Abstract
Background
Acute ethanol is known to affect cells and organs but the underlying molecular mechanisms are poorly explored. Recent developments highlight the potential importance of mitogen activated protein kinases, MAPKs (i.e. ERK1/2, p38 and JNK1/2) signaling, and histone modifications (i.e. acetylation, methylation and phosphorylation) in the actions of ethanol in hepatocytes. We have therefore investigated significance of these molecular steps in vivo using a model in which rats were acutely administered ethanol intraperitoneally (IP).
Methods
Ethanol was administered IP (3.5 gm/kg body weight) to 12 weeks old male Sprague–Dawley rats. Liver was subsequently removed at 1 and 4 hr. Serum was used for alcohol and ALT assays. At the time of the removal of liver, small portions of each liver were formalin-fixed and stained with hematoxylin and eosin (H&E) and used for light microscopy. Western blot analysis was done with specific primary antibodies for various parameters.
Results
There were clear differences at 1 and 4 hr in blood ethanol, ALT, steatosis, and cleaved caspase 3. Apoptosis at 1 h was followed by necrosis at 4 hr. Acute alcohol elicited a marked increase in the phosphorylation of ERK1/2 and moderate increases in the phosphorylation of p38 MAPK and JNK. Temporally different phosphorylation of histone H3 at ser-10 and ser -28 occurred and acetylation of histone H3 at lys 9 increased progressively.
Conclusions
There were distinct differences in the behavior of the activation of the three MAP kinases and histone modifications after acute short exposure of liver to ethanol in vivo. Although all three MAPKs were rapidly activated at 1 h, the necrosis, occuring at 4 h, correlated to sustained activation of ERK1/2. Transient activation of p38 is associated with rapid phosphorylation of histone H3 whereas prolonged activation of ERK1/2 is correlated to persistent histone H3 acetylation.
Keywords: Epigenetics, MAPK signaling, Alcoholic liver disease, Binge drinking, Steatosis
Alcoholic liver disease (ALD) is the most common hepatic disease in the western countries. More than 18 million adults in the United States abuse alcohol (Lucey et al., 2009, Purohit et al., 2009) and its chronic consumption causes liver damage leading to steatosis, alcoholic hepatitis, fibrosis, cirrhosis and leads to the development of hepatocellular carcinoma in susceptible individuals (Lucey et al., 2009, Purohit et al., 2009). Acute liver injury induced by ethanol is also drawing attention because the incidence of acute alcoholism or binge drinking is on the rise worldwide (Mathurin and Deltenre, 2009). Moreover, liver is sensitized to injury after acute ethanol in burn injury (Emanuele et al., 2009). Although mechanisms and mediators of chronic alcoholic liver injury have been extensively studied, little is known about the mechanisms underlying acute alcoholic liver injury.
The mitogen-activated protein kinase (MAPK) pathways represent a converging point for many signaling pathways, including tyrosine and serine/threonine kinases, G proteins, and calcium signaling (Roux et al., 2004). The most common MAPKs are the extracellular signal-regulated kinases ERK1 and ERK2 (also known as p44 and p42MAPK, respectively), p38MAPK, and c-Jun-N-terminal kinase/stress-activated protein kinases (JNK/SAPK). MAPKs regulate a variety of biologic processes, eg. cell growth and proliferation, chemotaxis, inflammation, steatosis, necrosis and apoptosis (Boutrous, 2008; Brown and Sacks, 2008). The activated MAP kinases translocate into nucleus and then phosphorylate transcription factors, and thereby regulate transcription. In addition to phosphorylating specific transcriptional factors, MAP kinases and their downstream kinases are implicated in alterations in chromatin environment by modulating the phosphorylation and acetylation of nucleosomal and chromatin proteins (Bártová et al., 2008, Delcuve et al., 2009). Histone H3 at ser-10 and ser-28 are rapidly and transiently phosphorylated during immediate-early response of mammalian cells to extracellular stimuli (Delcuve et al., 2009). There are a few kinases identified as histone H3 kinase, eg, mitogen-and stress-activated kinase-1 (MSK1), ribosomal S6 kinase 2 (RSK2), aurora B, and IkB kinase α (Bártová et al., 2008). RSK2, a downstream kinase of ERK1/2, is shown to be required for epidermal growth factor (EGF)-stimulated phosphorylation of histone H3 (Sassone-Corsi, et al., 1999). Histone H3 phosphorylation by p38 MAPK involves downstream kinase MSK-1 (Cheung, et al., 2000; Zhong et al., 2001). Acetylation of histone is also closely related to regulation of transcriptional activity (Delcuve et al., 2009). The regulation of histone acetylation may be indirect through phosphorylation of histone H3 by MAPKs (Sassone-Corsi, et al., 2000) or direct through phosphorylation of histone acetyltransfearses by MAPKs (Ait-Si-Ali S et al., 1999; Kawasaki et al., 2000; Merienne et al., 2001).
A role for MAPKs and epigenetic histone modifications in alcoholic organ damage is emerging (Aroor and Shukla, 2004; Shukla et al., 2008). However, the relationship between MAPK signaling and epigenetic histone modifications in acute alcoholic liver injury is not known. In primary culture of rat hepatocytes, ethanol caused moderate activation of ERK1/2 and marked activation of JNK (Lee et al., 2002). Although phospho-p38 MAPK was not affected in the cytosol, nuclear p38 MAPK phosphorylation was increased by ethanol (Lee and Shukla, 2007). Previously we have reported histone H3 lys 9 acetylation and histone H3 ser-10 and ser-28 phosphorylation in primary cultures of hepatocytes (Park et al., 2005; Lee and Shukla, 2007). In hepatocytes, histone H3 lys 9 acetylation was decreased by inhibition of ERK1/2 and JNK whereas histone H3 ser-10 and ser-28 phosphorylations were reduced by inhibition of p38 MAPK (Park et al., 2005; Lee and Shukla, 2007). Although the activation of MAPKs and histone modifications by ethanol have been studied in vitro, very little is known about the activation of MAPKs and their relationship to histone acetylation and phosphorylation in liver acutely exposed to ethanol in vivo. This was investigated here.
MATERIALS AND METHODS
Reagents
Antibodies to phospho-ERK1/2, ERK1/2 protein, phospho-p38 MAPK, p38 MAPK protein, phospho-JNK1/2, JKN1/2 protein and calreticulin were purchased from Cell Signaling (Danvers, MA). Antibodies to phospho-H3 ser 10, Phospho-H3 ser 28, H3 protein and acetyl –H3 lys 9 were obtained from Millipore (Temecula, CA). Protease inhibitors cocktail (p8340), anti β-actin antibody and assay kit for triglycerides were obtained from the Sigma-Aldrich (St. Louis, MO).
Acute ethanol administration
Twelve week old male Sprague–Dawley rats, each weighing between 300–350 g, were purchased from Harlan Laboratories (Indianapolis, IN). They were housed under a 12-h/12-h light/dark cycle. All animals had free access to water and were permitted ad libitum consumption of standard laboratory rat chow. After one week, rats were divided into two groups: control and acute ethanol group. Ethanol was administered intraperitoneally (ip) (32%, v/v in saline 3.5 gm/kg body weight) and liver was subsequently removed at 1 and 4 hr. In the control group, ethanol was replaced by saline. Blood samples were collected at 1 hr and 4 hr after binge ethanol administration. A small portion of the liver was placed in formalin and remaining portion of the liver was frozen in liquid nitrogen and stored at −70°C. This study conformed to the Guide for the Care and Use of Laboratory Animals published by National Institutes of Health and the protocol for their use was approved by the University of Missouri Animal Care Committee.
Determination of serum ethanol and ALT
Blood alcohol was determined by alcohol dehydrogenase assay using kit from Genzyme Diagnostics Framingham, MA. Serum alanine aminotransferase (ALT) levels were determined by kinetic ALT assay in an automated analyzer.
Determination of hepatic triglyceride
For the determination of triglycerides, 100 mg of liver was homogenized in 8 volumes of hypotonic buffer containing 20 mM Tris, 50 mM EGTA and Sigma protease inhibitor cocktail ( p8340) followed by addition of one volume of 5 M NaCl. 3.75 ml of chloroform – methanol (2:1) was added and the resulting homogenate was vortexed and left for 30 min at room temperature. After addition of 1.25 ml chloroform and 1.25 ml of water, the contents were mixed well and centrifuged at 1000 g for 5 min. The lower organic phase was removed and evaporated under nitrogen gas. The resulting lipid material was dissolved in 1.0 ml of phosphate buffered saline containing 2% Triton X -100. One hundred microliters of Triton X-100 fraction was used for triglyceride estimation using the assay kit as detailed by the supplier.
Histopathology
After formalin fixation, specimens were sectioned and stained with hematoxylin and eosin (H&E) and used for light microscopy.
Preparation of cytosolic and nuclear extracts
The cytosolic and nuclear protein extracts were obtained following our previously published protocols with some modifications (Park et al., 2005, Aroor et al, 2009). All steps were conducted at 4°C. One gram of frozen liver was homogenized in lysis buffer containing (50 mM Tris. HCl. pH 7.4, 25 mM KCl, 5 mM MgCl2, 5 mM glycerophosphate, 1 mM EDTA, 1 mM Na-orthovanadate, 1 mM EGTA, 1 mM DTT, and Sigma protease inhibitor cocktail ( p8340). The homogenate was layered over 1.0 M sucrose cushion and centrifuged at 1600 g for 10 min at 4 °C. The supernanatant containing cytosolic proteins was transferred to a precooled microcentrifuge tube, frozen in liquid nitrogen and stored at −70C. The nuclear pellet was resuspended in 1.0 M sucrose containing 0.25 % NP-40. The nuclear suspension was passed through a 26-gauge needle 6 times, followed by centrifugation at 1600 g for 10 min. This step successfully removed contaminated endoplasmic reticulum and plasma membrane. The nuclear pellet was re-suspended again in 1.0M sucrose. After centrifugation at 1600 g for 10 min, the pellet containing nuclei was washed once with homogenization buffer. The nuclear fractions were examined under light microscope for purity of nuclei and were free of membrane contamination and other subcellular organelles. The isolated nuclei preparations were solubilized in high salt detergent buffer (0.5M NaCl, 1% Triton X-100, 1% deoxycholate and 0.1% SDS). The nuclear preparations were sonicated for 5 sec. After centrifugation at 14000 g for 10 min, the supernatant was used as nuclear fraction. The purity of cytosolic and nuclear fractions were verified by the presence of β tubulin and absence of histone H3 in the cytosolic fraction and absence of β tubulin and presence of histone H3 in the nuclear fraction as reported earlier (Lee and Shukla 2007). The nuclear preparations were also free from contamination with endoplasmic reticulum (ER) as evaluated by western blot with calreticulin, a marker for ER. For histone extraction, nuclear pellets were resuspended in 0.4 N HCl with 10 % glycerol and centrifuged at 12,000 g for 10 min. The supernatant fraction (acid-soluble) is carefully collected, precipitated with trichloroacetic acid (final concentration 20%, w/v), washed with acetone, dried under the vacuum and dissolved in distilled water. Protein concentrations in cytosolic and nuclear extracts were measured using the Bio-Rad DC protein assay.
Immunoblot analysis
The cytosolic extract (80 μg) and nuclear extracts (40 μg for MAPKs and 10 μg for histones) were subjected to 10% SDS-PAGE and electrophoretically transferred onto nitrocellulose membrane (Bio-Rad) using Bio-Rad Trans-Blot apparatus. The membrane was washed with 20 mM Tris, pH 7.5, containing 0.1% Tween 20 and 150 mM NaCl (TBST) and incubated with TBST containing 5% nonfat dry milk for 2 h at room temperature. The membrane was next incubated with antibody to phospho- or total p42/p44 ERK1/2, p38 MAPK, JNK 1/2 overnight at 4°C. For western blot of cleaved caspase 3, membrane was incubated with antibody to cleaved caspase 3 (1:1000 dilution). After washing with TBST, the membrane was incubated with secondary antibody conjugated horseradish peroxidase for 1 h at room temperature. The horseradish peroxidase was detected by enhanced chemiluminescence (ECL) (Supersignal, Pierce Chemical, Rockford, IL). The membrane treated with ECL reagent was exposed to x-ray film or scanned with a LAS-3000 imaging system (Fujifilm life science). Quantitation of the data was done using MultiGauge ™ software. The intensity of the chemiluminescence was always determined within the linear range of detection. For repeat immunoblotting, membrane was stripped using Restore Western blot stripping buffer (Pierce). Equal loading of protein was confirmed by determining β actin levels for cytosolic extracts and histone H3 protein levels for nuclear extracts. Levels of β-actin or histone H3 did not change after acute ethanol exposure.
Data analysis
All results are expressed as mean ± S.E and were obtained by combining data from individual experiments. Graph Pad PRISM (version 4) software was used for statistical analysis using the Student t test (two-tailed, unpaired). Differences with a P value of <0.05 were considered statistically significant.
RESULTS
Effect of acute ethanol binge on liver injury in rats
Intraperitoneal administration of 3.5 gm of ethanol/kg body weight to rats resulted in marked elevation of ethanol levels in the blood. It ranged 60–90 mmol/L at 1 hr and 36–66 mmol/L at 4 hr (Fig 1). The magnitude of increase seen in these experiments was similar to levels seen after heavy consumption of alcohol in humans (Dietrich and Harris, 1996). In one series of study, 7.2 % of patients with alcoholic intoxication (190 out of 1250 patients with alcohol detected in the blood) had blood levels exceeding 60 mmol/L with alcohol levels reaching up to 100 mmol/L in the blood in some patients (Rivara et al., 1993). Alcohol caused mild apoptosis at early time points (1 hr) as evaluated by the increased levels of cleaved caspase 3 (Fig 2A). There was no significant necrosis at 1 h (Fig 2A). However, at 4 h ethanol significantly increased hepatic necrosis as indicated by the release of ALT from hepatocytes; a 4.2 fold increase in serum ALT levels (Fig 2B). Histochemical examination of liver sections revealed mild steatosis in acute ethanol treated rat liver at 4 (Fig 2.C) but not at 1 h (data not shown). The presence of stetaosis is further supported by 1.7 fold increase in liver triglycerides at 4 hr after ethanol treatment (Fig. 2D).
Fig. 1. Serum ethanol levels after acute ethanol administration.
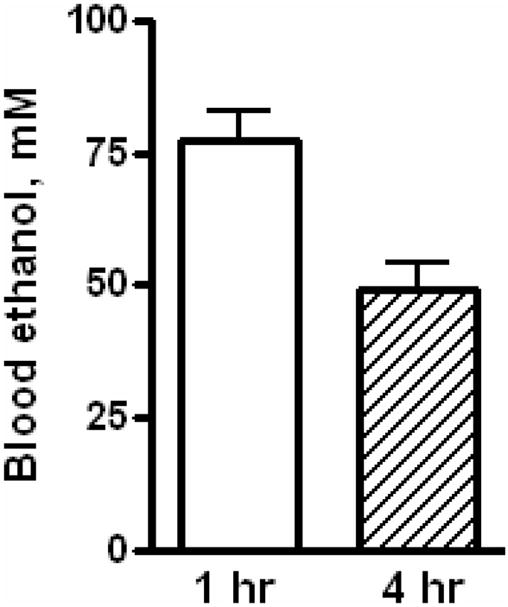
Rats were given IP a single ethanol binge dose (3.5 gm/kg) and the levels of serum ethanol were determined at 1 hr and 4 hr as described under materials and methods. Control represents animals given saline for binge control. Values are mean ± SE (n=5 rats).
Fig. 2. Serum ALT, cleaved caspase 3, and steatosis after acute ethanol administration.
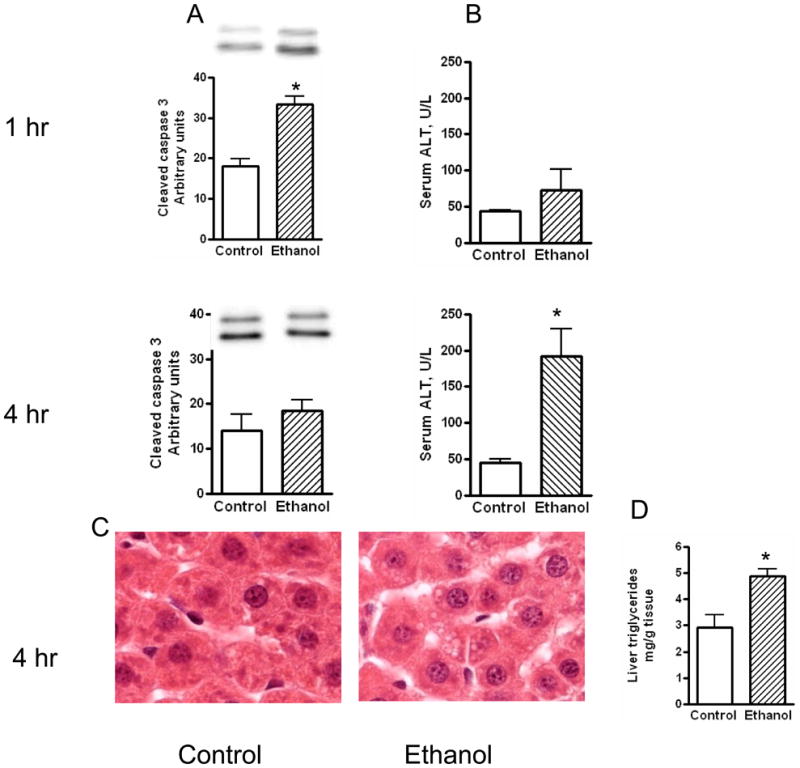
Rats were given IP a single ethanol binge dose (3.5 gm/kg) and the levels of serum ALT were determined at 1 hr and 4 hr as described under materials and methods. Cleaved caspase 3 levels were determined by western immunoblot at 1 hr and 4 hr after ethanol administration. Steatosis was monitored at 1hr and 4 hr after ethanol administration. Sections of liver samples were stained with hematoxylin and eosin. Liver triglycerides were determined at 4 hr by colorimetric method. Control represents animals given saline for binge control. Values are mean ± SE (n=4 to 5 rats) * significant compared to control group (p<0.05).
Activation of mitogen activated protein kinases after ethanol binge
We have determined the activation of MAPK by evaluating western immunoblots of phosphorylated MAPKs. The sample loading were similar for individual samples as evaluated by western blot for β actin (data not shown). Moreover, the protein levels of MAPK did not change between the control and ethanol treated groups (data not shown). We have previously shown a relatively modest increase in the phosphorylation of ERK1/2 and marked increase in phosphorylation of JNK in the cytosolic fractions at 1 hr after addition of ethanol to cultured primary hepatocytes (Lee et al., 2005; Lee and Shukla, 2007). In contrast to vitro findings, acute administration of ethanol in vivo produced a large increase in the phosphorylation of ERK1/2 levels and a smaller increase in phospho-JNK1/2 after 1 hr of ethanol administration (Fig. 3). The mean increases in phospho-ERK1 and phospho-ERK2 levels were 6.0 fold & 9.2 fold, respectively. The magnitude of increase in phospho-JNK 1 and JNK 2 levels (2.1 and 2.4 fold, respectively) were less pronounced than increase in phospho-ERK1/2. Ethanol activated p38 MAPK with a 1.5 fold increase in the levels of cytosolic phosphorylated p38 MAPK at 1hr. When MAPKs were monitored at 4 hr, activation of ERK1/2 was still significantly elevated (2.3 fold for ERK1 and 5.1 fold for ERK2) compared to control although the magnitude of increase was blunted at 4 hr as compared to 1 hr. In contrast to ERK1/2, the activation of JNK and p38 MAPK were transient and the levels of phosphorylated JNK and p38 MAPK at 4 hr were not significantly different from control group. These results suggest marked and prolonged activation of ERK1/2 by acute ethanol treatment in liver in vivo.
Fig. 3. Levels of phosphorylated ERK1/2, p38 MAPK and JNK1/2 in cytosolic extracts.
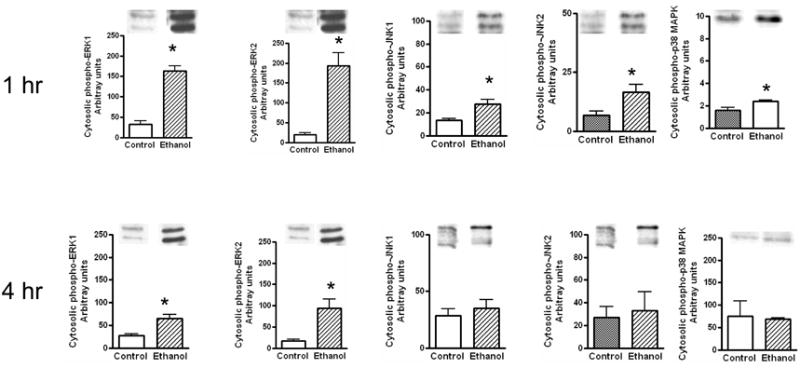
Ethanol binge was administered as described in Fig. 1 The levels of phosphorylated ERK1/2, p38 MAPK and JNK1/2 in cytosolic cell extracts were determined at 1 hr and 4 hr. Control represents animals given saline for binge control. Values are mean ± SE (n= 3 to 5 rats) * significant compared to control group (p<0.05)
The effects of ethanol on changes in the levels of phosphorylated-MAPKs in the nuclear extracts of liver after acute ethanol administration are shown in Fig. 4. Loading of samples were similar for control and ethanol treated samples as assessed by western immunoblot of histone H3 or β-actin protein levels. The increase in the levels of phosphorylated ERK1/2 in the nuclear extracts was significantly higher (4.7 for ERK1 and 4.9 fold for ERK2) at 1 hr. Moreover, the increases in the levels of phosphorylated ERK 1/2 were also significant (2.6 fold and 2.4 fold) at 4 hr. Increases in the levels of phosphorylated JNK1/2 (1.45 and 1.9 fold respectively) and p38 MAPK (1.5 fold) after 1 hr of ethanol treatment in the nuclear compartment was also moderate. At 4 hr, the levels of phosphorylated JNK1/2 and p38 returned to near normal values.
Fig. 4. Levels of phosphorylated ERK1/2, p38 MAPK and JNK1/2 in nuclear extracts.
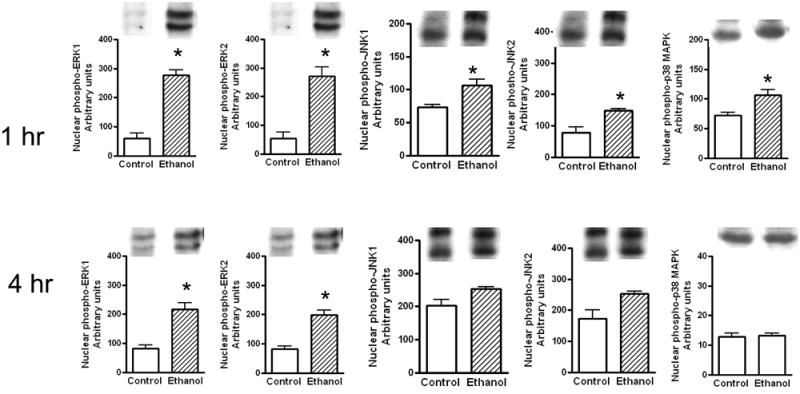
Ethanol binge was administered as described in Fig. 1. The levels of phosphorylated ERK1/2, p38 MAPK and JNK1/2 in nuclear extracts were determined at 1 hr and 4 hr as described under materials and methods. Control represents animals given saline for binge control. Values are mean ± SE (n=3 to 4 rats) * significant compared to control group (p<0.05).
Acetylation and phosphorylation of histone H3
We next determined the phosphorylation of histone H3 at ser-10 and ser-28 at 1hr and 4r hr after IP ethanol administration. As shown in Fig. 5 the phosphorylation of histone H3 at ser-10 and ser -28 increased significantly at 1 hr. Phosphorylation of histone H3 ser-28 (1.9 fold increase) was slightly higher compared to phosphorylation of histone H3 ser-10 (1.6 fold increase). The levels of these phosphorylations returned to near control levels at 4 hr. In contrast to transient nature of histone phosphorylation, alcohol caused sustained increase in histone acetylation. Acetylation of histone H3-lys 9 at 1hr was marginally increased but was significant at 4 hr (1.4 fold, Fig. 5). A comparative assessment of the changes in the levels of phosphorylated MAPKs in the cytosolic and nuclear fractions and changes in the levels of modified histones is given in Table 1.
Fig. 5. Levels of phosphorylated histone and acetylated histone after acute ethanol administration.
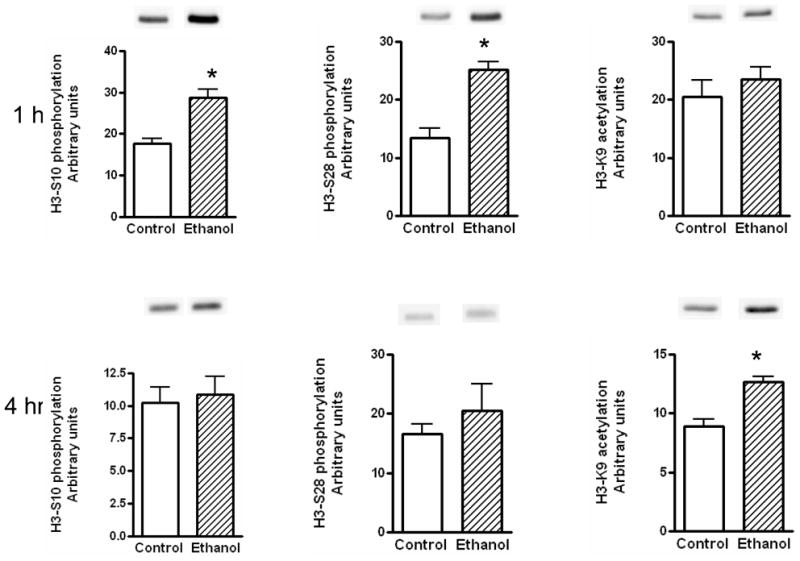
Rats were given IP a single ethanol binge dose (3.5 gm/kg) and levels of phosphorylated H3 ser-10 & ser-28 and acetylated H3 Lys 9 in nuclear extracts were determined at 1 hr and 4 hr as described under materials and methods. Values are mean ± SE (n=4 to 5 rats) *significant compared to control group (p<0.05).
Table 1.
MAPK activation and histone modifications after acute alcohol treatment of rats
| 1 hr | 4 hr | |
|---|---|---|
| Injury | ||
| Apoptosis | ++ | ± |
| Steatosis | ± | + |
| Necrosis | ± | ++ |
| MAPK phosphorylation: Cytosolic | ||
| ERK1 | ++++ | ++ |
| ERK2 | ++++ | ++ |
| JNK1 | ++ | + |
| JNK2 | ++ | + |
| p38 MAPK | ++ | + |
| MAPK phosphorylation: Nuclear | ||
| ERK1 | ++++ | ++ |
| ERK2 | ++++ | ++ |
| JNK1 | ++ | + |
| JNK2 | ++ | + |
| p38 MAPK | + | ± |
| Epigenetic histone modifications | ||
| H3 Ser 10 phosphorylation | ++ | ± |
| H3 Ser 28 phosphorylation | ++ | ± |
| H3 K9 acetylation | + | ++ |
DISCUSSION
To the best of our knowledge, no study has been reported on the activation pattern of liver cell MAPK during acute ethanol binge in vivo. This study has assessed the relationship among MAPK activation, histone modifications and liver injury induced by acute intraperitoneal ethanol administration. We noted some contrasting effects of acute ethanol on MAPK signaling in vivo compared to the effects of ethanol on MAPK activation in primary cultured rat hepatocytes (Lee et al., 2002; Lee and Shukla, 2007). While ERK1/2 activation was modest in vitro, increase in the phosphorylation of ERK1/2 by ethanol in vivo was more dramatic and marked. In contrast, phosphorylation of JNK1/2 was more marked in cultured hepatocytes whereas its activation in vivo after acute ethanol was moderate. In cultured hepatocytes, we have demonstrated increased phosphorylation of JNK by ethanol when ERK1/2 phosphorylation was inhibited by U-0126, a MEK1/2 inhibitor (Lee and Shukla, 2005). Recently, administration of resistin to endotoxin treated mice resulted in increased phosphorylation of ERK1/2 with concomitant decrease in phosphorylation in JNK1/2 (Beier et al., 2008). Therefore, marked activation of ERK1/2 with modest activation of JNK suggests the possibility of cross talk between these two signaling pathways after acute ethanol treatment in vivo. With regard to the phosphorylation of p38 MAPK, acute ethanol administration in vivo caused 1.6 fold increase in p38 MAPK in cytosolic extracts (Fig. 2) whereas no such increase in phosphorylation of p38 MAPK in the cytosolic fraction was observed in cultured hepatocytes (Lee and Shukla, 2007). However, when hepatocytes were cultured in the presence of serum, in contrast to our studies on serum free medium, increased phosphorylation of p38 MAPK was seen in cytosolic extracts (Zhang et al., 2007). These results suggest further modulation of ethanol induced MAPK activation by serum derived factors or cytokines in vitro and in vivo. In this regard, acute ethanol has been shown to cause hypoxia in liver (French, 2004) and hypoxia is associated with activation of three MAPKS in the cytoplasmic fraction in liver (Kaizu et al., 2008).
Pattern of caspase 3 cleavage and ALT at 1 and 4 h lead us to suggest that apoptosis precedes necrosis in acute binge. In hepatocyte cultures, transient activation of JNK1/2 was associated with transient apoptosis. The findings of in vitro are also seen in vivo after acute ethanol treatment. Moreover, marked activation of JNK1/2 correlated with increase in apoptosis. In contrast to marked apoptosis of hepatocytes, necrotic damage to hepatocytes were minimal in vitro as evaluated by LDH release (Weng and Shukla, 2000; Lee and Shukla 2005). On the other hand, acute ethanol in vivo caused significant increase in necrosis. This correlated with activation of ERK1/2 in vivo. Although ERK1/2 has been reported to mediate suppression of necrotic response in vitro and in vivo, ERK1/2 activation under hypoxic conditions has been shown to promote necrotic cellular injury in vivo and in vitro (Schattenberg et al., 2004, Sabbatini et al., 2006). Ethanol has been shown to induce hypoxic damage at high blood ethanol levels under both acute and chronic conditions in vivo (French, 2004).
Ethanol induced phosphorylation of histone in vivo. Acetylation of histone H3 lys 9 also increased after IP administration of ethanol. Although the type of MAPKs involved in ethanol induced histone phosphorylation and acetylation in vivo remain to be determined, our studies with hepatocyte suggested regulation of histone phosphorylation by p38 MAPK, and histone acetylation by ERK1/2 and JNK1/2 (Park et al., 2005; Lee and Shukla, 2007). The pattern of histone acetylation and phosphorylation by ethanol also demonstrated distinct temporal changes. Although the time course of histone acetylation induced by ethanol at 1 hr and 4 hr in vitro and in vivo are comparable, the magnitude of histone acetylation in vitro was more marked compared to in vivo. The increased acetylation in vitro may be due to marked activation of JNK1/2 in vitro since histone acetylation was dependent on both ERK1/2 and JNK1/2 activation in hepatocytes (Park et al, 2005). Interestingly, histone H3 ser-10 and ser-28 phosphorylations were evident at 1 hr and declined at 4 hr where as histone aectylation increased progressively with further increase at 4 hr both in vitro (Park et al., 2005; Lee and Shukla, 2007) and in vivo (the present study). The persistent acetylation observed at 4 hr may be due to persistent accumulation of phosphorylated ERK/2 in hepatocyte nucleus or due to MAPK independent effects of ethanol metabolite acetate as observed in in vitro hepatocyte cultures (Park et al., 2005). In contrast, the transient nature of histone H3 ser-10 and ser-28 phosphorylation may be linked to transient activation of p38 MAPK in the nuclear compartment after ethanol treatment. Although we have consistently observed ethanol induced H3 lys 9 acetylation, significant increase in H3K9 acetylation was not demonstrated after acute bolus administration of ethanol orally in another study (Bardag-Gorce et al., 2009). The discrepancy may be attributed to their administration of dextrose as control for ethanol binge. We have observed that histone is acetylated after administration of dextrose alone orally as bolus (unpublished observations).
The issue of how MAP kinase cascades can transduce the acetylation of nucleosomal and chromatin proteins in response to extracellular signals, remains to be known. MAPKs may cause increased phosphorylation of histone, and phosphorylated histone has been shown to be a better substrate for histone acetylation (Merienne et al., 2001). The HAT GCn5 displays a strong preference for phosphorylated histone H3 over non-phosphorylated histone H3 as substrate in vitro, suggesting that histone H3 phosphorylation can affect the efficiency of subsequent acetylation reactions (Cheung et al., 2000). Interestingly, GCn5 has been implicated in ethanol induced histone acetylation (Choudhury and Shukla, unpublished). Other human HATs such as PCAF and p300 also prefer phosphorylated histone H3 as substrate (Clayton et al., 2003). Taken together it is apparent that histone H3 is phosphorylated via MAP kinase cascades. It can be speculated that histone kinases downstream of MAP kinase, such as Rsk, are activated by ERK1/2 and that the resulting histone phosphorylation triggers further histone acetylation. However, ethanol induced phosphorylation of histone H3 was not inhibited by ERK1/2 (Lee and Shukla, 2007). Moreover, inhibition of p38 MAPK pathway in hepatocytes inhibits ethanol induced phosphorylation without affecting histone acetylation (Lee and Shukla, 2007). MAPKs phosphorylate several types of HATs (e.g., CBP, p300, ATF-2, and SRC-1) and directly increase their enzymaticactivities (Ait-Si-Ali et al., 1999; Kawasaki et al., 2000; Li et al., 2001). p300, a substrate of ERK1/2 in vivo and in vitro (Li et al., 2003) has been shown to acetylate histone H3.
Epigenetic histone modifications contribute to inflammatory response, necrosis, and apoptosis under diverse types of tissue injury (Waring, 1997; Tikoo et al., 2001, Bártová et al., 2008, Delcuve et al., 2009). The role of epigenetic histone modifications in alcoholic organ damage is poorly understood (Shukla et al., 2008). Histone H3 phosphorylation by ethanol in hepatocytes had no influence on apoptosis (Lee and Shukla, 2007). On the other hand, modifications of histones have been shown to be relevant to transcriptional activation induced by ethanol. Histone H3 acetylation is linked to ethanol induced regulation of ADH1 gene expression in vitro (Park et al., 2005; Pal-Bhadra et al., 2007) and expression of lipogenic genes after chronic ethanol treatment in vivo (You et al., 2008). We believe that differential behaviors of histone acetylation and transient increase in histone H3 phosphorylation may determine activation of different sets of genes, perhaps, in a sequential manner.
In summary, acute administration of ethanol caused a marked increase in the phosphorylation of ERK1/2 and modest increase in the phosphorylation of p38 MAPK and JNK. Increased phosphorylation of ERK1/2 and JNK1/2 correlated to significant acetylation of histone H3 K9, and the increased phosphorylation of p38 MAPK correlated to increased phosphorylation of histone H3 ser-10 and ser-28. Taken together, these results suggest the existence of molecular axis between MAPK and epigenetic histone modifications in ethanol induced acute alcoholic liver injury in vivo.
Acknowledgments
This work was supported by NIH grant AA11962.
References
- Ait-Si-Ali S, Carlisi D, Ramirez S, Upegui-Gonzalez LC, Duquet A, Robin PR, Rudkin B, Harel-Bellan A, Trouche D. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999;262:157–162. doi: 10.1006/bbrc.1999.1132. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Lee YJ, Shukla SD. Activation of MEK 1/2 and p42/44 MAPK by angiotensin II in hepatocyte nucleus and their potentiation by ethanol. Alcohol. 2009;43:315–322. doi: 10.1016/j.alcohol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Dedes J, Li J, French BA, French SW. Chronic ethanol feeding alters hepatocyte memory which is not altered by acute feeding. Alcohol Clin Exp Res. 2009;33:684–692. doi: 10.1111/j.1530-0277.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bártová E, Krejcí J, Harnicarová A, Galiová G, Kozubek S. Histone modifications and nuclear architecture: a review. J Histochem Cytochem. 2008;56:711–721. doi: 10.1369/jhc.2008.951251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE. New role of resistin in lipopolysaccharide-induced liver damage in mice. J Pharmacol Exp Ther. 2008;325:801–808. doi: 10.1124/jpet.108.136721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Brown MD, Sacks DB. Compartmentalized MAPK pathways. Handb Exp Pharmacol. 2008;186:205–35. doi: 10.1007/978-3-540-72843-6_9. [DOI] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–58. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Rastegar M, Davie JR. Epigenetic regulation. J Cell Physiol. 2009;219:243–250. doi: 10.1002/jcp.21678. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, Emanuele MA, Morgan MO, Sulo D, Yong S, Kovacs EJ, Himes RD, Callaci JJ. Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. J Burn Care Res. 2009;30:482–488. doi: 10.1097/BCR.0b013e3181a28df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW. The role of hypoxia in the pathogenesis of alcoholic liver disease. Hepatol Res. 2004;29:69–74. doi: 10.1016/j.hepres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Schiltz L, Chiu R, Itakura K, Taira K, Yokoyama KK. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195–200. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, et al. Protection of transplant induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway down regulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236–G244. doi: 10.1152/ajpgi.00144.2007. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J Pharmacol Exp Ther. 2002;301:908–914. doi: 10.1124/jpet.301.3.908. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur J Pharmacol. 2005;508:31–45. doi: 10.1016/j.ejphar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J Pharmacol. 2007;573:29–38. doi: 10.1016/j.ejphar.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol. 2001;21:8213–24. doi: 10.1128/MCB.21.23.8213-8224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Yang SH, Maeda Y, Sladek FM, Sharrocks AD, Martins-Green M. MAP kinase phosphorylation-dependent activation of Elk-1 leads to activation of the co-activator p300. EMBO J. 2003;22:281–291. doi: 10.1093/emboj/cdg028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- Merienne K, Pannetier S, Harel-Bellan A, Sassone-Corsi P. Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities. Mol Cell Biol. 2001;21:7089–7096. doi: 10.1128/MCB.21.20.7089-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Jackson DE, Mamatha L, Park PH, Shukla SD. Distinct methylation patterns in histone H3 at Lys-4 and Lys-9 correlate with up- & down-regulation of genes by ethanol in hepatocytes. Life Sci. 2007;81:979–987. doi: 10.1016/j.lfs.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PH, Lim RW, Shukla SD. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. Am J Physiol Gasterointest Liver Physiol. 2005;289:G1124–G1136. doi: 10.1152/ajpgi.00091.2005. [DOI] [PubMed] [Google Scholar]
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivara PF, Jurkovich GJ, Gurney JG, Seguin D, Fligner CL, Ries R, Raisys VA, Copass M. The magnitude of acute and chronic alcohol abuse in trauma patients. Archives of Surgery. 1993;128:907–913. doi: 10.1001/archsurg.1993.01420200081015. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg JM, Wang Y, Rigoli RM, Koop DR, Czaza MJ. CYP2E1 overexpression alters hepatocyte death from menadione and fatty acids by activation of ERK1/2 signaling. Hepatology. 2004;39:444–455. doi: 10.1002/hep.20067. [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Santillo M, Pisani A, Paternò R, Uccello F, Serù R, Matrone G, Spagnuolo G, Andreucci M, Serio V, Esposito P, Cianciaruso B, Fuiano G, Avvedimento EV. Inhibition of Ras/ERK1/2 signaling protects against postischemic renal injury. Am J Physiol Renal Physiol. 2006;290:F1408–1415. doi: 10.1152/ajprenal.00304.2005. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32:1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Tikoo K, Lau SS, Monks TJ. Histone H3 phosphorylation is coupled to poly-(ADP-ribosylation) during reactive oxygen species-induced cell death in renal proximal tubular epithelial cells. Mol Pharmacol. 2001;60:399–402. doi: 10.1124/mol.60.2.394. [DOI] [PubMed] [Google Scholar]
- Waring P, Khan T, Sjaarda A. Apoptosis induced by gliotoxin is preceded by phosphorylation of histone H3 and enhanced sensitivity of chromatin to nuclease digestion. J Biol Chem. 1997;272:17929–17936. doi: 10.1074/jbc.272.29.17929. [DOI] [PubMed] [Google Scholar]
- Weng Yu-I, Shukla SD. Ethanol alters angiotensin II stimulated mitogen activated protein kinase in hepatocytes: agonist selectivity and ethanol metabolic independence. Eur J Pharmacol. 2000;398:323–331. doi: 10.1016/s0014-2999(00)00313-7. [DOI] [PubMed] [Google Scholar]
- You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr. 2008;138:497–501. doi: 10.1093/jn/138.3.497. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venugopal SK, He S, Liu P, Wu J, Zern MA. Ethanol induces apoptosis in hepatocytes by a pathway involving novel protein kinase C isoforms. Cell Signal. 2007;19:2339–2350. doi: 10.1016/j.cellsig.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Zhong S, Zhang Y, Jansen C, Goto H, Inagaki M, Dong Z. MAP kinases mediate UVB-induced phosphorylation of histone H3 at serine 28. J Biol Chem. 2001;276:12932–12937. doi: 10.1074/jbc.M010931200. [DOI] [PubMed] [Google Scholar]


