averaged for each Group and Timepoint within the paracingulate (PCG) cluster identified on the left. Error bars represent standard errors of the mean. * p<.05.
Table 1 denotes all clusters with significant interaction terms, as well at the results of paired t-tests and effect sizes of signal intensity differences between Time 1 and Time 2 scans in the MDD group in clusters with significant interaction effects. Areas that showed significant decreases in activation following psychotherapy included the paracingulate gyrus, right orbital frontal cortex, right frontal pole, left Heschl’s gyrus, left occipital pole, bilateral postcentral gyrus, bilateral precentral gyrus, left superior, left middle, and right inferior posterior temporal gyrus, and right anterior supramarginal gyrus.
Table 1.
Clusters showing significant Group (Depressed, Nondepressed) × Time (Time 1, Time 2) interactions for the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast. The second-to-last column indicates results of two-tailed paired t-tests (i.e., Time 2 – Time 1) conducted on signal intensity t-values from MDD participants on clusters identified as significant from interaction tests. Negative t values denote a significant decrease in the MDD group after psychotherapy and positive t-values denote a significant increase in the MDD group after psychotherapy. The final column indicates the effect size of the paired t-test.
| Region | Brodmann’s Area | Size (mm3) | Z Max | Coordinates |
Effect of BATD: t (p) | Effect Size of BATD effect | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Frontal Orbital Cortex (Right) | 48 | 2.1 | 20 | 16 | −14 | −2.14 (0.04) | 0.82 | |
| Frontal Pole | ||||||||
| Right | 10 | 40 | 2.05 | 24 | 72 | 0 | 1.36 (0.19) | 0.55 |
| Right | 56 | 2.29 | 28 | 56 | 6 | −2.69 (0.01) | 0.93 | |
| Right | 10 | 120 | 2.31 | 24 | 58 | 14 | −3.15 (0.00) | 1.03 |
| Left | 40 | 2.1 | −40 | 48 | 30 | 0.18 (0.86) | 0.06 | |
| Heschl’s Gyrus (Left) | 104 | 2.38 | −42 | −18 | 2 | −3.58 (0.005) | 1.36 | |
| Inferior Frontal Gyrus, pars triangularis (Right) | 152 | 2.41 | 48 | 26 | 0 | 2.38 (0.03) | 0.85 | |
| Occipital Pole (Left) | 40 | 2.39 | −32 | −96 | 26 | −2.22 (0.04) | 0.92 | |
| Pallidum (Right) | 96 | 2.52 | 18 | −6 | 4 | −1.84 (0.08) | 0.67 | |
| Paracingulate Gyrus (Left) | 9 | 64 | 2.17 | −8 | 48 | 14 | −4.44 (0.001) | 0.54 |
| Postcentral Gyrus | ||||||||
| Right | 88 | 2.1 | 22 | −42 | 54 | −3.41 (0.006) | 1.14 | |
| Right | 168 | 2.38 | 8 | −36 | 60 | −4.14 (0.001) | 1.41 | |
| Left | 40 | 40 | 2.08 | −48 | −30 | 60 | −2.35 (0.03) | 0.96 |
| Left | 136 | 2.4 | −36 | −30 | 66 | −2.06 (0.05) | 0.81 | |
| Precentral Gyrus | ||||||||
| Right | 264 | 2.27 | 26 | −12 | 64 | −2.41 (0.02) | 0.87 | |
| Right | 6 | 192 | 2.83 | 52 | −2 | 30 | −3.17 (0.009) | 1.17 |
| Left | 272 | 2.35 | −50 | 10 | 32 | 2.29 (0.03) | 0.82 | |
| Left | 168 | 2.4 | −28 | −20 | 48 | −1.72 (0.10) | 0.67 | |
| Left | 4 | 80 | 2.15 | −40 | −14 | 54 | −2.92 (0.01) | 1.09 |
| Temporal Gyrus (Superior, Posterior, Left) | 72 | 2.1 | −62 | −16 | 2 | −2.38 (0.03) | 0.99 | |
| Temporal Gyrus (Inferior, Posterior, Right) | 224 | 2.29 | 54 | −40 | −18 | −2.88 (0.01) | 0.98 | |
| Temporal Gyrus (Middle, Posterior, Left) | 21 | 72 | 2.33 | −70 | −30 | −8 | −2.54 (0.02) | 1.00 |
| Supramarginal Gyrus (Anteior, Right) | 64 | 2.24 | 36 | −38 | 36 | −2.30 (0.03) | 0.97 | |
We investigated whether the magnitudes of pretreatment signal intensity in any cluster identified by significant 2 (Group: MDD, nondepressed) × 2 (Time: Time 1, Time 2) interaction terms (see Table 1) on the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast predicted change in depressive symptoms within the MDD group. This post-hoc analysis did not correct for the number of correlation analyses performed, though this analysis was restricted to clusters showing significant interaction terms (i.e., this was not a whole-brain covariate analysis). The only region to show a significant correlation was the paracingulate gyrus cluster illustrated in Figure 3. Figure 4 depicts a scatterplot of this relation, and suggests that individuals with greater changes in BDI scores (i.e., those with greater symptom reductions after psychotherapy) were those with lower pretreatment signal intensities to [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast. No significant relations emerged between Time 2 depressive symptoms and Time 2 brain imaging data within the MDD group, likely due at least in part to the restriction of range produced by symptom remission in the majority of MDD cases after psychotherapy.
Figure 4.
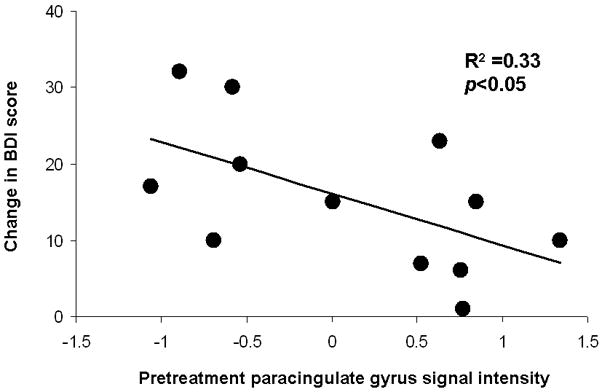
Scatterplot depicting relations between change in BDI score (i.e., Pre-treatmement-Post-Treatment) and pretreatment paracingulate gyrus (cluster depicted on the left side of Figure 3) signal intensity from the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast in the MDD group.
Discussion
The purpose of the present study was to evaluate the effects of Behavioral Activation Therapy on prefrontal brain function in response to cognitive control stimuli presented in sad versus neutral contexts in outpatients with MDD. Time 1 data, reported previously (Dichter, Felder & Smoski 2009) revealed that MDD outpatients recruited prefrontal brain regions to a greater extent to cognitive control stimuli presented in sad contexts than in neutral contexts. These pretreatment data are consistent with conceptualizations of the prefrontal cortex as a mediator of affect regulation in emotional contexts (Ochsner et al. 2004) as well as empirical evidence that behavioral performance in the context of increased task demands requires greater compensatory prefrontal neuronal activity (Adler et al. 2001). Thus, it appeared as though patient with MDD required relatively greater cognitive “effort” to disengage from sad stimuli to engage with cognitive control stimuli.
Primary hypotheses of the present study were that symptom remission due to psychotherapy in outpatients with MDD would be accompanied by a concomitant decrease in the magnitude of prefrontal activation required to successfully respond to cognitive control stimuli in sad contexts versus neutral contexts. Findings supported hypotheses. A number of prefrontal regions showed decreased activation in sad contexts after treatment in the MDD group, including the paracingulate gyrus, right orbital frontal cortex, right frontal pole, and left postcentral gyrus. These findings are consistent with other reports of increased prefrontal activity following antidepressant treatment in response to cognitive control stimuli presented within sad contexts in MDD (Benedetti et al. 2009; Miskowiak et al. 2009).
Decreased activation in the right paracingulate gyrus is noteworthy, given a large literature demonstrating that metabolism in this region predicts treatment response in an array of functional and metabolic imaging paradigms (e.g., Kennedy et al. 2007; Konarski et al. 2007; Ressler & Mayberg 2007). The finding of decreased right orbital frontal cortex activation may be conceptualized within the role of this region to mediate emotional evaluations (Wright et al. 2008) and known linkages between orbital frontal cortex dysfunction and MDD in a number of contexts (see Drevets, Price & Furey 2008 for a review). Finally, the right frontal pole is a mediator of executive control of cognitive operations (John et al. 2009) and has been suggested to be a cognitive “gateway” that prioritizes information processing (Burgess, Dumontheil & Gilbert 2007).
Covariate analyses within the MDD group revealed that pretreatment paracingulate gyrus activation to the [Targets within Sad Blocks] minus [Targets within Neutral Blocks] contrast predicted change in symptoms scores. The direction of this effect is consistent with the conceptualization that MDD is associated with relatively greater prefrontal activation to targets embedded within sad blocks that reflects greater cognitive “effort” to disengage from sad stimuli (Dichter, Felder & Smoski 2009): those with greater pretreatment signal intensity showed less symptom changes, whereas those with smaller pretreatment signal intensity showed greater symptom changes. Though the post-hoc nature of these analyses warrants replication, these findings suggest that greater paracingulate gyrus activation in the present context may be a marker of treatment response.
Unexpectedly, the right pars triangularis showed increased activation in the MDD group after psychotherapy. This region was not indentified in Dichter et al (2009) as a region that differentiated groups at baseline. This region is involved in language, motor function, and imitation of action in others (Molnar-Szakacs et al. 2005), and change in activation in this region after psychotherapy in MDD bears replication.
This is the first study to date to evaluate the effects of psychotherapy on cognitive control in affective contexts in MDD. The central finding of reduced prefrontal activation after psychotherapy stands in contrast to some of the available data addressing the effects of psychopharmacologic intervention in MDD, where prefrontal activation increases are typically observed (Fales et al. 2009; Fu et al. 2004; Langenecker et al. 2007; Norbury et al. 2007) (see Benedetti et al. 2009; Miskowiak et al. 2009 for exceptions). Although these studies utilized different tasks and samples, the effects of psychotherapy and psychopharmacologic antidepressants on regional brain function are often in opposite directions, despite equivalent degrees of symptom reduction (Brody, Saxena et al. 2001; Goldapple et al. 2004). It may be the case that various treatment modalities initially affect brain sites differentially, yet all may result in a net change in neural functioning that results in symptom remission. Clearly, a full test of this model would require multiple brain imaging assessments over time to track the chronometry of brain responses to different treatment modalities.
We note that a number of brain regions reactive to psychotherapy were clearly outside of emotion processing and cognitive control structures. Additionally, the present sample and design had a number of characteristics that limit interpretability. First, the present study did not include wait-list or placebo control groups, and thus functional brain changes in the MDD group may have been due to other variables, such as spontaneous improvement of symptoms over time instead of to the BATD intervention. Additionally, the addition of another form of antidepressant treatment would allow for isolation of effects to the BATD modality specifically. The relatively broader age range of the MDD group is an additional limitation of this study. Finally, post-hoc tests of the effects of BATD in the MDD group were not corrected for multiple comparisons, and findings regarding the effects of BATD warrant replication. Despite these limitations, the present study suggests that Behavioral Activation Therapy for depression may normalize neural functioning in cognitive control and affective contexts.
Acknowledgments
The authors would like to thank Todd Harshbarger and Syam Gadde for assistance with image analysis, Prue Cuper and Shian-Ling Keng for assistance with data collection, and MRI technologists Susan Music, Natalie Goutkin, and Talaignair Venkatraman for assistance with data acquisition. This investigation was supported by grants from the National Institute of Mental Health (MH078145) and the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) to G. Dichter. Assistance for this study was provided by the Neuroimaging Core of the UNC Neurodevelopmental Disorders Research Center. G. Dichter was supported by Postdoctoral Research in Neurodevelopmental Disorders, NICHD T32-HD40127, a career development award from UNC-Chapel Hill, NIH/NCRR K12 RR023248 (Orringer), and by NIMH K23 MH081285. M. Smoski was supported by NIMH T32-MH070448, a NARSAD Young Investigator award, a career development award from Duke University Medical Center, NICHD K12 HD043446, and by NIMH K23 MH087754.
Footnotes
Contributors
G. Dichter and M Smoski designed the study and wrote the protocol.
J. Felder managed the literature searches and analyses.
G. Dichter, J. Felder, and M Smoski undertook the statistical analysis.
G. Dichter wrote the first draft of the manuscript.
All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42(4):266–72. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition: DSM-IV. Washington, DC: 1994. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Colombo C, Smeraldi E. Changes in medial prefrontal cortex neural responses parallel successful antidepressant combination of venlafaxine and light therapy. Arch Ital Biol. 2009;147(3):83–93. [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3:129–36. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. Journal of Behavioral Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6(2):102–12. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631–40. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11(7):290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, O’Neill SC, Tolpin LH. Daily affective reactivity as a prospective predictor of depressive symptoms. J Pers. 2005;73(6):1687–713. doi: 10.1111/j.0022-3506.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, Parrish BP, Wenze SJ, Beck JS. Negative affective spillover from daily events predicts early response to cognitive therapy for depression. J Consult Clin Psychol. 2008;76(6):955–65. doi: 10.1037/a0014131. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord. 2009;114(1–3):131–42. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658–70. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112(1–3):206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Res. 2004;20(1):67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version; Administration Booklet. American Psychiatric Press; Washington, D.C: 1996. [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61(9):877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goplerud E, Depue RA. Behavioral response to naturally occurring stress in cyclothymia and dysthymia. J Abnorm Psychol. 1985 May;94(2):128–39. doi: 10.1037//0021-843x.94.2.128. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63(4):369–76. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. J Child Psychol Psychiatry. 2009;50(3):307–16. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Hamilton MA. A rating scale for depression. Journal of Neurology and Neurosurgery in Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Department of Sociology, Yale University, Working paper; New Haven, CT: 1975. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46(12):2904–13. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: Procedures, principles, and progress. Clinical Psychology Review. 2003;23:699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, Gortner E, Prince SE. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64(2):295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- John JP, Burgess PW, Yashavantha BS, Shakeel MK, Halahalli HN, Jain S. Differential relationship of frontal pole and whole brain volumetric measures with age in neuroleptic-naive schizophrenia and healthy subjects. Schizophr Res. 2009;109(1–3):148–58. doi: 10.1016/j.schres.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164(5):778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, Kennedy SH, McIntyre RS, Rafi-Tari S, Soczynska JK, Mayberg HS. Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res. 2007;155(3):203–10. doi: 10.1016/j.pscychresns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. Technical Report A-6. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62(11):1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009 doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the International Affective Picture System. Behav Res Methods. 2005;37(4):626–30. doi: 10.3758/bf03192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, Harmer CJ. Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology (Berl) 2009;207(1):133–42. doi: 10.1007/s00213-009-1641-1. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb Cortex. 2005;15(7):986–94. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. British Journal of Psychiatry. 2007;190:531–2. doi: 10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. J Abnorm Psychol. 2003;112(2):203–11. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of Mood Disorders. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50(1):1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Mood and Emotion in Major Depression. Current Directions in Psychological Science. 2005;14(3):167–70. [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63(7):749–55. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol. 1997;19(4):587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimage. 1999;10(2):91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Wang L, Labar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163(2):143–55. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5(1):12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wright P, Albarracin D, Brown RD, Li H, He G, Liu Y. Dissociated responses in the amygdala and orbitofrontal cortex to bottom-up and top-down components of emotional evaluation. Neuroimage. 2008;39(2):894–902. doi: 10.1016/j.neuroimage.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99(17):11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Sava S, Dahlgren MK. Mood disorders. Neuroimaging Clin N Am. 2007;17(4):511–21. ix. doi: 10.1016/j.nic.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(3):111–9. [PubMed] [Google Scholar]


