Abstract
Intravenous immunoglobulin (IVIG)-resistant Kawasaki disease (KD) patients comprise at least 20% of treated patients and are at higher risk of coronary artery abnormalities. If identified early in the course of the disease, such patients may benefit from additional anti-inflammatory therapy. The aim of this study was to compare the transcript abundance between IVIG-resistant and – responsive KD patients to identify biomarkers that might differentiate between these two groups and to generate new targets for therapies in IVIG-resistant KD patients. We compared the transcript abundance profiles of whole blood RNA on Agilent arrays from acute and convalescent KD subjects and age-similar, healthy controls. KD subjects were stratified as IVIG-resistant or – responsive based on response to initial IVIG therapy. Transcript abundance was higher for IL-1 pathway genes (IL-1 receptor, interleukin receptor associated kinase, p38 mitogen-activated protein kinase), and MMP-8. These findings point to candidate biomarkers that may predict IVIG-resistance in acute KD patients. The results also underscore the importance of the IL-1 pathway as a mediator of inflammation in KD and suggest that IL-1 or its receptor may be reasonable targets for therapy, particularly for IVIG-resistant patients.
Keywords: Kawasaki disease, gene expression, intravenous immunoglobulin
1. Introduction
Transcript abundance can be a window into the host response to an inflammatory stimulus. From previous transcript abundance studies of Kawasaki disease (KD), the leading cause of acquired heart disease in children, we have learned of the dynamic nature of the host response in the first week following onset of fever. Investigators have focused on groups of genes in pathways implicated in inflammation, endothelial cell injury, and cardiomyocyte injury [1-5].
Treatment with intravenous immunoglobulin (IVIG) remains the mainstay of therapy for KD and reduces the rate of coronary aneurysm formation from 25% to 3-5% [6]. Resistance to IVIG therapy appears to be increasing with reported resistance rates of 20-30% in Japan and the U.S. [7-9]. IVIG-resistance is a major risk factor for development of aneurysms and early identification of patients at risk would allow timely administration of additional anti-inflammatory therapies [8, 10]. Although clinical scoring systems to identify potential IVIG-resistant KD patients have been validated for Japanese populations, such scoring systems were insufficiently robust to be clinically useful in an ethnically diverse population from the United States [8, 11-14]. Thus, a better understanding of the pathogenetic mechanisms underlying IVIG-resistance is needed.
We compared whole blood transcript levels among acute KD subjects prior to initial IVIG administration, the same subjects during convalescence, and healthy controls. KD subjects were stratified for analysis based on clinical response to IVIG. We performed a sequential, pairwise comparison of transcript abundance. From this analysis, we identified novel biological pathways that characterized acute KD and transcript signatures associated with IVIG-resistance. This analysis highlights the role of cytokine networks involving IL-1 and matrix metalloproteinase-8 (MMP-8) and suggests a role for IL-1 antagonists in the treatment of IVIG-resistant KD.
2. Subjects and Methods
2.1 Study populations and sample collection
KD patients (n=20) and healthy controls (n=9) were enrolled at Rady Children's Hospital San Diego after obtaining parental informed consent. KD subjects with a spectrum of illness days, ethnicities, and disease severity admitted to Rady Children's Hospital San Diego between 2003 and 2006 were chosen based on availability of acute and convalescent samples. The human subjects' protocol was reviewed and approved by the University of California, San Diego, Institutional Review Board. All patients diagnosed with KD had fever for at least 4 days and met at least 4 of 5 clinical criteria for KD (rash, conjunctival injection, cervical lymphadenopathy, oral mucosal changes, and changes in the extremities) or 3 of 5 criteria and coronary artery abnormalities documented on echocardiogram[15]. Blood was obtained at the time of diagnosis prior to initial IVIG treatment (acute phase) and again approximately 1 month following the resolution of the acute illness after the erythrocyte sedimentation rate (ESR) and platelet count had returned to normal (convalescent phase), from all but one subject from whom only an acute sample was available. Whole blood was collected in PAXgene tubes and total RNA was isolated as previously described [1]. Healthy age-similar controls were children <5 years of age undergoing elective minor orthopedic surgical procedures. Whole blood was collected in PAXgene tubes prior to general anesthesia.
Demographic and clinical characteristics of study subjects are presented in Table 1. Clinical laboratory evaluations included complete blood count and differential, C-reactive protein level, and ESR. Sex, age, illness day at sample collection (illness day 1= 1st calendar day of fever), response to IVIG, and echocardiographic measurement of coronary arteries were recorded for all KD patients. Measurements of the internal diameter of the right and left anterior descending coronary arteries were normalized based on body surface area and expressed as a z score (standard deviation units from the mean) [16, 17]. Arteries were classified as normal, dilated or aneurysmal (z score <2.5, ≥2.5 to <4.0, or ≥4.0, respectively). KD subjects were IVIG-resistant (n=8) if they had persistent or recrudescent fever at least 36 hours after the end of their IVIG infusion. IVIG-resistance was treated with either infliximab at 5 mg/kg IV (n=5) or a second dose of IVIG at 2 g/kg IV (n=3).
Table 1.
Demographic and clinical characteristics of study subjects.
| KD IVIG-Responsive (n=12) |
KD IVIG-Resistant (n=8) |
Healthy Controls (n=9) |
|
|---|---|---|---|
| Age at Onset, yr | 3.29 (0.33-9) |
1.85 (0.33-4.92) |
1.65 (0.39-4.47) |
| Male, n (%) | 5 (42) | 5 (63) | 3 (33) |
| Illness Day at sample collection |
6 (4-10) |
5 (4-7) |
NA |
| Ethnicity/Race, n (% of total) |
|||
| Asian | 1 (8.3) | 0 | 0 |
| Caucasian | 4 (33) | 2 (25) | 3 (33) |
| Hispanic | 3 (25) | 4 (50) | 3 (33) |
| Mixed | 4 (33) | 2 (25) | 3 (33) |
| Coronary Artery | 8 Normal | 3 Normal | NA |
| Statusa | 4 Dilated | 3 Dilated 2 Aneurysms |
|
| White blood cells × 103/mm3 |
13.3 (5.9-23) |
13.0 (7.6-19.2) |
NA |
| Neutrophils, % | 57 (22-76) |
54 (28-73) |
NA |
| Bands, % | 8 (0-28) |
17 (0-50) |
NA |
| Absolute | 8303 | 9888 | NA |
| Neutrophil Count | (4818-16269) | (4302-14976) | |
| Lymphocytes, % | 17 (5-70) |
18 (2-34) |
NA |
| Monocytes, % | 4.5 (0-17) |
5 (2-10) |
NA |
| zHemoglobinb | −0.73 (−4.33-1) |
−1.40 (−5.83-0) |
NA |
| Erythrocyte | 59.5 | 63.5 | NA |
| Sedimentation | (27-140) | (40-140) | |
| Rate, mm/h | |||
| C Reactive | 5.5 | 18 | NA |
| Protein, mg/dL | (0.3-18.4) | (5.1-44.2) | |
| Platelets × 103/mm3 |
404 (89-541) |
394 (85-516) |
NA |
| Alanine | 69 | 12.5 | NA |
| Aminotransferase, U/L | (6-725) | (24-161) | |
| Gamma Glutamyl | 64 | 109.5 | NA |
| Transferase, U/L | (9-220) | (6-216) | |
All data presented as median (range).
Only significant difference between IVIG-responsive and –resistant KD subjects, p=0.04 chi square test
zHemoglobin, standard deviation units from the mean based on age-adjusted normal values [44] NA= not available
2.2 Agilent Whole Human Genome Oligo Microarray Experiment
Aminoallyl cRNA was produced from 1 μg of total RNA using Fluorescent Linear Amplification Kit (Agilent Technologies) and aminoallyl-UTP. Cyanine (Cy) 3 or Cy5 was chemically conjugated to amninoallyl cRNA using Cy3 or Cy5 NHS ester (GE Life Sciences). All sample RNAs were labeled with Cy3 and compared to a human blood common reference RNA labeled with Cy5. Cy3 and Cy5 labeled cRNAs were hybridized to the Agilent G4112F Human Whole Genome Oligo Arrays (design number 14850) per the manufacturer's instructions except for the following modification: cRNAs containing 300 pmol of Cy3 and 150 pmol of Cy5 were used and the hybridization was done at 70°C. Hybridized microarrays were washed according to the manufacturer's protocol and scanned on an Agilent Microarray Scanner. Raw data were extracted from scanned array images using Agilent Feature Extraction 9.5.1.1. Ratios were calculated after subtracting the average of negative control signals (background) and normalization by non-linear Lowess normalization.
2.3 Quantitative RT-PCR
Whole blood mRNA levels for MMP-8 were measured in 8 IVIG-resistant and 12 IVIG-responsive KD subjects using TaqMan Gene Expression assay (Hs01029057_m1, Applied Biosystem). Relative abundance of the target transcripts was normalized to the expression level of the housekeeping gene, TATA box binding protein-associated factor RNA polymerase I B (TAF1B) (Hs01057259_m1, Applied Biosystem) as previously described [1].
2.4 MMP-8 Serum Levels
We measured acute and convalescent serum levels of MMP-8 in an independent cohort of 26 consecutive, unselected KD subjects. . The cohort characteristics were as follows: median age 2.9 years (range 0.37 to 11 years); 69% male; 19% Caucasian; 57% IVIG responders; 65% normal coronaries; 19% dilated coronaries; 15% aneurysms. MMP-8 levels were measured using the Fluorokine MAP Assay (R&D Systems) as per manufacturer's protocol. MMP-8 concentrations were calculated using Bio-Plex Manager 5.0 software with a 5 parameter curve fitting algorithm applied for standard curve calculations.
2.5 Statistical analysis
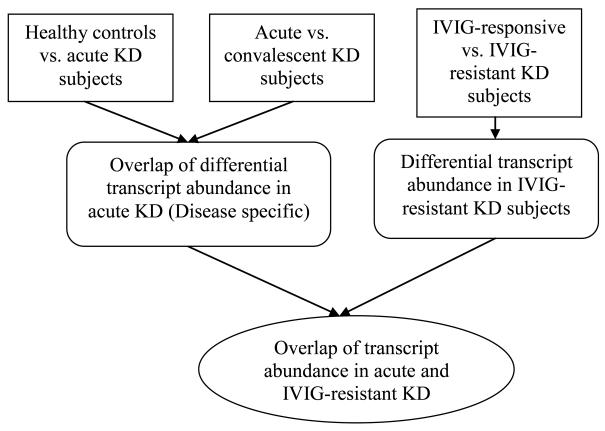
We first identified the transcripts that were differentially abundant between all KD subjects and healthy controls (Figure 1). Then we selected from those transcripts the ones that were also differentially abundant between acute and convalescent KD subjects. Lastly, we identified the KD-specific transcripts that were also differentially abundant between IVIG-responsive and –resistant subjects.
Figure 1.
Flow diagram of the groups compared for transcript abundance
Genes whose average raw probe intensities were lower than 100 across the samples analyzed were removed from further analysis. To identify genes with differentially abundant transcripts between the two groups of samples, a filter of average fold change >1.5 was applied. If the same probe sequence was on more than one spot on the microarray chip, the signal intensity for that probe was averaged. Comparisons between two groups for continuous variables were made with a Student's t-test or a Mann Whitney U test for parametric and non-parametric data, respectively. Non-parametric microarray data were log-transformed and analyzed by Student t-test. If more than one probe existed for a given gene, the average of the p values of the log-transformed data was reported. Results were considered statistically significant if p < 0.05. False Discovery Rate (FDR) q value was calculated using the formula (p * n / i) where p is the p value of the Student test of the probe, n is the number of total Student t tests, and i is the ascending sorted rank of the p value. Pearson's correlation and the correlation coefficients were calculated using Prism to evaluate the relationship between microarray and RT-PCR results. The overlap genes of two lists were obtained by using GeneSpring GX 7.3 software by Agilent® Technologies (www.agilent.com.) Hypergeometric probability tests were performed with R (http://cran.r-project.org/). Genes associated with probes of interest were identified and assigned to pathways by Ingenuity Pathway Analysis (Ingenuity® Systems, www.ingenuity.com).
3. Results
3.1 Increased MMP-8 transcript abundance in KD versus healthy control subjects
Microarray data were compared in 3 different pairwise analyses to identify sets of probes with > 1.5-fold differences between acute KD subjects and healthy controls, between acute and convalescent KD subjects, and between IVIG-resistant and –responsive acute KD subjects (Table 2). Of the 4,799 probes that were less abundant during the acute phase of KD compared with healthy controls, 2,200 probes were also more abundant during the convalescent phase of KD and thus represented disease-specific transcripts that were differentially abundant between the acute and convalescent phase (Figure 2). Of the 6,875 probes that were more abundant in acute KD subjects vs. healthy controls, 2,575 probes were more abundant in acute KD subjects vs. both convalescent KD subjects and healthy controls. Of the 4,775 acute-phase, disease-specific probes differentially expressed in acute KD subjects compared with convalescent KD subjects and healthy controls, we selected for analysis the 200 probes that had the largest fold difference in transcript abundance between acute KD subjects and healthy controls (maximum q value 3.23%, Supplemental Table 1). Of these probes, 173 were more abundant in acute KD subjects than in healthy controls and 27 were less abundant. Ingenuity Network Pathway Analysis revealed that the genes associated with these 200 probes were in Inflammatory Disease and Immunological Disease pathways (ranges of p values 2.96×10−16 to 9.52×10−3 and 6.22×10−15 to 9.52×10−3, respectively) and included MMP-8 as the most abundant transcript and nuclear factor kappa B (NF-κB) signaling proteins. Also included were S100 proteins (S100A8, S100A9 and S100A12) and carcinoembryonic antigen-related cell adhesion molecules (CEACAM 1 and CEACAM 8) that had been identified in previous microarray analyses by our group and others as being associated with IVIG resistance (Figure 3) [1, 2, 18-20]. Ingenuity Canonical Pathway Analysis found that the most shared pathway for these top 200 probe-related genes was the Toll-like receptor signaling pathway with a p value of 1.18×10−03 (Figure 4) [21].
Table 2.
Pairwise comparisons between healthy controls and acute and convalescent Kawasaki disease subjects who were either IVIG-resistant or IVIG-responsive.
| Healthy controls |
KD IVIG-resistant subjects |
KD IVIG-responsive subjects |
Number of Probes | ||||
|---|---|---|---|---|---|---|---|
| Pairwise comparisons* |
Acute | Convalescent | Acute | Convalescent | More abundant in all A vs. all B |
More abundant in all B vs. all A |
|
| (n=9) | (n=8) | (n=8) | (n=12) | (n=11) | |||
| I | A | B1 | B2 | 4799 | 6875 | ||
| II | A1 | B1 | A2 | B2 | 3123 | 2894 | |
| III | A | B | 1457 | 1915 | |||
Comparisons I and III were analyzed by independent group t tests; Comparison II was analyzed by a dependent group t test
Figure 2. Venn diagram of disease-specific transcripts differentially abundant in acute KD subjects (n=20) compared to healthy controls (n=9) and convalescent KD subjects (n=20).
A. Overlap of probes that were less abundant in acute KD subjects and more abundant in both healthy controls and convalescent KD subjects. B. Overlap of probes that were more abundant in acute KD subjects and less abundant in both healthy controls and convalescent KD subjects.
Figure 3. Ingenuity Network Pathway Analysis of MMP-8, S100 proteins (S100A8, S100A9 and S100A12), and carcinoembryonic antigen-related cell adhesion molecules (CEACAM 1 and CEACAM 8).
A grey shaded molecule refers to a probe that was more abundant in acute KD subjects vs. both convalescent KD subjects and healthy control.
Figure 4. Ingenuity Canonical Pathway Analysis of the genes associated with the top 200 probes that are differentially abundant between acute KD subjects and both convalescent KD subjects and healthy control.
A grey shaded molecule refers to a probe that was more abundant in acute KD subjects vs. both convalescent KD subjects and healthy control.
3.2 Transcript abundance profiles reveal a role for the IL-1 signaling pathway in IVIG– resistant KD patients
Comparison between IVIG-responsive and –resistant KD subjects revealed 3,372 probes that were differentially expressed between these two groups, with 1,457 probes more abundant and 1,915 probes less abundant in IVIG-resistant subjects (Table 2). We compared the 2,575 disease-specific probes more abundant in acute KD than in healthy controls and convalescent KD with the 1,457 probes more abundant in IVIG-resistant subjects versus IVIG-responsive subjects (Figure 5). Among the resulting 356 probes, in order of abundance, were MMP-8, IL1 receptor 2, S100A8, interleukin-1 receptor-associated kinase, S100A9, and CEACAM1 (p <2.2×10−16; maximum q value 3.81%, Supplemental Table 2). The Ingenuity Canonical Pathway Analysis found that these 356 probes were associated with a broad range of genes in the IL-1 signaling pathway that encoded proteins expressed at the cell surface, in the cytoplasm, and in the nucleus (Figure 6) [22-25].
Figure 5. Venn diagram of overlap between disease-specific transcripts differentially abundant in acute KD (n=20) and IVIG-resistant KD subjects (n=8).
Overlap of probes that were more abundant in acute KD and IVIG-resistant KD subjects than in IVIG-responsive KD subjects.
Figure 6. Ingenuity Canonical Pathway Analysis of the genes associated with the 356 probes that were increased in both acute KD and IVIG-resistant KD subjects.
A grey shaded molecule refers to a probe that was more abundant in acute KD subjects vs. both convalescent KD subjects and healthy control as well as more abundant in IVIG-resistant compared to IVIG-responsive KD subjects.
We also tested the reciprocal situation with transcripts that were less abundant in acute KD and more abundant in IVIG resistance and transcripts that were more abundant in acute KD and less abundant in IVIG resistance. There was no significant overlap between these probe sets. Therefore, acute KD subjects and IVIG-resistant subjects shared the same pattern of gene expression with the IVIG-resistant subjects having higher transcript abundance of disease-specific genes.
3.3 Transcript abundance and serum levels of MMP-8
The probe for MMP-8 had the highest fold difference in acute KD compared to both convalescent KD and healthy controls, as well as in IVIG-resistant KD subjects compared to IVIG-responsive subjects. RT-PCR for MMP-8 transcripts from IVIG-resistant and IVIG-responsive subjects showed a strong correlation with the microarray results (correlation coefficient r=0.91; p <0.0001, 95% CI 0.79 to 0.97), thus confirming elevated levels of MMP-8 mRNA in IVIG-resistant subjects (Figure 7).
Figure 7. Comparison of MMP-8 relative transcript abundance levels by RT PCR in IVIG-resistant and IVIG-responsive KD subjects.
Box plots demonstrate the median, 25th and 75th percentiles. Whiskers identify the 5th and 95th percentiles.
MMP-8 serum levels were measured in an independent cohort of 26 paired acute and convalescent KD subjects. A statistically significant difference in median levels of MMP-8 was seen in this comparison with a median of 47,672 pg/ml and 10,371 pg/ml in acute and convalescent KD serum samples, respectively. (p <0.0001) (Figure 8). The MMP-8 serum levels were also measured between IVIG-resistant and –responsive KD subjects. No difference was detected between the median MMP-8 levels of these two groups, although power to detect a difference was limited by the small sample size.
Figure 8. Comparison of MMP-8 serum levels in an independent cohort of acute and convalescent KD subjects.
Box plots demonstrate the median, 25th and 75th percentiles. Whiskers identify the 5th and 95th percentiles.
3.4 Abundance of S100 proteins and CEACAM1 transcripts in IVIG-resistant acute KD
Transcripts of genes reported to be differentially expressed in KD were compared across acute and convalescent IVIG-resistant and IVIG-responsive subjects as well as healthy controls. Signal intensity was low for CEACAM1, S100A12, S100A8, S100A9 and C1Q in convalescent IVIG-resistant and IVIG-responsive KD subjects and healthy controls compared to acute KD subjects. During the acute phase, IVIG-responsive and IVIG-resistant KD subjects had similar elevations in C1Q and S100A12 probe intensity levels, which decreased in the convalescent phase (data not shown). Probe intensity for CEACAM1, S100A8, and S100A9 was higher in IVIG-resistant subjects compared to IVIG-responsive subjects (p =0.027, 0.027, and 0.040 respectively) (Supplemental Figure 1).
4. Discussion
We analyzed whole blood transcript abundance in acute and convalescent KD subjects and healthy pediatric controls. In an Ingenuity Pathway Analysis, the 200 highest intensity probes associated with acute KD were from genes in pathways for inflammatory and immunological diseases. Toll-like receptor signaling was the most significant canonical pathway of these top 200 probes. Subjects with acute KD were further subdivided based on response to therapy. Analysis of the 356 probes that were elevated both between acute and convalescent KD subjects and between IVIG-responsive and –resistant subjects demonstrated the importance of the IL-1 signaling pathway. In addition, probes for MMP-8 had the highest intensity and MMP-8 was the most differentially abundant transcript when comparing IVIG-responsive and -resistant KD subjects. This finding was confirmed by RT-PCR. This is the first microarray study to demonstrate that the IL-1 pathway and MMP-8 are important in KD pathogenesis, and, therefore, potential targets for further study and therapeutic intervention.
While increased transcript abundance for other matrix metalloproteinases, including MMP-9, has been found by microarray analysis of whole blood from acute KD subjects, this is the first time that increased transcript abundance of MMP-8 has been reported. This is important because MMP-8, also known as neutrophil collagenase, cleaves type 1 collagen in the extracellular matrix. Levels of MMP-8 are elevated in ruptured infarct tissue as compared to control myocardial infarction tissue, thus demonstrating the role of MMP-8 in myocardial destruction which may also be a factor in KD [26]. The previous microarrays used by our group did not have MMP-8 probes on the chip (Lymphochip, Stanford University) [1, 27]. The high levels of MMP-8 expression in KD [28, 29] subjects suggest that this collagenase may be a potential therapeutic target. The antibiotic, doxycycline, has been shown to inhibit abdominal aortic aneurysm formation in animals models and in limited human clinical trials by inhibiting matrix metalloproteinases [30-33]. A recent randomized, dose escalation study in adults with abdominal aortic aneurysms demonstrated reduced MMP-8 and MMP-9 levels in plasma as well as reduced neutrophil infiltration into the aortic wall in subjects treated with doxycycline compared to control subjects prior to surgical resection of the aneurysm [34]. These data suggest that doxycycline may be an effective adjuvant therapy for KD patients.
The increased transcript abundance of several genes in the IL-1 pathway in IVIG-resistant subjects is a novel finding and suggests that IL-1 may also be a therapeutic target in KD. Higher transcript abundance for IL-1 pathway genes was also found in a comparison of acute KD subjects with febrile control subjects with documented adenovirus infection [27]. A variety of biologic agents target the IL-1 pathway and include anakinra, an IL-1 receptor antagonist, the fusion protein rilonacept (Arcalyst®), and the monoclonal antibody canakinumab (Ilaris®) [28, 35, 36]. Targeted anti-IL-1 therapy may be a promising adjuvant therapy for acute KD. Given the acute, self-limited nature of KD, only a single dose would likely be required, which would circumvent the side effects associated with chronic use.
NF-κB is a critical transcription factor for genes that encode proinflammatory cytokines, so it was not surprising that several of the genes from the list of the top 200 probes with differential intensity between acute and convalescent KD subjects are members of this signaling pathway. NF-κB signaling has been shown to decrease in CD14+ monocytes/ macrophages from IVIG-responsive KD subjects after IVIG infusion [37]. The NF-κB signaling cascade is activated by several proinflammatory cytokines including tumor necrosis factor (TNF)-α which is a target in a current clinical trial for treatment of acute KD [38].
The rate of IVIG-resistance is increasing in Japan and the U.S. [7-9]. Since IVIG-resistance is associated with an increased rate of coronary artery aneurysms, it would be important to identify IVIG-resistant KD patients before initiation of therapy as they may benefit from additional anti-inflammatory treatment [8]. Our microarray analysis identified several genes that may be important both in predicting IVIG resistance and in defining disease-specific therapeutic targets for IVIG-resistant KD patients. Increased transcript abundance of the neutrophil-associated calcium binding proteins S100A8 and A9 in IVIG-resistant KD subjects confirms the role of activated neutrophils in the acute phase of KD. S100A8 and A9 have also been shown to regulate adhesion of neutrophils and monocytes to endothelial cells, a process that is likely important in the vasculitis of KD [20]. Given the role of the S100A8/A9 heterodimer in activating interleukin-1 receptor associated kinase-1 and NF-κB, which induce TNFα, it is not surprising that increased transcript abundance of these genes is associated with more inflammation and IVIG-resistance [39]. Increased transcript abundance of S100A9 was reported in acute vs. convalescent KD peripheral blood mononuclear cells [2, 3, 40]. The increased mRNA levels of several S100 proteins, including S100A8 and A9, have been previously confirmed by RT-PCR [2, 4, 41]. Persistent elevation of the plasma S100A8/S100A9 heterocomplex in acute KD has been associated with coronary artery aneurysms [2]. Taken together, these data emphasize the role of S100A8 and A9 in KD disease pathogenesis and suggest that these proteins may be useful biomarkers for identifying IVIG-resistant KD patients.
Our study confirms previous work by our group documenting the differential expression of CEACAM1 in IVIG-resistant vs. –responsive KD patients [1]. The CEACAM1 protein appears to have a role in angiogenesis via the vascular endothelial growth factor (VEGF) pathway [42]. Elevated plasma levels of VEGF have been detected in acute KD and immunohistochemistry has demonstrated VEGF in coronary arteries, where it may mediate the prominent neovascularization seen in the arterial wall [43, 44]. Increased CEACAM1 expression has also been associated with delayed neutrophil apoptosis, which suggests that apoptosis may be an important pathway for decreasing inflammation in KD [18, 45, 46]. Therefore, measuring concentrations of the alternatively spliced variants of CEACAM1 in plasma may reveal it to be a useful biomarker in predicting IVIG-resistance.
We recognize several limitations to our study. The cohort size was small for both our KD subjects and healthy controls and the small number of subjects with coronary artery abnormalities limited our power to detect a difference between groups. Transcript abundance patterns in whole blood may not accurately reflect gene expression patterns in the tissues where the pathologic changes associated with this vasculitis occur.
Conclusions
The increasing rate of IVIG-resistance in KD patients lead us to seek clues from whole blood transcript abundance patterns to identify biomarkers of IVIG-resistance and new targets for therapies in IVIG-resistant patients. Higher MMP-8,S100A8, S100A9, and CEACAM1 transcript levels were associated with IVIG-resistance, suggesting that these may be potential biomarkers for identifying these KD patients who are at highest risk for coronary artery aneurysms and might benefit from additional anti-inflammatory therapy. The novel finding that the IL-1 pathway and MMP-8 are important in acute KD suggests these as potential therapeutic targets.
Supplementary Material
Comparison of log transformed probe intensity of CEACAM1, S100A8, and S100A9 transcript abundance in IVIG-resistant subjects (acute and convalescent), IVIG-responsive subjects (acute and convalescent), and healthy controls. Box plots demonstrate the median, 25th and 75th percentiles. Whiskers identify the 5th and 95th percentiles. * Significant difference between acute IVIG-resistant subjects and IVIG-responsive subjects with p =0.027, 0.027, and 0.040 for CEACAM1, S100A8, and S100A9, respectively.
Acknowledgements
We thank DeeAnna Scherrer for technical assistance, Joan Pancheri for the data and sample collection, the Baylor Institute for Immunology Research NIAID Cooperative Centers Luminex Core Facility (Dr. John E. Connolly, director) for analysis of MMP-8 levels, Dr. David Relman and Dr. Stephen Popper for helpful discussion of this manuscript, and the families who participated in this study. The authors have received permission to use the copyrighted figures (Fig 3, 4, and 6) from Ingenuity Pathways Analysis. This work supported in part by grants from the National Institutes of Health/National Heart Lung Blood Institute awarded to JCB (HL69413 and K24 HL074864).
Abbreviations
- KD
Kawasaki disease
- IVIG
intravenous immunoglobulin
- ESR
erythrocyte sedimentation rate
- IL
interleukin
- MMP
matrix metalloproteinase
- CEACAM
carcinoembryonic antigen-related cell adhesion
- NF-κB
Nuclear factor kappa B
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Popper SJ, Shimizu C, Shike H, Kanegaye JT, Newburger JW, Sundel RP, Brown PO, Burns JC, Relman DA. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 2007;8(12):R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe J, Jibiki T, Noma S, Nakajima T, Saito H, Terai M. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J Immunol. 2005;174(9):5837. doi: 10.4049/jimmunol.174.9.5837. [DOI] [PubMed] [Google Scholar]
- 3.Nomura I, Abe J, Noma S, Saito H, Gao B, Wheeler G, Leung DY. Adrenomedullin is highly expressed in blood monocytes associated with acute Kawasaki disease: a microarray gene expression study. Pediatr Res. 2005;57(1):49. doi: 10.1203/01.PDR.0000147745.52711.DD. [DOI] [PubMed] [Google Scholar]
- 4.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ma X, Shimazu M, Otoguro T, Kobayashi K. Differential gene expression of S100 protein family in leukocytes from patients with Kawasaki disease. Eur J Pediatr. 2005;164(7):427. doi: 10.1007/s00431-005-1664-5. [DOI] [PubMed] [Google Scholar]
- 5.Furuno K, Takada H, Yamamoto K, Ikeda K, Ohno T, Khajoee V, Mizuno Y, Hara T. Tissue inhibitor of metalloproteinase 2 and coronary artery lesions in Kawasaki disease. J Pediatr. 2007;151(2):155. doi: 10.1016/j.jpeds.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324(23):1633. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 7.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17(12):1144. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uehara R, Belay ED, Maddox RA, Holman RC, Nakamura Y, Yashiro M, Oki I, Ogino H, Schonberger LB, Yanagawa H. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008;27(2):155. doi: 10.1097/INF.0b013e31815922b5. [DOI] [PubMed] [Google Scholar]
- 10.Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, Melish ME, Jackson MA, Asmar BI, Lang DJ, Connor JD, Capparelli EV, Keen ML, Mamun K, Keenan GF, Ramilo O. Infliximab Treatment of Intravenous Immunoglobulin-Resistant Kawasaki Disease. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113(22):2606. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J Pediatr. 2000;137(2):177. doi: 10.1067/mpd.2000.107890. [DOI] [PubMed] [Google Scholar]
- 13.Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166(2):131. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 14.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 15.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 16.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133(2):254. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 17.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 18.Singer BB, Klaile E, Scheffrahn I, Muller MM, Kammerer R, Reutter W, Obrink B, Lucka L. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur J Immunol. 2005;35(6):1949. doi: 10.1002/eji.200425691. [DOI] [PubMed] [Google Scholar]
- 19.Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168(10):5139. doi: 10.4049/jimmunol.168.10.5139. [DOI] [PubMed] [Google Scholar]
- 20.Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166(7):4678. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 21.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22(20):7158. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheper GC, Morrice NA, Kleijn M, Proud CG. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol Cell Biol. 2001;21(3):743. doi: 10.1128/MCB.21.3.743-754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18(45):6087. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 25.Blanco-Aparicio C, Torres J, Pulido R. A novel regulatory mechanism of MAP kinases activation and nuclear translocation mediated by PKA and the PTP-SL tyrosine phosphatase. J Cell Biol. 1999;147(6):1129. doi: 10.1083/jcb.147.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Borne SW, Cleutjens JP, Hanemaaijer R, Creemers EE, Smits JF, Daemen MJ, Blankesteijn WM. Increased matrix metalloproteinase-8 and -9 activity in patients with infarct rupture after myocardial infarction. Cardiovasc Pathol. 2009;18(1):37. doi: 10.1016/j.carpath.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Popper SJ, Watson VE, Shimizu C, Kanegaye JT, Burns JC, Relman DA. Gene Transcript Abundance Profiles Distinguish Kawasaki Disease from Adenovirus Infection. J Infect Dis. 2009;200(4):657. doi: 10.1086/603538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilowite N, Porras O, Reiff A, Rudge S, Punaro M, Martin A, Allen R, Harville T, Sun YN, Bevirt T, Aras G, Appleton B. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28(2):129. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, Anderson JP, Wanderer AA, Firestein GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364(9447):1779. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Boyle JR, McDermott E, Crowther M, Wills AD, Bell PR, Thompson MM. Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg. 1998;27(2):354. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 32.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23(3):483. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 33.Lau AC, Duong TT, Ito S, Wilson GJ, Yeung RS. Inhibition of matrix metalloproteinase-9 activity improves coronary outcome in an animal model of Kawasaki disease. Clin Exp Immunol. 2009;157(2):300. doi: 10.1111/j.1365-2249.2009.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Hussien H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, Lindeman JH. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49(3):741. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, Weinstein SP, Belomestnov P, Yancopoulos GD, Stahl N, Mellis SJ. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 36.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 37.Ichiyama T, Yoshitomi T, Nishikawa M, Fujiwara M, Matsubara T, Hayashi T, Furukawa S. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol. 2001;99(3):373. doi: 10.1006/clim.2001.5026. [DOI] [PubMed] [Google Scholar]
- 38.Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, Melish ME, Jackson MA, Asmar BI, Lang DJ, Connor JD, Capparelli EV, Keen ML, Mamun K, Keenan GF, Ramilo O. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153(6):833. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8(3):R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns JC. S100 proteins in the pathogenesis of Kawasaki disease. J Am Coll Cardiol. 2006;48(6):1265. doi: 10.1016/j.jacc.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Hirono K, Foell D, Xing Y, Miyagawa-Tomita S, Ye F, Ahlmann M, Vogl T, Futatani T, Rui C, Yu X, Watanabe K, Wanatabe S, Tsubata S, Uese K, Hashimoto I, Ichida F, Nakazawa M, Roth J, Miyawaki T. Expression of myeloid-related protein-8 and -14 in patients with acute Kawasaki disease. J Am Coll Cardiol. 2006;48(6):1257. doi: 10.1016/j.jacc.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 42.Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5(2):311. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- 43.Terai M, Yasukawa K, Narumoto S, Tateno S, Oana S, Kohno Y. Vascular endothelial growth factor in acute Kawasaki disease. Am J Cardiol. 1999;83(3):337. doi: 10.1016/s0002-9149(98)00864-9. [DOI] [PubMed] [Google Scholar]
- 44.Freeman AF, Crawford SE, Cornwall ML, Garcia FL, Shulman ST, Rowley AH. Angiogenesis in fatal acute Kawasaki disease coronary artery and myocardium. Pediatr Cardiol. 2005;26(5):578. doi: 10.1007/s00246-005-0801-2. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimoto H, Takeshita S, Nakatani K, Kawamura Y, Tokutomi T, Sekine I. Delayed apoptosis of circulating neutrophils in Kawasaki disease. Clin Exp Immunol. 2001;126(2):355. doi: 10.1046/j.1365-2249.2001.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsujimoto H, Takeshita S, Nakatani K, Kawamura Y, Tokutomi T, Sekine I. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin Immunol. 2002;103(2):161. doi: 10.1006/clim.2002.5209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of log transformed probe intensity of CEACAM1, S100A8, and S100A9 transcript abundance in IVIG-resistant subjects (acute and convalescent), IVIG-responsive subjects (acute and convalescent), and healthy controls. Box plots demonstrate the median, 25th and 75th percentiles. Whiskers identify the 5th and 95th percentiles. * Significant difference between acute IVIG-resistant subjects and IVIG-responsive subjects with p =0.027, 0.027, and 0.040 for CEACAM1, S100A8, and S100A9, respectively.