Abstract
Background
Despite advances in our understanding of excessive alcohol intake-related tissue injury and modernization of the management of septic patients, high morbidity and mortality due to infectious diseases in alcohol abusers remain a prominent challenge. Our previous studies have shown that milk fat globule epidermal growth factor-factor VIII (MFG-E8), a protein required to opsonize apoptotic cells for phagocytosis, is protective in inflammation. However, it remains unknown whether MFG-E8 ameliorates sepsis-induced apoptosis and organ injury in alcohol-intoxicated rats. The purpose of this study was to determine whether recombinant murine MFG-E8 (rmMFG-E8) attenuates organ injury after acute alcohol exposure and subsequent sepsis.
Methods
Acute alcohol intoxication was induced in male adult rats by a bolus injection of intravenous alcohol at 1.75 g/kg BW, followed by an intravenous infusion of 300 mg/kg BW/h of alcohol for 10h. Sepsis was induced at the end of 10-h alcohol infusion by cecal ligation and puncture (CLP). rmMFG-E8 or vehicle (normal saline) was administered intravenously three times (i.e., at the beginning of alcohol injection, the beginning of CLP, and 10h post-CLP) at a dose of 20 μg/kg BW each. Blood and tissue samples were collected 20h after CLP in alcoholic animals for various measurements.
Results
Acute alcohol exposure per se did not affect the production of MFG-E8; however, it primed the animal and enhanced sepsis-induced MFG-E8 downregulation in the spleen. Administration of rmMFG-E8 reduces alcohol/sepsis-induced apoptosis in the spleen, lungs, and liver. In addition, administration of rmMFG-E8 after alcohol exposure and subsequent sepsis decreases circulating levels of TNF-α and IL-6 and attenuates organ injury.
Conclusions
rmMFG-E8 attenuates sepsis-induced apoptosis and organ injury in alcohol-intoxicated rats.
Keywords: Ethanol, Sepsis, MFG-E8, Apoptosis, Inflammation
Introduction
Alcohol is the third leading cause of preventable death in the U.S. Over 18 million Americans, ages 18 and older, suffer from alcohol abuse or dependence. Alcohol abusers are susceptible to a wide range of infectious diseases (Cook 1998;Brown et al., 2006;Waldschmidt et al., 2008). The increase in both frequency and severity of infections in ethanol-consuming individuals is attributed to ethanol-induced immune dysfunction (Cook 1998). Alcohol exposure compromises multiple aspects of the immune system. In particular, cells of the monocyte lineage seem to be extremely sensitive to the immunomodulatory effects of ethanol (Bautista 1998;Szabo 1998;Bautista and Spitzer 1999). In alcoholic liver disease, Kupffer cell function is dysregulated, leading to inefficient phagocytosis, the increased production of proinflammatory cytokines (Tilg et al., 1992;McClain et al., 2005), free radical production (Bautista 1998;Bautista and Spitzer 1999), and the release of suppressive factors (Tilg et al., 1992;Annoni et al., 1992).
Sepsis is also a significant health problem and a major cause of death in intensive care units (ICU) worldwide (Powers and Jacobi 2006;Howell and Tisherman 2006;Jenkins 2006;Remick 2007). Evidence indicates that in the US alone, more than 750,000 people develop sepsis each year with an overall mortality rate of 28.6% (Angus et al., 2001). It is reasonable to conclude that sepsis in alcoholics is associated with even higher mortality. Studies in septic patients and animals have demonstrated that several cell types (e.g., lymphocytes, vascular endothelial cells, and enteric epithelial cells) undergo apoptosis, and excessive apoptosis has severe pathological consequences for the immune system (Ayala et al., 1998;Mahidhara and Billiar 2000;Wesche et al., 2005;Hotchkiss et al., 2005a;Hotchkiss et al., 2005b). Apoptotic cells are prone to undergoing secondary necrosis if they are not effectively removed by phagocytes (Fink and Cookson 2005). Without proper clearance, apoptotic cells will undergo secondary necrosis and have the potential to pose a great harm to the host, as they then release potentially harmful inflammatory and toxic mediators, further impairing the septic condition (Hanayama et al., 2002;Scaffidi et al., 2002;Bell et al., 2006;Miksa et al., 2009).
Our recent studies have shown that milk fat globule epidermal growth factor-factor VIII (MFG-E8, also called lactadherin in humans), an opsonizing protein essential for the engulfment of apoptotic bodies, decreases significantly during sepsis. In fact, the downregulation of MFG-E8 is responsible for reduced apoptotic cell clearance under such conditions (Miksa et al., 2009). Administration of MFG-E8-containing exosomes or recombinant murine MFG-E8 (rmMFG-E8) increases phagocytosis of apoptotic cells, reduces proinflammatory cytokines, and improves survival in a rodent model of polymicrobial sepsis. However, it remains unknown whether MFG-E8 ameliorates sepsis-induced organ injury in alcohol-intoxicated rats. The purpose of this study was to determine whether rmMFG-E8 attenuates apoptosis and organ injury after alcohol exposure and subsequent sepsis.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats (250-275g) purchased from Charles River Laboratories (Wilmington, MA) were housed in a light-controlled room with a 12-h light/dark cycle and allowed free access to water and standard rat chow. Rats were acclimatized for at least 1 week before experimentation. All animals received humane care in compliance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of The Feinstein Institute for Medical Research.
Animal model of alcohol exposure followed by polymicrobial sepsis
Prior to alcohol exposure, male Sprague-Dawley rats were fasted overnight but allowed water ad libitum. Acute alcohol exposure was performed according to Bautista (Bautista 2002) with some modification as we have recently described (Chaung et al., 2008). Briefly, rats were anesthetized with isoflurane inhalation. A catheter (PE-50 tubing) was placed in a jugular vein. Acute alcohol intoxication was induced by a bolus injection of alcohol at 1.75 g/kg BW (in 1 ml normal saline) via the jugular venous catheter, followed by an intravenous infusion of 300 mg/kg/h of alcohol for 10h (total dose 4.75 g/kg BW) which was administered without anesthesia, by use of a specialized animal restraint allowing free movement of the rat within the cage. This produced an average blood alcohol level of >135 mg/dL without any lethality (Chaung et al., 2008). Sepsis was induced at the end of the 10-h alcohol infusion by cecal ligation and puncture (CLP), a well-described clinically relevant model of polymicrobial sepsis (Chaudry et al., 1979;Fink and Heard 1990;Deitch 1998;Wang and Chaudry 1998). In brief, a 2-cm ventral midline abdominal incision was performed under anesthesia. The cecum was then exposed, ligated just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The incision was afterwards closed in layers and the animals received 3 ml/100g BW normal saline subcutaneously (i.e., fluid resuscitation). Sham-operated animals underwent the same procedures (i.e., cannulation and exposure of their abdominal cavity) with the exception that normal saline instead of alcohol was injected and their cecum was neither ligated nor punctured.
Quantitative RT-PCR
RNA was extracted from spleen tissue samples using TRIzol Reagent (Invitrogen, Carlsbad, CA). 5μg of RNA was reverse transcribed to cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) and amplified by quantitative PCR (Q-PCR) using SYBR green PCR Master Mix (Applied Biosystems). The following primer sets were used: MFGE8 (107 bp, Gene Bank NM_012811) sense 5′ TGA GGA ACA AGG AAC CAG 3′, antisense: 5′ GGA AGG ACA CGC ACA TAG 3′, G3PDH (100 bp, Gene Bank XM_579386), sense: 5′ ATG ACT CTA CCC ACG GCA AG 3′, antisense: 5′ CTG GAA GAT GGT GAT GGG TT 3′.
Administration of rmMFG-E8
In order to determine whether MFG-E8 has any beneficial effects after alcohol and sepsis, recombinant murine MFG-E8 (rmMFG-E8; R&D Systems, Minneapolis, MN) was administered intravenously three times (at the beginning of alcohol injection, the beginning of CLP, and 10h post-CLP) at a dose of 20 μg/kg BW (in 1 ml normal saline) each time (total dosage 60 μg/kg BW) via either jugular or femoral vein catheters. Vehicle-treated animals received an equivalent volume of fluid (i.e., normal saline). Sham-operated animals were exposed to neither alcohol/sepsis nor rmMFG-E8 treatment. Blood and tissue (i.e., the spleen, lungs and liver) samples were collected 20h after CLP in the alcoholic animals for various measurements.
TUNEL assay
The presence of apoptotic cells in the spleen, lungs, and liver was demonstrated using a terminal deoxynucleotide transferase dUTP nick-end labeling (TUNEL) staining kit (Roche Diagnostics, Indianapolis, IN). Briefly, tissue samples were fixed in 10% phosphate buffered formalin, embedded into paraffin and sectioned at 6 μm following standard histology procedures. Tissue sections were dewaxed, rehydrated and equilibrated in Tris buffered saline (TBS). The sections were then digested with 20 μg/ml proteinase K for 20 min at room temperature. The sections were then washed and incubated with a mixture containing terminal deoxynucleotidyl transferase and fluorescence-labeled nucleotides and examined under a fluorescence microscope. The negative control was performed by incubating slides in the mixture containing only deoxynucleotidyl transferase as recommended by the provider.
Western blotting analysis of cleaved caspase-3 protein levels
Spleen, lung, and liver samples (0.1 g) were lysed and homogenized in 1 ml of lysis buffer (10 mM Tris-buffered saline, 1 mM EDTA, 1 mM EGTA, 2 mM sodium orthovanadate, 0.2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 1% Triton X-100) for 30 min on ice and cleared by centrifugation at 12,000 g for 15 min at 4°C. Twenty-five micrograms of protein were fractionated on a 4-12% Bis-Tris gel and transferred to a 0.2-μm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 5% milk for 1 h. Blots were then incubated with cleaved caspase-3 antibodies (1:1,000) (Asp175, Cell Signaling Technology) overnight at 4°C. Finally, the blots were washed in TBST three times for 15 min, incubated with horseradish peroxidase-linked anti-rabbit immunoglobulin G for 1 h at room temperature, and washed five times in TBST for 10 min. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) was applied according to the manufacturer's instructions, and the membranes were exposed briefly to radiography film. The band densities were normalized by β-actin with the use of the Bio-Rad Image System.
Determination of serum levels of TNF-α and IL-6
The concentrations of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in the serum were quantified by using commercially obtained enzyme-linked immunosorbent assay (ELISA) kits specific for rat-TNF-α and IL-6 (BioSource International, Camarillo, CA). The assays were carried out according to instructions provided by the manufacturer. All samples were tested in duplicates.
Measurement of myeloperoxidase (MPO) activity
MPO activity in the lungs was determined by using the peroxidase-catalyzed reaction as described previously (Wu et al., 2008). All samples were tested in duplicates.
Determination of serum levels of organ injury markers
Serum levels of lactate and lactate dehydrogenase (LDH) were determined by using assay kits. All assays were performed according to instructions provided by the manufacture (Pointe Scientific, Canton, MI).
Statistical analysis
All data were expressed as means ± SE and compared by one-way or two-way ANOVA and the Student-Newman-Keuls test. Differences in values were considered significant if P < 0.05.
Results
Effects of acute alcohol exposure on sepsis-induced organ injury and inflammatory responses in rats
As shown in Table 1, acute alcohol exposure has no major effects on plasma levels of lactate, a marker for systemic hypoxia, in either normal or septic rats. Similarly, acute alcohol exposure alone had no major effects on plasma levels of TNF-α and IL-6 as compared to sham-operation (i.e., no alcohol no CLP). Sepsis-induced TNF-α and IL-6 production, on the other hand, was slightly higher in acute alcohol intoxicated rats (Alcohol+CLP) than normal rats (CLP). However, these increases were not statistically significant. Pulmonary MPO activity, an indication of neutrophil infiltration in the lungs, was almost doubled after acute alcohol exposure in normal rats. However, sepsis-induced neutrophil infiltration in the lungs was only slightly higher in acute alcohol intoxicated rats (Alcohol+CLP) than normal rats (CLP).
Table 1. Effects of acute alcohol exposure (Alcohol) on CLP induced organ injury and inflammatory responses in rats.
| Sham | Alcohol | CLP | Alcohol+CLP | |
|---|---|---|---|---|
| Lactate (mg/dL) | 9.9±1.31 | 10.8±0.89 | 22.0±2.26*# | 23.3±1.47*# |
| TNF-α (pg/ml) | 5.6±0.23 | 5.0±1.27 | 13.7±2.46*# | 17.7±1.91*# |
| IL-6 (pg/ml) | 49.6±3.43 | 47.9±0.96 | 201.8±45.04*# | 242.6±59.81*# |
| Pulmonary MPO (U/g) | 33.6±5.38 | 98.5±9.70* | 136.9±5.41*# | 142.3±6.18*# |
Data are expressed as means ± SE (n=5-6/group) and compared by one-way ANOVA and Student-Newman-Keuls method:
P<0.05 versus sham-operated animals;
P<0.05 versus Alcohol alone animals.
Alterations in splenic MFG-E8 gene expression after alcohol exposure and subsequent sepsis
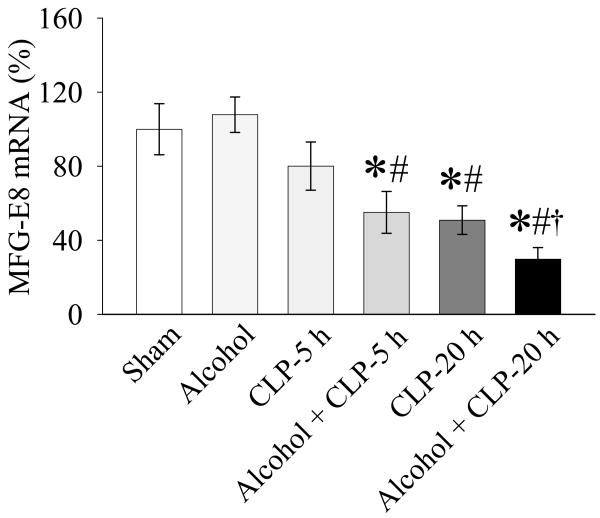
As shown in Fig. 1, infusion of alcohol for 10h did not significantly alter MFG-E8 expression. Although splenic MFG-E8 gene expression decreased by 19.9% at 5h after CLP in the absence of alcohol, such a reduction is not statistically significant from the sham control. In contrast, MFG-E8 gene expression in the spleen decreased by 45.0% at 5h after CLP in alcohol-exposed animals as compared to that in sham-operated animals (P<0.05, Fig. 1). MFG-E8 gene expression was further downregulated by 70.3% at 20h after CLP in alcohol-exposed animals, as compared to only 49.1% decrease at 20h after CLP without alcohol exposure (Fig. 1). The levels of MFG-E8 gene expression in Alcohol+CLP-20 h animals were even significantly lower than those in CLP alone animals, suggesting that alcohol possesses the priming effect which sensitizes the animal more susceptible to the second hit (sepsis).
Figure 1.
Alterations in MFG-E8 gene expression in the spleen in sham-operated animals (Sham), acute alcohol intoxicated rats (Alcohol), CLP animals at 5 or 20 h after CLP, and acute alcohol exposure plus CLP (Alcohol+CLP) animals at 5 or 20 h after CLP. Data are expressed as means ± SE (n=5-7/group) and compared by one-way ANOVA and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus Alcohol alone animals; †P<0.05 versus CLP alone animals.
rmMFG-E8 decreases apoptosis in various tissues after alcohol exposure and subsequent sepsis
Apoptosis was detected in situ using the TUNEL assay. As shown in Figures 2A-D, TUNEL-positive cells in the spleen increased markedly at 20h post-CLP in alcohol-exposed animals with vehicle treatment as compared to sham-operated animals. Administration of rmMFG-E8, however, significantly decreased the number of apoptotic cell in the spleen after the double-hit of alcohol plus sepsis (Figs. 2C & 1D). Similar results (increased apoptosis after alcohol/sepsis and prevention of apoptosis by rmMFG-E8) were also observed in the lungs (Figs. 3A-D) and liver (Figs. 4A-D). To further confirm this beneficial effect of rmMFG-E8, cleaved caspase-3 was used as an additional measure of apoptosis. Tissue cleaved caspase-3 was measured by Western blot analysis, and β-actin was used to ensure equal protein loading. As shown in Figure 5, cleaved caspase-3 levels increased in animals experiencing the double-hit, and administering rmMFG-E8 treatment decreased cleaved caspase-3 in the spleen, lungs, and liver. Thus, rmMFG-E8 effectively reduces apoptosis in various tissues after alcohol exposure and sepsis.
Figure 2.
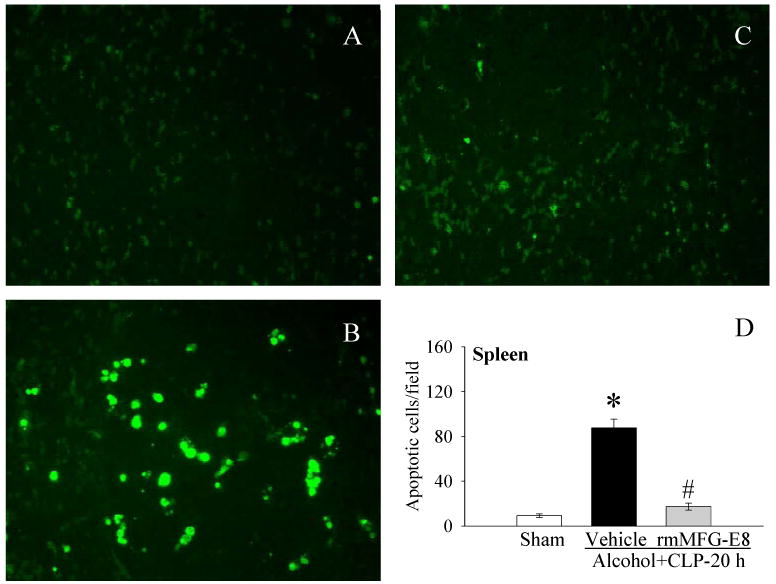
The splenic sections were stained with TUNEL (green fluorescent). Photomicrographs of rat splenic sections from A, sham; B, Alcohol+CLP, and C, Alcohol+CLP+rmMFG-E8; D, alterations in the numbers of TUNEL-positive cells in the spleen in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 4/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group. Original magnification: 200 ×.
Figure 3.
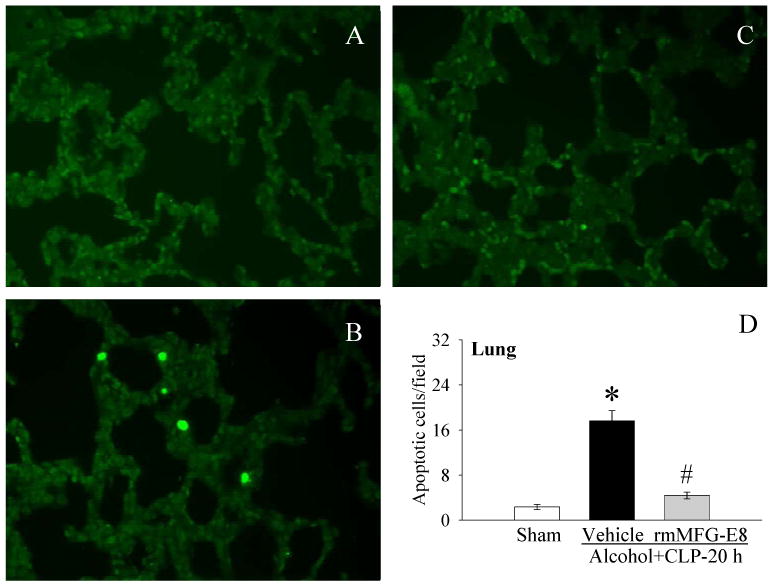
The pulmonary sections were stained with TUNEL. Photomicrographs of rat pulmonary sections from A, sham; B, Alcohol+CLP, and C, Alcohol+CLP+rmMFG-E8; D, alterations in the numbers of TUNEL-positive cells in the lungs in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 4/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group. Original magnification: 200 ×.
Figure 4.
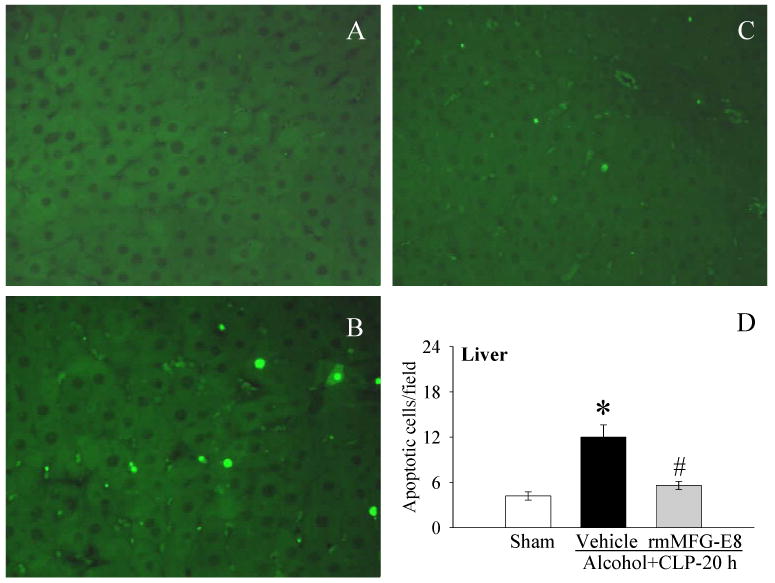
The hepatic sections were stained with TUNEL. A, Photomicrographs of rat hepatic sections from A, sham; B, Alcohol+CLP, and C, Alcohol+CLP+rmMFG-E8; D, alterations in the numbers of TUNEL-positive cells in the liver in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 4/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group. Original magnification: 200 ×.
Figure 5.
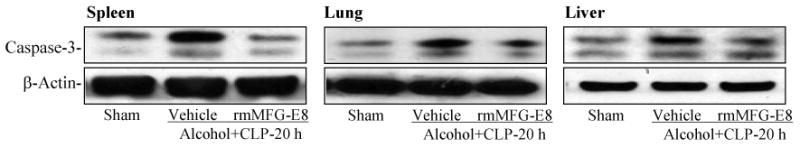
Alterations in cleaved caspase-3 in the spleen, lungs and liver in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Representative gels of 3 independent observations are presented.
rmMFG-E8 downregulates proinflammaotory cytokines after alcohol exposure and subsequent sepsis
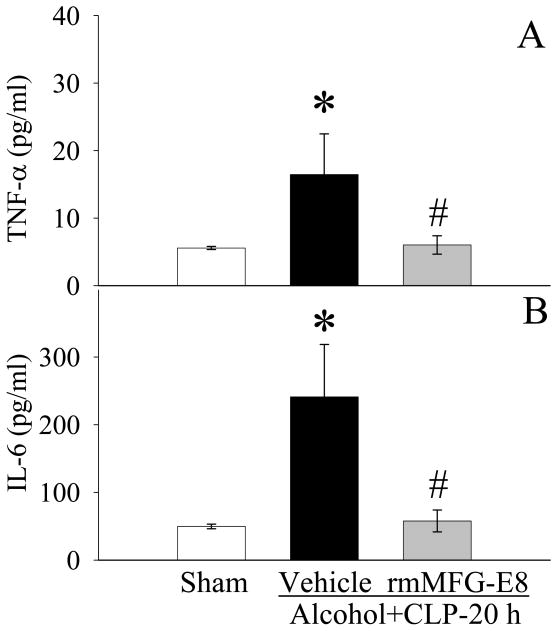
As shown in Figure 6A, TNF-α levels in the serum increased by 194.7% 20h after CLP in animals exposed to alcohol (P<0.05). Administration of rmMFG-E8 significantly decreased serum TNF-α levels by 63.4% (Fig. 6A). Similarly, serum IL-6 levels increased markedly after alcohol exposure and subsequent sepsis, and importantly, administration of rmMFG-E8 prevented the aforementioned increases (P<0.05, Fig. 6B).
Figure 6.
Alterations in serum levels of TNF-α (A) and IL-6 (B) in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 6-7/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group.
rmMFG-E8 reduces neutrophil infiltration in the lungs after alcohol exposure and subsequent sepsis
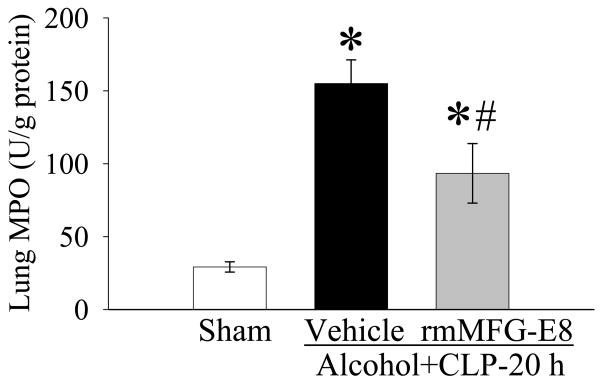
Tissue levels of myeloperoxidase (MPO) activities were assayed to reflect neutrophil accumulation. Neutrophils are a potential source of oxygen free radicals, which can cause tissue damage. As shown in Figure 7, alcohol/sepsis increased lung MPO activities by 429.8%, and rmMFG-E8 treatment decreased activity by 39.7%, suggesting that rmMFG-E8 reduces tissue neutrophil accumulation.
Figure 7.
Alterations in pulmonary levels of myeloperoxidase (MPO) activity in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 6-7/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group.
rmMFG-E8 attenuates tissue injury after alcohol exposure and subsequent sepsis
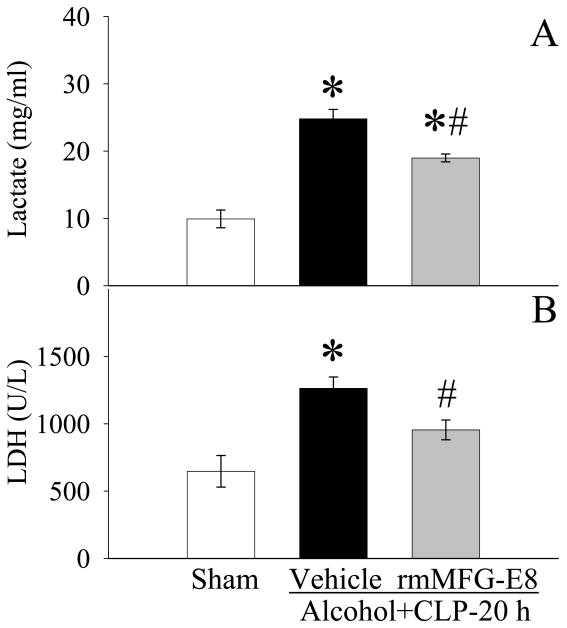
As shown in Fig. 8A, circulating levels of lactate (a measure of decreased tissue perfusion or hypoxia) increased markedly 20h after CLP in alcohol-exposed animals. Administration of rmMFG-E8 significantly reduced lactate levels despite the fact that such levels were statistically higher than those in sham-operated animals. The activity of LDH in the circulation, another marker of cell injury, was increased after the double-hit, but administration of rmMFG-E8 was able to decrease LDH (Fig. 8B).
Figure 8.
Alterations in serum levels of lactate (A) and lactate dehydrogenase (LDH) (B) in sham-operated rats and at 20 h after CLP in acute alcohol-intoxicated rats treated by normal saline or rmMFG-E8. Data are presented as means ± SE (n = 6-7/group) and compared by one-way ANOVA and Student-Newman-Keuls Method: *P < 0.05 vs. Sham group; #P < 0.05 vs. Vehicle group.
Discussion
Alcohol abusers are susceptible to a wide range of infectious diseases. Despite advances in our understanding of excessive alcohol-intake related tissue injuries and improvements in the management of septic patients, controlling the high morbidity and mortality rate due to infectious diseases in alcohol abusers remains a prominent challenge (Waldschmidt et al., 2008). Therefore, there is a dire need for therapy and treatments geared directly toward treating sepsis in alcoholics. Using a rat model of alcohol exposure and subsequent polymicrobial sepsis, the current study clearly shows that administration of rmMFG-E8 reduces sepsis-induced apoptosis, inflammation and organ injury in alcohol-intoxicated rats.
MFG-E8 is a secreted protein (Hanayama et al., 2002), which is expressed in mammary glands, secreted from the glandular epithelial cells in milk fat globules, and is possibly involved in the uptake of milk fat globules in the gut (Taylor et al., 1997;Oshima et al., 1999;Akakura et al., 2004). Recently, Hanayama et al. discovered that MFG-E8 plays a major role in the clearance of apoptotic cells (Hanayama et al., 2002;Hanayama et al., 2004;Hanayama et al., 2006). Cells undergoing apoptosis present several “eat-me” signals on their surface, the best-described being the phosphatidylserine (PS). PS is normally expressed on the inner leaflet of the plasma membrane and is exposed only if the cell undergoes apoptosis (Savill et al., 2003;Asano et al., 2004). It can be recognized by several surface proteins on phagocytes, including PS receptors, annexins (Fan et al., 2004), and CD36 (Tait and Smith 1999). The apoptotic cells then adhere to the cell surface of the phagocytes and are closely attached to them (Zullig and Hengartner 2004). For the engulfment of apoptotic bodies, however, interaction between the exposed PS on the surface of apoptotic cells and a specific integrin [αvβ3 (vitronectin receptor) or αvβ5] expressed on the phagocytes is required (Zullig and Hengartner 2004). Such a process is mediated by MFG-E8 (Hanayama et al., 2002;Zullig and Hengartner 2004). Without MFG-E8, full engulfment and the removal of apoptotic cells cannot be completed (Hanayama et al., 2004). Our recent studies have shown that a deficiency in MFG-E8 is detrimental in sepsis (Miksa et al., 2009). Mice lacking MFG-E8 accumulate 2-3 times as many apoptotic cells above the basal level and have a 60% higher mortality rate than wild-type mice (Miksa et al., 2009). In the current study, we found that acute alcohol exposure per se does not affect the production of MFG-E8; however, it primes the animal and enhances sepsis-induced MFG-E8 downregulation in the spleen. It is possible that the molecular basis of alcohol's priming effect on MFG-E8 downregulation induced by sepsis is its upregulatory effect on CD14 and TLR4 (data not shown). However, whether co-administration of alcohol at the time of CLP without continued infusion would have a similar result remains to be determined.
Apoptotic cells from the spleen, thymus, peripheral blood mononuclear cells, and even the liver can be detected after alcohol exposure (Neuman et al., 2002). Studies in septic patients and animals have also revealed the excessive apoptosis presents under such conditions (Ayala et al., 1998;Mahidhara and Billiar 2000;Wesche et al., 2005;Hotchkiss et al., 2005a;Hotchkiss et al., 2005b). Loss of immune cells is believed to contribute to the immune suppression that is linked to disease pathogenesis and resulting mortality. Indeed, recent reports suggest a causative link between profound lymphocyte loss due to apoptosis and poor outcome (Le et al., 2002;Bilbault et al., 2004). Inhibition of apoptosis has recently been suggested as a promising therapeutic approach for the prevention and treatment of sepsis (Wesche et al., 2005;Hotchkiss and Nicholson 2006;Weber et al., 2009). There is no doubt that prevention of lymphocyte apoptosis can have a profound positive effect on survival in sepsis models. However, inhibition of apoptosis requires early or even pre-treatment. Therefore, the clinical application of this idea is extremely limited and often impractical. Our current study has shown that administration of rmMFG-E8 reduced apoptosis in various organs after alcohol and sepsis. Since MFG-E8 does not decrease the number of apoptotic cells through the direct modulation of apoptotic pathways but rather through an increased clearance of apoptotic cells (Miksa et al., 2006), pre- or early treatment is not required for its application. In this regard, it may be used in alcoholic patients with established sepsis.
Sepsis is marked by a systemic inflammatory response. Balanced inflammatory responses are essential elements of a successful host response after injury (Moretta 2002). However, excessive and sustained inflammatory responses can cause severe tissue damage and may lead to multiple organ damage and septic shock (Martin et al., 2003;Riedemann and Ward 2003). Systemic increases of proinflammatory cytokines in sepsis have been previously associated with high mortality rates (Lotze and Tracey 2005). Studies using inhibitors of these cytokines demonstrated increased survival in experimental sepsis (Tracey et al., 1987;Qin et al., 2006). However, the effects of antagonistic anti-cytokine therapies often failed clinically, and in many cases, the efficacy of these treatments was dependent on the severity of sepsis (Wesche-Soldato et al., 2007). Therefore, modulation of cytokine production rather than direct inhibition may be more effective in sepsis treatment. In our rat model of alcohol/sepsis, increases in TNF-α and IL-6 were observed at 20 h after CLP. We have shown here that rmMFG-E8 suppressed sepsis-induced increases in TNF-α and IL-6 in alcohol-intoxicated rats. Historically, apoptosis has been seen as an orderly process of cell suicide that, unlike necrosis, does not elicit inflammation (Fadok et al., 1998). Recently it has become clear, however, that apoptotic cells eventually undergo secondary necrosis and stimulate an inflammatory response if they are not removed by phagocytosis (Scaffidi et al., 2002;Bell et al., 2006). Under physiological conditions, a secondary (post-apoptotic) necrosis of apoptotic cells can be prevented through their fast removal by phagocytes (e.g., macrophages, endothelial cells) in tissues and circulation (Zullig and Hengartner 2004;Lauber et al., 2004;Kim et al., 2005). Therefore, potential harm from apoptotic cells caused by the leakage of their dangerous contents (e.g., cytokines, enzymes, etc.) due to secondary necrosis can be abrogated (Gershov et al., 2000;Takahashi et al., 2005;Wu et al., 2005). In this way, the downregulatory effects of rmMFG-E8 on TNF-α and IL-6 may be related to its ability to stimulate the removal of excessive apoptotic cells under such conditions. Our recent study (Miksa et al., 2008) has shown that MFG-E8-mediated apoptotic cell phagocytosis results in an inhibition of mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NFκB) signaling pathways. Since MAPK and NF-κB are pro-inflammatory pathways in macrophages, the inhibition of these pathways may be the underlining mechanisms of MFG-E8's anti-inflammatory effects.
In the current study, we also found that acute alcohol exposure alone had no major effects on plasma levels of TNF-α and IL-6 as compared to sham-operation. Sepsis-induced TNF-α and IL-6 production, on the other hand, was slightly higher in acute alcohol intoxicated rats than normal rats. The effects of acute alcohol exposure on cytokine production are complicated. A recent study has shown that acute alcohol attenuates toll like receptors (TLR)-4- but not TLR-2-induced TNF-α production (Oak et al., 2006). In contrast, acute alcohol augments TNF-α production when both TLR-2 and TLR-4 ligands were present (Oak et al., 2006). Both TLR-2 and TLR-4 ligands are present in CLP-induced polymicrobial sepsis, which may be responsible for the slightly higher levels of TNF-α and IL-6 in Alcohol+CLP rats than CLP alone rats.
In summary, the present study has shown that rmMFG-E8 reduces alcohol/sepsis-induced apoptosis in the spleen, lungs, and liver. In addition, administration of rmMFG-E8 after alcohol exposure and subsequent sepsis decreases circulating levels of TNF-α and IL-6 and attenuates organ injury. In our future studies, we will determine the optimal dose(s) of MFG-E8 in treating alcohol and sepsis. In addition, investigation of delayed administration of MFG-E8 is needed in order to mimic the clinical situation, where pre-treatment is often impossible.
Acknowledgments
This study was supported by NIH grants R01 GM057468, R01 GM053008, and R01 AG028352 (P. Wang).
References
- Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292:403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Annoni G, Weiner FR, Zern MA. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992;14:259–264. doi: 10.1016/0168-8278(92)90168-o. [DOI] [PubMed] [Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459–467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Xin XY, Ayala CA, Sonefeld DE, Karr SM, Evans TA, Chaudry IH. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood. 1998;91:1362–1372. [PubMed] [Google Scholar]
- Bautista AP. Acute ethanol binge followed by withdrawal regulates production of reactive oxygen species and cytokine-induced neutrophil chemoattractant and liver injury during reperfusion after hepatic ischemia. Antioxid Redox Signal. 2002;4:721–731. doi: 10.1089/152308602760598864. [DOI] [PubMed] [Google Scholar]
- Bautista AP. The role of Kupffer cells and reactive oxygen species in hepatic injury during acute and chronic alcohol intoxication. Alcohol Clin Exp Res. 1998;22:255S–259S. doi: 10.1111/j.1530-0277.1998.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Bautista AP, Spitzer JJ. Role of Kupffer cells in the ethanol-induced oxidative stress in the liver. Front Biosci. 1999;4:D589–D595. doi: 10.2741/bautista. [DOI] [PubMed] [Google Scholar]
- Bell CW, Jiang W, Reich CF, III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- Bilbault P, Lavaux T, Lahlou A, Uring-Lambert B, Gaub MP, Ratomponirina C, Meyer N, Oudet P, Schneider F. Transient Bcl-2 gene down-expression in circulating mononuclear cells of severe sepsis patients who died despite appropriate intensive care. Intensive Care Med. 2004;30:408–415. doi: 10.1007/s00134-003-2118-z. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30:1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- Chaung WW, Jacob A, Ji Y, Wang P. Suppression of PGC-1alpha by Ethanol: Implications of Its Role in Alcohol Induced Liver Injury. Int J Clin Exp Med. 2008;1:161–170. [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell. 2004;15:2863–2872. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun. 2006;9:162–172. doi: 10.1159/000090780. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin Infect Dis 41 Suppl. 2005a;7:S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005b;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- Howell G, Tisherman SA. Management of sepsis. Surg Clin North Am. 2006;86:1523–1539. doi: 10.1016/j.suc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Jenkins I. Evidence-based sepsis therapy: a hospitalist perspective. J Hosp Med. 2006;1:285–295. doi: 10.1002/jhm.116. [DOI] [PubMed] [Google Scholar]
- Kim S, Chung EY, Ma X. Immunological consequences of macrophage-mediated clearance of apoptotic cells. Cell Cycle. 2005;4:231–234. doi: 10.4161/cc.4.2.1421. [DOI] [PubMed] [Google Scholar]
- Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- Le TY, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Mahidhara R, Billiar TR. Apoptosis in sepsis. Crit Care Med. 2000;28:N105–N113. doi: 10.1097/00003246-200004001-00013. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- McClain C, Barve S, Joshi-Barve S, Song Z, Deaciuc I, Chen T, Hill D. Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:180S–188S. doi: 10.1097/01.alc.0000189276.34230.f5. [DOI] [PubMed] [Google Scholar]
- Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, Yang WL, Ravikumar TS, Wang P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–748. [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, Wang Z, Wang H, Ravikumar TS, Tracey KJ, Wang P. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor VIII. J Immunol. 2009;183:5983–5990. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- Neuman MG, Katz GG, Malkiewicz IM, Mathurin P, Tsukamoto H, Adachi M, Ishii H, Colell A, Garcia-Ruiz C, Fernandez-Checa JC, Casey CA. Alcoholic liver injury and apoptosis--synopsis of the symposium held at ESBRA 2001: 8th Congress of the European Society for Biomedical Research on Alcoholism, Paris, September 16, 2001. Alcohol. 2002;28:117–128. doi: 10.1016/s0741-8329(02)00243-4. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem Biophys Res Commun. 1999;254:522–528. doi: 10.1006/bbrc.1998.0107. [DOI] [PubMed] [Google Scholar]
- Powers J, Jacobi J. Pharmacologic treatment related to severe sepsis. AACN Adv Crit Care. 2006;17:423–432. doi: 10.4037/15597768-2006-4007. [DOI] [PubMed] [Google Scholar]
- Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Ward PA. Anti-inflammatory strategies for the treatment of sepsis. Expert Opin Biol Ther. 2003;3:339–350. doi: 10.1517/14712598.3.2.339. [DOI] [PubMed] [Google Scholar]
- Savill J, Gregory C, Haslett C. Cell biology. Eat me or die. Science. 2003;302:1516–1517. doi: 10.1126/science.1092533. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Szabo G. Monocytes, alcohol use, and altered immunity. Alcohol Clin Exp Res. 1998;22:216S–219S. doi: 10.1097/00000374-199805001-00002. [DOI] [PubMed] [Google Scholar]
- Tait JF, Smith C. Phosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J Biol Chem. 1999;274:3048–3054. doi: 10.1074/jbc.274.5.3048. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2006 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2008;42:137–142. doi: 10.1016/j.alcohol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chaudry IH. A single hit model of polymicrobial sepsis: cecal ligation and puncture. Sepsis. 1998;2:227–233. [Google Scholar]
- Weber P, Wang P, Maddens S, Wang PS, Wu R, Miksa M, Dong W, Mortimore M, Golec JM, Charlton P. VX-166: a novel potent small molecule caspase inhibitor as a potential therapy for sepsis. Crit Care. 2009;13:R146. doi: 10.1186/cc8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8:493–500. doi: 10.2174/138945007780362764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE. 2008;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- Zullig S, Hengartner MO. Cell biology. Tickling macrophages, a serious business. Science. 2004;304:1123–1124. doi: 10.1126/science.1099161. [DOI] [PubMed] [Google Scholar]