Abstract
The repair of cytochrome oxidase depletion during the treatment of copper deficiency was studied in the rat. The purpose of this study was to distinguish the role of new cell production from the possibly more specific role of mitochondrial turnover in determining the rate of this repair.
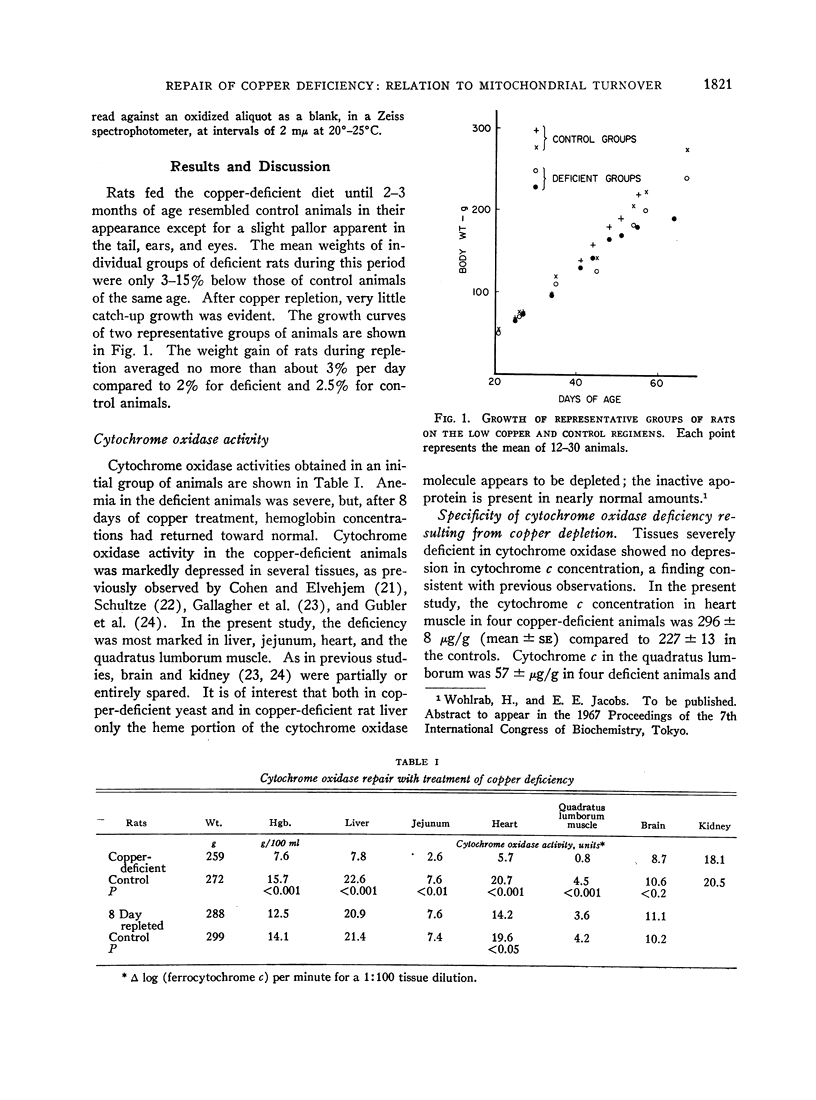
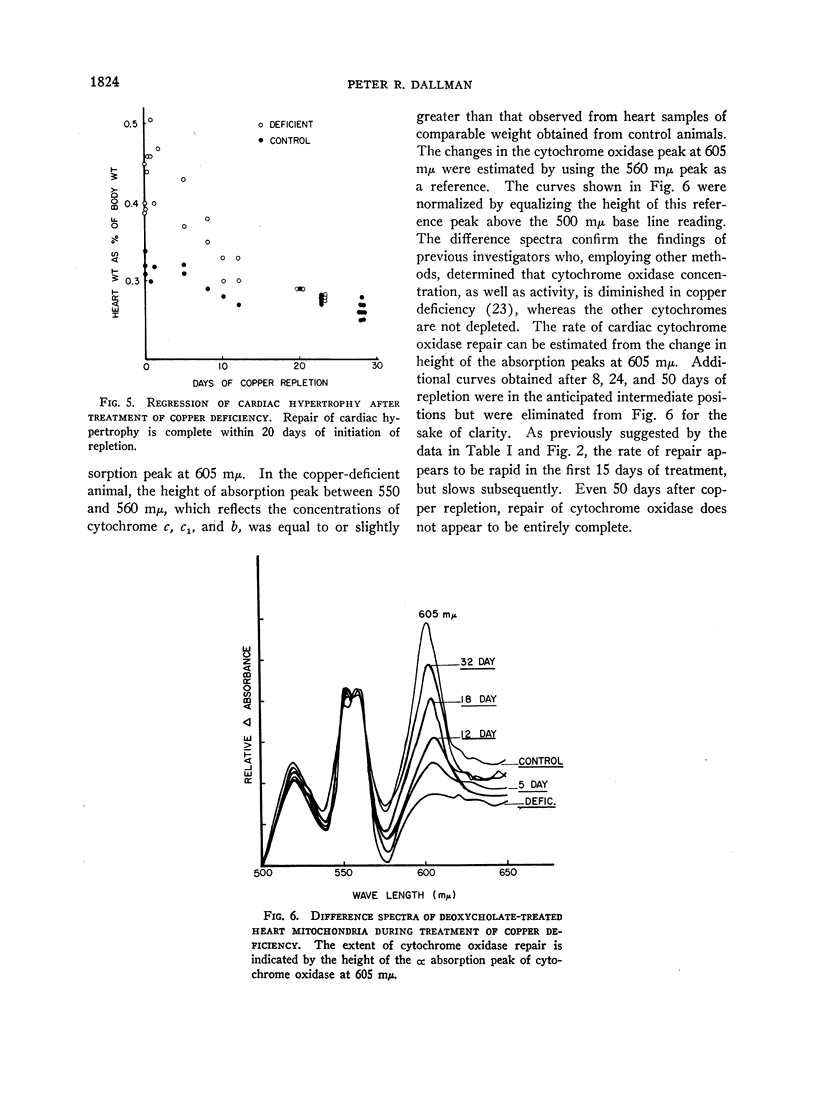
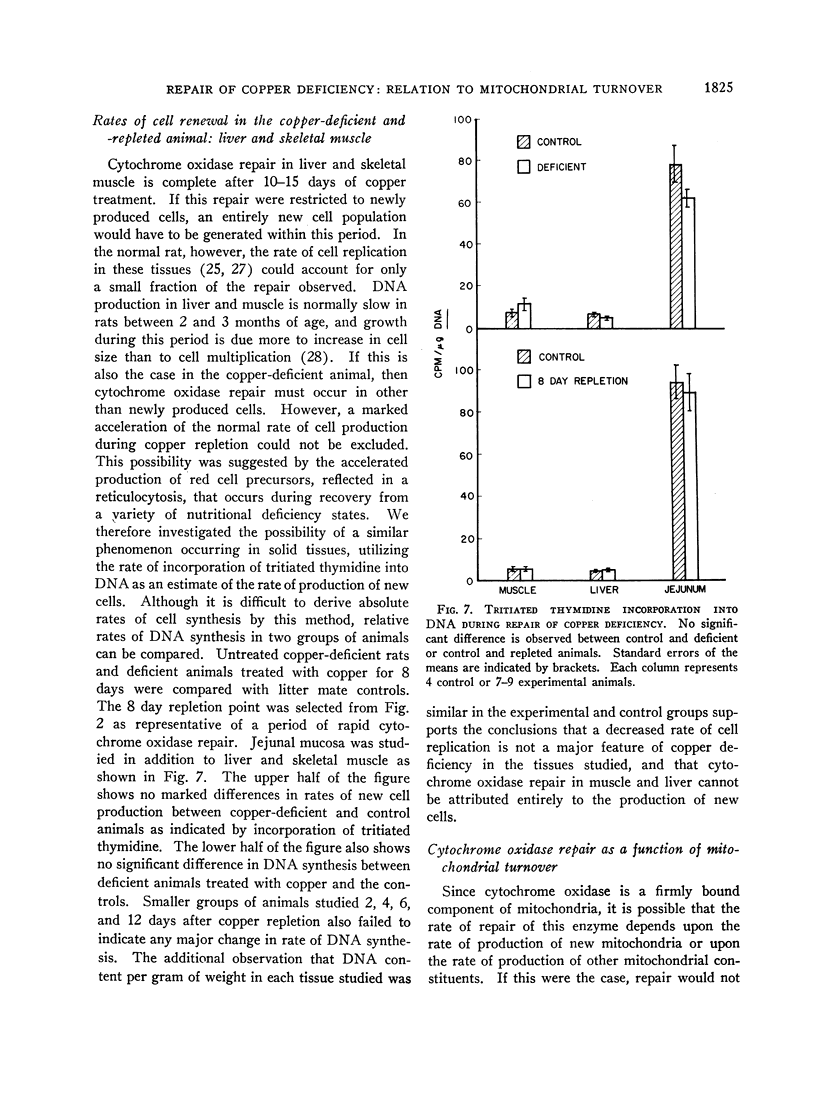
In rats on a copper-deficient regimen until 2.5-3 months of age, activities of cytochrome oxidase expressed as per cent of control were as follows: skeletal muscle (quadratus lumborum), 18%; heart, 27%; liver, 34%; and intestinal mucosa, 34%. After 2-3 days of dietary supplementation with cupric acetate, repair of decreased cytochrome oxidase activity in intestinal mucosa is complete. Histochemical studies indicated that this repair starts in the newly differentiating cells at the base of the villus and then progresses toward the tip of the villus at a rate approximating the normal rate of migration of the mucosal cells. In liver and skeletal muscle, cytochrome oxidase activity returned to control values after 10-15 days of treatment with cupric acetate. In heart muscle, control values were approached more slowly as indicated both by activity of the enzyme and by mitochondrial difference spectra which reflect enzyme concentration.
Although cytochrome oxidase repair in the intestine appeared to be limited by the rate of production of new mucosal cells, the rate of repair in liver and skeletal muscle was several times too rapid to be accounted for by known rates of new cell production. Incorporation of tritiated thymidine into DNA in these tissues in both the deficiency state and during repair indicated no major differences in new cell production compared to that of control animals. However, the time required for cytochrome oxidase repair in liver was similar to the turnover reported for other mitochondrial constituents in this tissue. The rate of cytochrome oxidase repair may therefore be more directly determined by the rate of synthesis of new mitochondrial material than by the rate of production of new cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
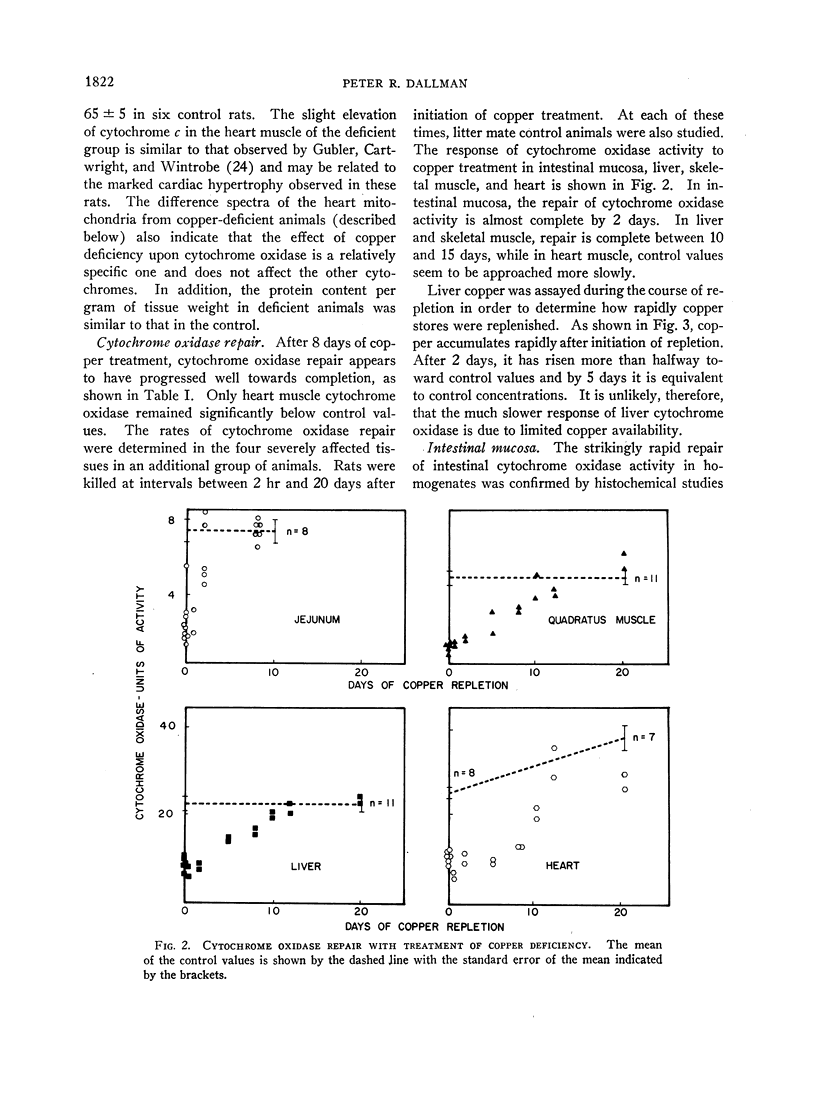
- BURSTONE M. S. Modifications of histochemical techniques for the demonstration of cytochrome oxidase. J Histochem Cytochem. 1961 Jan;9:59–65. doi: 10.1177/9.1.59. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., GUBLER C. J., WINTROBE M. M. Studies on copper metabolism. XX. Enzyme activities and iron metabolism in copper and iron deficiencies. J Biol Chem. 1957 Jan;224(1):533–546. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- CROSBY W. H., MUNN J. I., FURTH F. W. Standardizing a method for clinical hemoglobinometry. U S Armed Forces Med J. 1954 May;5(5):693–703. [PubMed] [Google Scholar]
- DALLMAN P. R., SCHWARTZ H. C. DISTRIBUTION OF CYTOCHROME C AND MYOGLOBIN IN RATS WITH DIETARY IRON DEFICIENCY. Pediatrics. 1965 Apr;35:677–686. [PubMed] [Google Scholar]
- Dallman P. R., Schwartz H. C. Myoglobin and cytochrome response during repair of iron deficiency in the rat. J Clin Invest. 1965 Oct;44(10):1631–1638. doi: 10.1172/JCI105269. [DOI] [PMC free article] [PubMed] [Google Scholar]
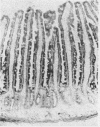
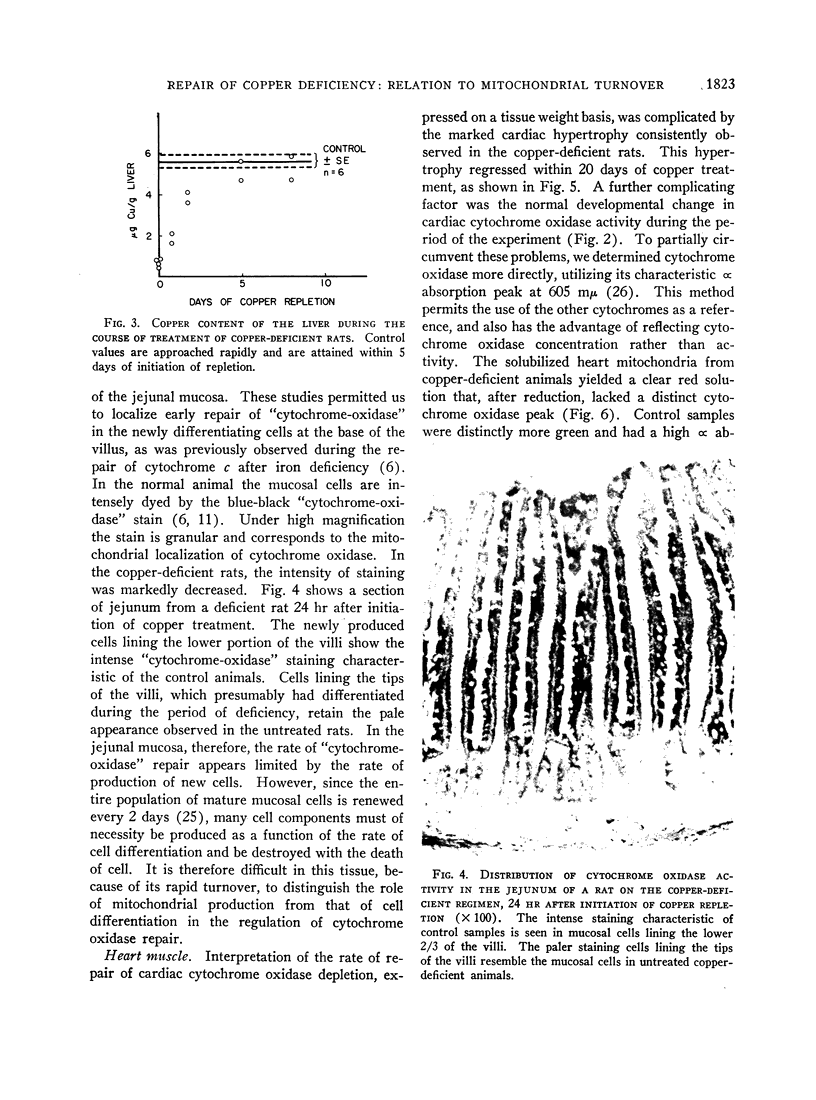
- Dallman P. R., Sunshine P., Leonard Y. Intestinal cytochrome response with repair of iron deficiency. Pediatrics. 1967 Jun;39(6):863–870. [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- FREER M., CAMPLING R. C., BALCH C. C. Factors affecting the voluntary intake of food by cows. 4. The behaviour and reticular motility of cows receiving diets of hay, oat straw and oat straw with urea. Br J Nutr. 1962;16:279–295. doi: 10.1079/bjn19620030. [DOI] [PubMed] [Google Scholar]
- Granick S., Gibor A. The DNA of chloroplasts, mitochondria and centrioles. Prog Nucleic Acid Res Mol Biol. 1967;6:143–186. doi: 10.1016/s0079-6603(08)60526-7. [DOI] [PubMed] [Google Scholar]
- Heath C. W., Strauss M. B., Castle W. B. QUANTITATIVE ASPECTS OF IRON DEFICIENCY IN HYPOCHROMIC ANEMIA: (The Parenteral Administration of Iron). J Clin Invest. 1932 Nov;11(6):1293–1312. doi: 10.1172/JCI100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lusena C. V., Depocas F. Heterogeneity and differential fragility of rat liver mitochondria. Can J Biochem. 1966 May;44(5):497–508. doi: 10.1139/o66-060. [DOI] [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Neubert D., Bass R., Helge H. Umsatzgeschwindigkeit der DNS in Mitochondrien von Warmblüterzellen. Naturwissenschaften. 1966 Jan;53(1):23–24. doi: 10.1007/BF00607636. [DOI] [PubMed] [Google Scholar]
- OWEN C. A., Jr DISTRIBUTION OF COPPER IN THE RAT. Am J Physiol. 1964 Aug;207:446–448. doi: 10.1152/ajplegacy.1964.207.2.446. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL H. L., KIM Y. S. GLUCOCORTICOID STIMULATION OF THE BIOSYNTHESIS OF GLUTAMIC-ALANINE TRANSAMINASE. Proc Natl Acad Sci U S A. 1963 Nov;50:912–918. doi: 10.1073/pnas.50.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman D. E. The fractionation of proteins from ox-heart mitochondria labelled in vitro with radioactive amino acids. Biochem J. 1964 Apr;91(1):59–64. doi: 10.1042/bj0910059. [DOI] [PMC free article] [PubMed] [Google Scholar]