Abstract
The Free and Cued Selective Reminding Test (FCSRT) is used widely to identify very mild dementia; three alternative scoring procedures have been proposed based on free recall, total recall, and cue efficiency. We compared the predictive validity of these scoring procedures for the identification of very mild prevalent dementia (CDR=0.5), of incident dementia and for distinguishing AD and nonAD dementias. We tested 244 elderly African American and Caucasian primary care patients at 18 month intervals using a screening neuropsychological battery that included the FCSRT and a comprehensive diagnostic neuropsychological battery. Median follow-up was 2.6 years. Dementia diagnoses were assigned using standard criteria without access to the results of the screening battery. There were 50 prevalent and 28 incident dementia cases. At scores selected to provide specificities of 90%, free recall was more sensitive to incident and prevalent dementia than the other two measures. Patients with impaired free recall were 15 times more likely to have a prevalent dementia and their risk of future dementia was four times higher than patients with intact free recall. Neither race nor education affected prediction though older patients were at increased risk of future dementia. Total recall was more impaired in AD dementia than in nonAD dementias. The results indicate that using the FCSRT, free recall is best measure for detecting prevalent dementia and predicting future dementia. Total recall impairment supports the diagnosis of AD rather than nonAD dementia.
Introduction
Memory testing is critical to identifying dementia because current criteria for the diagnosis of any dementia, irrespective of subtype, requires memory impairment.1 Impaired memory is one of the earliest manifestations of Alzheimer's Disease (AD), the most common form of late-life dementia. The Free and Cued Selective Reminding Test with Immediate Recall, hereafter called the Free and Cued Selective Reminding Test (FCSRT), has been used to identify prevalent dementia 2-7, predict future dementia 8-11, identify those patients with mild cognitive impairment (MCI) destined to develop Alzheimer's Disease (AD) 12, 13, and distinguish AD from nonAD dementias. 14-16
Unlike most other memory tests, the FCSR begins with a study phase designed to control attention and cognitive processing to identify memory impairment that is not secondary to other cognitive deficits. 17, 18 Patients identify pictured items (e.g., grapes, vest) in response to category cues (fruit, clothing). In the test phase, subjects are asked to recall the items they learned (free recall). The category cues are used to prompt recall of items not retrieved by free recall to generate a score termed cued recall. The sum of free and cued recall is termed total recall. These procedures have a strong theoretical and empirical foundation discussed elsewhere. 4, 17, 18 Controlled learning remediates the mild retrieval deficits that occur in many healthy elderly individuals 19 but has only modest benefits in patients with dementia 20, thus enhancing the FCSRT's discriminative validity for the diagnosis of dementia in comparison to tests that do not control the conditions of learning.
Most of the research on the FCSRT has been conducted with community volunteers from population-based studies2, 3, 8, 9, 12, 21 or with patients from subspecialty memory disorder practices. 6, 7, 11, 13 Recently, we have studied these procedures in the primary care setting, where most seniors receive their medical care. 16, 22, 23 Three measures derived from the FCSRT have been proposed to detect dementia: free recall, total recall, (the sum of free and cued recall) and cue efficiency (the ratio of cued recall successes to the number of cued recall attempts). Performance on the FCSRT for patients in our primary care study was re-evaluated at 18-month intervals over the next four years. Using this prospective cohort, we sought to determine the best FCSRT measure for answering three increasingly common questions about elderly primary care patients: Does the patient have a dementia? (i.e., prevalent dementia); Is the patient likely to develop a dementia in the next few years? (i.e., prediction of incident dementia); and Is the dementia due to AD or not? (i.e., etiology).
Both cued recall measures, total recall and cue efficiency, have been used with high accuracy to identify prevalent dementia and AD in previous studies. 4-6 Both measures were expected to outperform free recall in prediction of prevalent dementia. In contrast, free recall was expected to outperform the cued recall measures as a predictor of incident dementia. In our prior prospective study of community residing elderly, subjects with normal total recall but impaired free recall developed dementia at dramatically higher rates than subjects with intact free recall. 8 With regard to distinguishing AD from the nonAD dementias, cued recall measures were expected to outperform free recall given the considerable evidence of impaired cued recall in AD patients but not in patients with nonAD dementias. 14-16 Free recall was not expected to be useful in distinguishing AD from nonAD dementias since all patients with dementia would have impaired free recall.
Methods
Clinical setting
The current study took place in the Geriatric Ambulatory Practice (GAP), an urban academic primary care practice staffed by geriatricians at Montefiore Medical Center in the Bronx, New York. All study procedures were approved by the local institutional review board. Recruitment methods, test procedures, and diagnostic determination at cross-section have been described in detail elsewhere. 22, 23 Because our focus was on identification of very mild (CDR=0.5) and mild dementia (CDR=1.0), we excluded patients scoring less than 18 on the Mini Mental Status Exam (MMSE) 24 at baseline, on the grounds that scores in this range would be more likely to accompany moderate or severe dementia. Recruitment began in January, 2003 and final follow-up was completed in July, 2007. Masters-level psychology assistants evaluated patients at approximately 18 month intervals with (1) the two-stage screening strategy that included the FCSRT in the second stage; (2) an independent diagnostic battery of neuropsychological tests (Table 1) and (3) informant responses to a structured clinical interview covering six domains of cognitive and daily functioning.25 During routine clinical visits, GAP geriatricians blinded to the results of all neuropsychological testing used CDR box scores 25 to rate the cognitive and functional status of their patients. Of 356 patients from the inception cohort, 249 (70%) were followed prospectively and comprised the sample reported here. Compared to the 107 patients without follow-up, the prospective sample had more women and less education (12.15 versus 13.25 years), and higher free recall (27.43 versus 25.43, p=.06). MMSE scores were identical (26.6).
Table 1.
Diagnostic Battery.
| Patient Evaluation | Instrument |
|---|---|
| Memory | CERAD verbal recall40 |
| CERAD figure recall40 | |
| Name and address recall30 | |
| Event recall41 | |
| FCSRT Spatial Location Memory42 | |
| Executive functions | WORLD backwards24 |
| Fluency using fruits and vegetables37 | |
| CERAD problem solving25 | |
| Intrusions in CERAD recall40 | |
| Subtracting 7's24 | |
| Months backwards30 | |
| Other Cognitive functions | Orientation24 |
| Clock Drawing43 | |
| CERAD Figure Copy40 | |
| Naming8* | |
| Counting up, counting down30 | |
| Self reported ADLs44 | |
| Mood | Geriatric Depression Scale (Yesavage, 1986)45 |
CERAD: Consortium to Establish a Registry in Alzheimer's Disease
FCSRT: Free and Cued Selective Reminding Test
ADL: Activities of Daily Living
Before administering the FCSRT, the 16 pictures are shown one at a time for naming
Diagnostic procedures
At baseline and at each subsequent cross-sectional evaluation, a consensus panel consisting of a neuropsychologist (EG), a geriatrician, and a geriatric psychiatrist used results of the independent diagnostic battery of neuropsychological tests and the informant interviews to determine presence or absence of dementia according to DSM IV criteria for dementia. 1 To avoid diagnostic circularity, members of the consensus panel did not have access to the screening test results including the FCSRT, MMSE scores, or to the CDR ratings assigned by the patient's provider. After the final follow-up evaluation, the scores from each preceding cross-sectional evaluation, including the diagnostic battery, informant interviews, and the provider's CDR ratings were reviewed by the neuropsychologist (EG) without knowledge of the diagnostic outcome assigned at cross-section. Using all available longitudinal information except FCSRT and MMSE scores, EG assigned the final diagnosis using DSM IV criteria and a final CDR score. Patients were classified as having no dementia, a dementia present at baseline (i.e., prevalent dementia) or dementia present only at follow-up (i.e., incident dementia). Patients with dementia were subtyped by the neurologist (AS) through chart review using established criteria for probable or possible AD 26, probable or possible vascular dementia 27, probable or possible Lewy Body dementia 28, and frontotemporal dementia 29. Subtyping decisions were based on detailed review of the patients’ paper and computerized medical records, including social and family history, vascular and other risk factors, medications, and laboratory results; neuroimaging reports were available for the majority of patients (82%; 68% had CT, 9% had MRI, 23% had both). Particular attention was paid to the onset, nature, and development of neurological and cognitive complaints. Subtyping was accomplished without knowledge of FCSRT scores. A diagnosis of dementia was recorded in the medical record of 23% of the patients with dementia.
Free and Cued Selective Reminding Test with Immediate Recall (FCSRT)18, 22
The FCSRT begins with a study phase in which subjects are asked to search a card containing four pictures (e.g., grapes) for an item that goes with a unique category cue (e.g., fruit). After all four items are identified, immediate cued recall of just those four items is tested, providing retrieval practice while the items are still in working memory. The search is performed again for items not retrieved by cued recall. The search procedure is continued for the next group of four items until all 16 items have been identified and retrieved in immediate recall. The study procedure is followed by three trials of recall, each consisting of free recall followed by cued recall for items not retrieved by free recall for a maximum score of 48. Items not retrieved by cued recall are re-presented. Each separate trial is followed by 20 seconds of interference. The three measures being evaluated here include free recall (the cumulative sum of free recall from the three trials; range 0-48), total recall (the cumulative sum of free recall + cued recall from the three trials, range 0-48), and cue efficiency (total recall-free recall)/(48-free recall, range 0.0-1.0).
Statistical procedures
Baseline scores on free recall, total recall, and cue efficiency from the prospective cohort were the main predictors. Receiver operating characteristic (ROC) curves were generated to assess the trade-off between sensitivity and specificity across a range of cut-scores. The diagnostic accuracy of tests can by compared by examining the area under the ROC curve (AUC). This method may be insensitive to differences at high sensitivity and specificity. An alternative approach is to hold specificity constant at a clinically relevant value and then compare sensitivities across screening measures using a within sample test such as McNemar's test. Herein, we take both approaches. The optimal cut-score for a screening test is determined by the benefits and consequences of a positive screen. As screening positive creates tremendous anxiety for patients and their families and significant expense, herein we evaluate a strategy that minimizes false positives by choosing cut-scores which yield a specificity of 90%.
Binary logistic regression was used to model each FCSRT measure separately as the main predictor of prevalent dementia, including age, education and race as covariates because of their well-known associations with dementia. Cox proportional hazards models were used to model incident dementia, permitting adjustment for variable follow-up time. Finally, using prevalent cases of dementia, we compared mean FCSRT measure (Mann-Whitney U test) to determine which measure was best for differentiating between AD and nonAD dementias. Patients with AD or mixed dementia (AD+VaD) comprised the AD group; the nonAD dementia group was comprised of patients with VaD or other dementias. Patients whose dementia subtype could not be determined were excluded from these comparisons.
Results
Baseline sample characteristics
Table 2 contrasts participants who were dementia free at baseline (n=194) to those with prevalent dementia (n=50) based on DSM-IV criteria. The dementia free group was separated by CDR score into 101 (53%) patients with normal cognition (CDR 0.0) and 91 (47%) patients with questionable cognition (CDR 0.5). Patients with CDR scores of 0.5 at baseline were older and had significantly lower MMSE and FCSRT scores than patients with normal cognition.
Table 2.
Characteristics of study participants at baseline..
| Dementia-Free @ Baseline | Dementia @ Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | CDR 0 | CDR 0.5 | p | All | AD only | AD+VaD | VaD only | Other/Undetermined | p | |
| N | 194 | 101 | 91 | 50 | 23 | 9 | 10 | 8 | ||
| Age (SD) | 78.3 (6.9) | 76.7 (6.3) | 80.2 (7.0) | 0.0007* | 82.4 (6.8) | 84.6 (7.1) | 82.0 (5.1) | 79.4 (7.0) | 80.8 (6.4) | 0.2** |
| Education (years) (SD) | 12.5 (3.3) | 13.1 (3.2) | 12.0 (3.3) | 0.019* | 10.6 (4.3) | 10.7 (4.0) | 10.0 (3.3) | 11.1 (5.6) | 10.5 (4.8) | 0.9** |
| Sex (% F) | 84 | 94 | 73 | 0.0001*** | 90 | 87 | 89 | 90 | 100 | 0.84**** |
| Race (% AA) | 48.5 | 54 | 41 | 0.077*** | 56 | 61 | 44 | 60 | 50 | 0.91**** |
| MMSE | 27.3 (2.7) | 28.07 (2.2) | 26.6 (3.0) | 0.0001* | 24.5 (3.7) | 24.3 (3.0) | 25.0 (3.8) | 23.3 (5.19) | 26.0 (2.6) | 0.52** |
| Free Recall | 30.1 (6.3) | 32.7 (5.5) | 27.4 (6.1) | <.0001* | 19.1 (7.3) | 17.6 (8.2) | 17.1 (5.0) | 22.1 (4.0) | 22.0 (8.6) | 0.18** |
| Total Recall | 47.5 (1.6) | 47.9 (0.45) | 47.0 (2.1) | 0.0001* | 41.5 (8.9) | 39.1 (10.5) | 39.9 (8.4) | 45.8 (4.0) | 44.5 (6.4) | 0.095** |
| Cue Efficiency | .978 (.055) | .994 (.022) | .962 (.073) | 0.0001* | .813 (.227) | .760 (.257) | .750 (.237) | .923 (.137) | .896 (.156) | 0.79** |
Mann-Whitney test
Kruskal-Wallis test
Pearson chi-squared test
Fisher exact test
SD: standard deviation
F: Female
AA: African American
MMSE: Mini Mental State Examination
Notes: Two patients who were diagnosed with dementia at baseline were re-classified as dementia-free at final follow-up and are not included in the CDR breakdown but are included in the statistics for all dementia free patients at baseline.
The p-values reflect the comparisons between the dementia free groups and among the dementia subgroups.
There were 50 cases of prevalent dementia, most (86%) with very mild dementia defined by CDR scores of 0.5 25. Five cases were excluded from these analyses due to the presence of moderate dementia, defined as CDR 2.0, which was outside the scope of our focus on very mild dementia. Dementia subtypes included 11 patients with probable AD, 12 with possible AD, nine with mixed dementia (AD + VaD), 10 with VaD, six patients with other dementias and two with insufficient evidence to determine dementia subtype. There were no differences among the dementia subgroups in demography or performance (Table 2).
Initial analyses indicated nearly complete overlap between total recall and cue efficiency: they were nearly perfectly correlated (Spearman correlation r=.988); their areas under the ROC curves were virtually identical both for prevalent dementia (0.78 versus 0.77) and for incident dementia (0.65 for each measure), and their sensitivities to both prevalent and incident dementia were similar when specificities were fixed at 90%. For this reason, we chose to not analyze cue efficiency further, opting to focus on total recall because it is easier to conceptualize and to compute than cue efficiency and so would be more amenable to widespread use in primary care clinical settings.
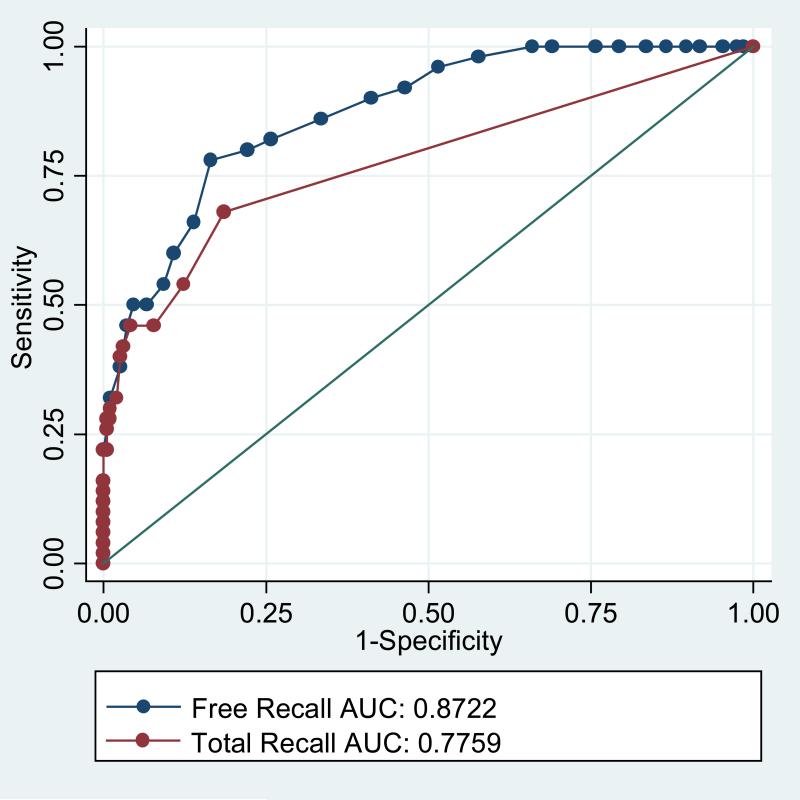
Figure 1 shows the ROC curves for free recall and total recall for prevalent dementia across the full range of possible cut scores. Higher values for sensitivity across a range of values for specificity and the larger values for area under the curve (0.87 versus 0.78, p=.006) indicates that free recall is more discriminating for prevalent dementia than cued recall. The cut score on free recall was set to ≤24 because this value fixed specificity at 90%. For total recall a cut-score of ≤ 46 (out of 48) fixed specificity at 90%. With specificities equated, free recall had 78% sensitivity for prevalent dementia, compared to a sensitivity of 54% for total recall (McNemar Test, p=.004).
Figure 1.
Receiver operating characteristic curves for prevalent dementia comparing free and total recall.
Table 3 presents the logistic regression models for predicting prevalent dementia using FCSRT measures as the main predictors and age, race, and years of education as covariates. Patients with impaired free recall were 15 times more likely to have a prevalent dementia than patients with intact free recall (Panel 3A). Neither age (p=.08), race (p=.10), nor education (p=.19) were significantly associated with dementia status. Patients with impaired total recall were 5.8 times more likely to have a prevalent dementia than patients with intact total recall (Panel 3B). Older patients were at higher risk (p=0.009); neither race (.47) nor education (.15) modified risk. When both free recall and total recall were included as predictors in the model (Panel 3C), the odds ratios declined: free recall dropped to 11.2 (p=.000) and total recall dropped to 2.4 (p=.04). The model was unaffected by age (p=.13), race (p=.19), and education (p=.27).
Table 3.
Logistic regression models for predicting prevalent dementia using free recall (3A), total recall (3B), both free and total recall (3C).
| Odds ratio | Lower .95 CI | Upper .95 CI | P | |
|---|---|---|---|---|
| 3A | ||||
| Free<=24 | 15.112 | 6.680 | 34.186 | .000 |
| Age | 1.059 | .993 | 1.129 | .080 |
| AA race | 2.091 | .865 | 5.056 | .102 |
| Years Educ | .924 | .823 | 1.040 | .191 |
| 3B | ||||
| Total<=46 | 5.836 | 2.770 | 12.294 | .000 |
| Age | 1.078 | 1.019 | 1.142 | .009 |
| AA race | 1.343 | .606 | 2.976 | .467 |
| Years Educ | .926 | .832 | 1.029 | .153 |
| 3C | ||||
| Free<=24 | 11.235 | 4.765 | 26.493 | .000 |
| Total<=46 | 2.443 | 1.040 | 5.736 | .040 |
| Age | 1.052 | .986 | 1.122 | .128 |
| AA race | 1.823 | .743 | 4.472 | .190 |
| Years Educ | .936 | .832 | 1.053 | .271 |
Incident dementia
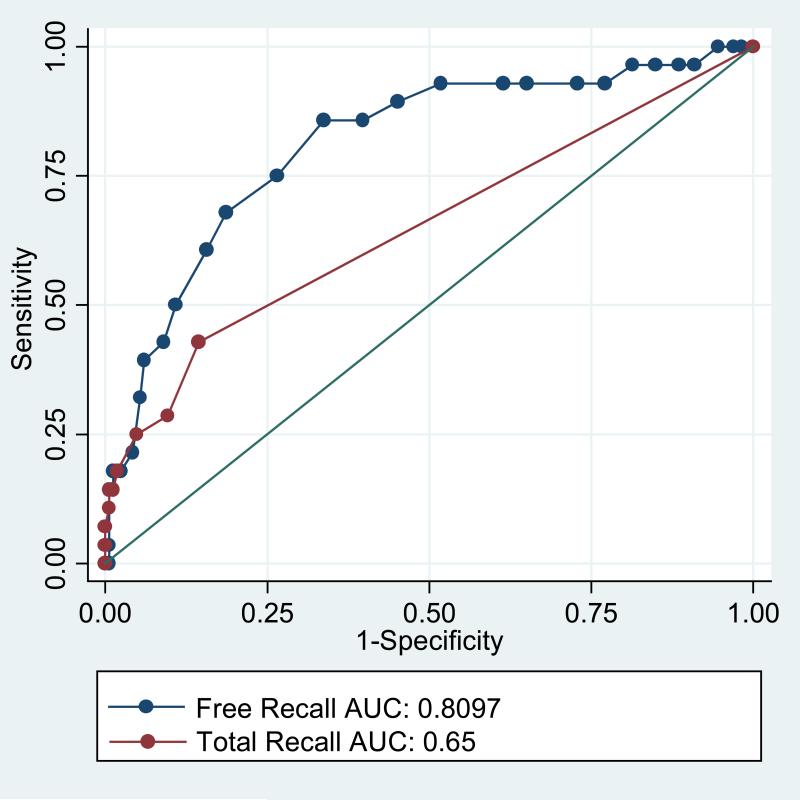
Table 4 displays the baseline characteristics for all 194 patients who did not meet DSM-IV criteria at baseline and separately by those who remained dementia-free throughout follow-up (n=166) and those who developed incident dementia (n=28). Patients with incident dementia were older, more likely to be Caucasian, and had lower FCSRT scores than patients who remained dementia free. MMSE scores were the same. Follow-up time was not different. Figure 2 displays the ROC curves for free recall and total recall for incident dementia. Higher values for sensitivity across a range of values for specificity and the larger values for area under the curve (0.81 versus 0.65, p=.006) indicates that free recall is more discriminating for incident dementia than total recall.
Table 4.
Characteristics of study participants who did not meet DSM-IV criteria for dementia at baseline.
| All | Dem-Free at FU | Dem at FU | p | |
|---|---|---|---|---|
| N | 194 | 166 | 28 | |
| Age (SD) | 78.3 (6.9) | 77.4 (6.6) | 83.25 (6.0) | 0.0001* |
| Education (years) (SD) | 12.5 (3.3) | 12.5 (3.4) | 12.4 (2.9) | 0.75* |
| Sex (% F) | 84 | 84 | 82 | 0.78**** |
| Race (% AA) | 48.5 | 53 | 21 | 0.0039*** |
| MMSE | 27.3 (2.7) | 27.4 (2.8) | 27.0 (2.5) | 0.29* |
| Free Recall | 30.1 (6.3) | 31.2 (5.8) | 24.2 (6.1) | <0.0001* |
| Total Recall | 47.5 (1.6) | 47.7 (1.0) | 46.2 (3.1) | 0.0002* |
| Cue Efficiency | .978 (.055) | .985 (.041) | .938 (.097) | 0.0002* |
| Follow up time (SD) | 2.76 (.90) | 2.74 (.91) | 2.83 (.84) | 0.06* |
Mann-Whitney test
Pearson chi-squared test
Fisher exact test
SD: standard deviation
F: Female
AA: African American
MMSE: Mini Mental State Examination
Note: The p-values reflect comparisons between patients with and without dementia at follow-up.
Figure 2.
Receiver operating characteristic curves for incident dementia comparing free and total recall.
Table 5 presents results from a series of Cox proportional hazards analyses modeling time to incident dementia using FCSRT measures as the main predictors and age, race, and education as covariates. Patients with impaired free recall were four times more likely than patients with intact free recall to have developed dementia at anytime during the follow-up period (Panel 5A). Older age increased the risk (p=.03) but race (.20) and education (.32) did not. The model for predicting incident dementia from total recall was similar. Patients with impaired total recall were nearly four times more likely to develop incident dementia than patients with intact total recall (Panel 5B). Older age increased the risk (p=.03) but race (.08) and education (.55) did not. When both predictors were included in the model (Panel 5C), free recall remained a statistically-significant predictor of incident dementia (p =.005) but total recall did not (p=0.10). Neither age (p=.12), education (p=0.62), nor race (p=0.15) significantly modified prediction.
Table 5.
Cox Proportional Hazards Models for predicting incident dementia using free recall (5A), total recall (5B), free and total recall (5C).
| Exp(coef) | Lower .95 CI | Upper .95 CI | p | |
|---|---|---|---|---|
| 5A | ||||
| Free<=24 | 4.024 | 1.821 | 8.89 | .006 |
| Age | 1.069 | 1.006 | 1.14 | .03 |
| AA race | .504 | .177 | 1.44 | .20 |
| Years Educ | .940 | .832 | 1.06 | .32 |
| 5B | ||||
| Total<=46 | 3.996 | 1.432 | 11.15 | .008 |
| Age | 1.066 | 1.004 | 1.13 | .03 |
| AA race | .379 | .128 | 1.12 | .08 |
| Years Educ | .964 | .855 | 1.09 | .55 |
| 5C | ||||
| Free<=24 | 3.397 | 1.450 | 7.96 | .005 |
| Total<=46 | 2.462 | .830 | 7.30 | .10 |
| Age | 1.051 | .988 | 1.12 | .12 |
| AA race | .443 | .149 | 1.31 | .14 |
| Years Educ | .968 | .855 | 1.10 | .61 |
Distinguishing AD from nonAD dementias
The 16 patients with nonAD dementias displayed significantly higher total recall (45.0, 95% CI: 42.2, 47.8) than the 32 patients with AD (39.8; 95% CI: 35.8, 42.9) (p=0.029). While free recall was higher for patients with nonAD dementias (21.5, 95% CI: 18.1, 24.9) than for patients with AD (17.5; 95% CI: 14.8, 20.1), the difference was not significant (p=0.063).
Discussion
We compared three scores derived from the FCSRT (the free recall, total recall and cue efficiency) in their ability to identify prevalent dementia, predict future (incident) dementia and distinguish AD and nonAD dementias in a primary care setting. The near complete overlap between cued recall measures led us to focus on total recall because its simplicity would be more amenable to widespread use in primary care clinical settings. Free recall outperformed total recall in predicting future dementia as we expected. The unexpected finding was that free recall outperformed total recall in identifying prevalent dementia. Total recall showed the expected advantage in distinguishing patients with AD dementias from patients with nonAD dementias.
Based on measures of the area under the ROC curves and McNemar's test, free recall outperformed total recall in distinguishing patients with prevalent dementia from patients who were dementia free at baseline. When specificity of the measures was fixed at 90%, sensitivity to prevalent dementia was 78% for free recall and 54% for total recall. Logistic regression provided further evidence for the advantage of free recall over total recall as a predictor of prevalent dementia. Patients with impaired free recall (≤ 24) were 15 times more likely to have a prevalent dementia than patients with intact free recall compared to an odds ratio of 5.6 for patients with impaired total recall (<=46). When total recall was added as a predictor in the model, the odds ratio for each measure was reduced.
A similar picture emerged in the prediction of incident dementia. The area under the ROC curve was significantly higher for free recall than for total recall. Though both free recall and total recall predicted incident dementia equally well in separate Cox models, when both measures were included in the same model, only free recall was a significant predictor of future dementia. The same cut score used here to indicate impaired free recall was optimally discriminating for predicting dementia over five years in a community residing elderly cohort. 8
Whereas free recall outperformed total recall in identifying incident and prevalent dementia in this primary care cohort, total recall displayed the expected advantage in distinguishing AD from nonAD dementias. In AD, impairments in total recall and cue efficiency result from poor information storage that is not remediable by controlled learning and cued recall procedures. In contrast, the memory deficit in patients with nonAD dementias (e.g. vascular [VaD], Lewy body, frontotemporal) usually occurs secondary to executive dysfunction that adversely impacts strategies for encoding and retrieving information. Unlike AD, this memory deficit can be ameliorated with controlled learning and cued recall 6, 14, making total recall a useful adjunct to other clinical indicators in subtyping patients.
The advantage of free recall over total recall in identifying prevalent dementia was unexpected, given that in multiple previous studies total recall has been used with high accuracy to identify prevalent dementia and AD 2, 5, 6. It is possible that the nature of the current study may account for this unexpected result. Compared to previously-reported findings, our study accorded greater emphasis to very mild dementia (CDR =0.5), was set in a primary-care practice rather than a memory-disorders clinic, used all-cause dementia as an outcome instead of AD, and differed in the administration of the FCSRT.
Most patients (86%) in the current cohort had very mild dementia. In our prior studies of the FCSRT 4, 18 patients likely had more advanced dementia, as indicated by their average of 15 errors on the Blessed Information-Memory- Concentration test 30; in the current clinical rating system they likely would correspond to patients with moderately severe dementia (CDR 2.0). In the prior study, impaired total recall correctly classified 97% of 120 individuals, including 50 prevalent cases. In the current study, half of the patients with prevalent dementia had intact cued recall, thereby limiting its sensitivity relative to free recall.
Second, the setting of our study differed from previous studies conducted by French researchers in which cue efficiency outperformed free recall in identifying prevalent dementia 6, 13. In these studies, patients were recruited from memory disorder clinics where samples were enriched by patients with have mild cognitive Impairment (MCI) and thus were at increased risk of future dementia. In contrast, the current study was conducted in a primary care clinic where 44% of the study participants had normal cognition (CDR = 0.0). These sample differences can differentially affect the operating characteristics of the measures.
A third difference is that we predicted all-cause dementia, whereas other studies have traditionally emphasized prediction of AD. In these studies cued recall measures, including total recall and cue efficiency, outperformed free recall in predicting prodromal and early AD 5, 13. If the goal is to distinguish AD patients from patients who are dementia free, the best predictor is cued recall which is intact in normal aging 19, 31. Because the retrieval deficits that characterize nonAD dementias are likely to be remediated with controlled learning and cued recall, cued recall will be insensitive to predicting all-cause dementia. As a consequence, free recall is a more sensitive predictor of all-cause dementia.
The final difference is in the nature of the to-be-remembered items; the French version of the FCSRT uses words whereas our administration has traditionally used pictures. The cut scores for incident dementia used in the French studies were lower than those used here13. The lower cut scores for words than pictures on all the FCSRT measures is consistent with the dual encoding theory of memory 32. Pictures are remembered better than words because their representations in memory include both verbal and visual codes while words are encoded only verbally. A recent study of the picture superiority affect confirms its presence in community residing older adults (aged 60-97) in both free recall and recognition 33. Our current data suggest that patients with very mild dementia can retrieve this more richly elaborated memory trace by cued recall as well as patients without dementia but not in the absence of cues, rendering free recall more sensitive than total recall to incident dementia.
The absence of significant race and education effects in models predicting incident and prevalent dementia from free recall and total recall suggests that these FCSRT measures can be used to classify patients in disparity groups without adjustment for race and education differences. This is especially important in primary care where socio/demographic diversity is common and disease presentation is heterogeneous. The lack of accurate and efficient methods to identify mild dementia in ethnically and educationally diverse patients has been a barrier to primary care screening. 34
For a test with continuous scores, like the FCSRT, cut-scores are typically selected so that individuals can be classified as having screened positive or negative. As these cut-scores become more restrictive, the specificity of the screening test increases at the price of sensitivity. The optimal balance between sensitivity and specificity depends upon the nature of the illness being detected, the risks and burden of the procedures for definitive diagnosis, the benefits of early detection, and the risks of detection failures. We made high specificity a priority for several reasons. First, sensitivity remains quite good even at high specificity. Second, low specificity would result in many false positive screens, needlessly worrying patients. Third, false positive screening would lead to costly follow-up tests. Finally, in a world of symptomatic and not disease modifying treatment, diagnostic delay while undesirable, does not cause great harm. If treatment improves, clinicians may find that accepting a lower specificity with a corresponding increase in sensitivity is optimal for particular contexts or applications.
In addition to its high specificity, the FCSRT has the added advantages of low cost and ease of administration.35 The FCSRT takes 12-15 minutes to administer, so it may not be practical to screen all patients over the age of 65 with this instrument. To optimize efficiency, we developed three two stage strategies (with the FCSRT as the second stage) that have high sensitivity (first stage) and specificity (FCSRT in the second stage) for very mild dementia in primary care22, 23. One strategy, which we call the Alzheimer's Disease Screen for Primary Care (ADS-PC),23 consisting of the Memory Impairment Screen 36 and Animal Fluency 37 followed by the FCSRT for patients who screen positive in the rapid first stage, outperformed the MMSE in identifying early dementia, and worked equally well in African American and Caucasian patients and in patients who differed by educational level. The ADS-PC was highly efficient: only 30% of patients tested had a positive screening result in the first stage and needed to undergo the FCSRT. Moreover, only 10% of non-cases were misidentified as having dementia by the ADS-PC, an advantage over other sensitive screens that misidentify 50% of non-cases as having dementia.38
There are several caveats to the current results. The operating characteristics of the FCSRT measures must be assessed in other primary care cohorts. It is possible that optimal cut scores will vary from sample to sample. Different versions of the FCSRT have been used by other investigators. The study phase used in the Mayo clinic normative study 21 presents all 16 pictures at once rather than four at a time as we do here. This does not allow for immediate recall which provides retrieval practice while the items are still in working memory. Without immediate recall, specificity of the test may be reduced. French researchers use the same study procedures as we do including immediate recall but they use words rather than pictures. The FCSRT procedure used by Spanish researchers in the NEURONORMA Project does not include immediate recall and uses words rather than pictures, resulting in much lower scores for both free recall and total recall in their normative sample. 39
As new treatments and preventive approaches for AD emerge, they will be implemented in primary care settings where the majority of older adults receive their care. Identifying very mild dementia with tools such as the ADS-PC will be essential to implementing the next generation of symptomatic treatments and the disease-modifying treatments of the future to the seniors who need them.
Acknowledgments
The Free and Cued Selective Reminding Test with Immediate Recall (FCSRT-IR) is copyrighted by the Albert Einstein College of Medicine and one author (EG) receives a small percentage of any royalties on the FCSRT when it is used for commercial purposes. This work is supported in part by grants AG017854 and AG03949. Dr. Sanders is supported in part by the CTSA Grants UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
This work was supported in part by grants AG017854 and AG03949. Dr. Sanders is supported in part by the CTSA Grants UL1 RR025750 and KL2 RR025749 and NCRR Grant TL1 RR025748.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association Press; Washington, D.C.: 1994. [Google Scholar]
- 2.Ferris SH, Aisen PS, Cummings J, et al. ADCS Prevention Instrument Project: Overview and initial results. Alzheimer's Disease and Associated Disorders, Supplement. 2006 doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- 3.Gebner R, Reischies FM, Kage A, Geiselmann B. In an epidemiological sample the apolipoprotein E4 allele is associated to dementia and loss of memory function only in the very old. Neuroscience Letters. 1997;222:29–32. doi: 10.1016/s0304-3940(97)13334-1. al e. [DOI] [PubMed] [Google Scholar]
- 4.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG. Memory function in very early Alzheimer's disease. Neurology. 1994 May 1;44(5):867–872. doi: 10.1212/wnl.44.5.867. 1994. [DOI] [PubMed] [Google Scholar]
- 6.Tounsi H, Deweer B, Ergis A, et al. Sensitivity to Semantic Cuing: An Index of Episodic Memory Dysfunction in Early Alzheimer Disease. Alzheimer Disease and Associated Disorders. 1999;13(1):38–46. doi: 10.1097/00002093-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Tuokko H, Crockett D. Cued recall and memory disorders in dementia. Journal of Clinical and Experimental Neuropsychology. 1989;11:278–294. doi: 10.1080/01688638908400889. [DOI] [PubMed] [Google Scholar]
- 8.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 9.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's Disease. Psychology and Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995 April 26;273(16):1274–1278. 1995. [PubMed] [Google Scholar]
- 11.Robert PH, Berr C, Volteau M, et al. Neuropsychological performance in mild cognitive impairment with and without apathy. Dementia and Geriatric Cognitive Disorders. 2006;21:192–197. doi: 10.1159/000090766. [DOI] [PubMed] [Google Scholar]
- 12.Grober E, Hall C, Lipton RB, Zonderman A, Resnick S, Kawas C. Memory Impairment, Executive Dysfunction, and Intellectual Decline. Journal of the International Neuropsychological Association. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology. 2007 November 6;69(19):1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. 2007. [DOI] [PubMed] [Google Scholar]
- 14.Traykov L, Baudic S, Raoux N, et al. Patterns of memory impairment and perseverative behavior discriminate early Alzheimer's disease from subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229-230:75. doi: 10.1016/j.jns.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch Neurol. 1993 April 1;50(4):374–379. doi: 10.1001/archneur.1993.00540040036010. 1993. [DOI] [PubMed] [Google Scholar]
- 16.Grober E, Hall C, Sanders A, Lipton RB. Free and Cued Selective Reminding distinguishes Alzherimer's disease from vascular dementia. Journal of the American Geriatrics Society. 2008;56(5):944–946. doi: 10.1111/j.1532-5415.2008.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buschke H. Cued recall in Amnesia. Journal of Clinical and Experimental Neuropsychology. 1984;6(4):433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- 18.Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- 19.Grober E, Merling A, Heimlich T, Lipton RB. Comparison of selective reminding and free and cued selective reminding in the elderly. J Clinical and Experimental Neuropsychology. 1997;19:643–654. doi: 10.1080/01688639708403750. [DOI] [PubMed] [Google Scholar]
- 20.Buschke H, Sliwinski M, Kuslansky G, Lipton RB. Aging, encoding specificity, and memory change in the Double Memory Test. Neurology. 1995;1:483–493. doi: 10.1017/s1355617700000576. [DOI] [PubMed] [Google Scholar]
- 21.Ivnik RJ, Smith GE, Lucas JA. Free and cued selective reminding test: MOANS norms. Journal of Clinical and Experimental Neuropsychology. 1997;19:676–691. doi: 10.1080/01688639708403753. [DOI] [PubMed] [Google Scholar]
- 22.Grober E, Hall C, McGinn M, et al. Neuropsychological strategies for detecting early dementia. Journal of the International Neuropsychological Society. 2008;14:130–142. doi: 10.1017/S1355617708080156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grober E, Hall C, Lipton RB, Teresi J. Primary care screen for early dementia. Journal of the American Geriatrics Society. 2008;56:206–213. doi: 10.1111/j.1532-5415.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. The Mini-Mental State: a practical method for grading cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC. Clinical Dementia Rating: Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer's Disease: Report of the NINCDS-ADRA work group under the auspices of Department of Health and Human Services Task force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. al e. [DOI] [PubMed] [Google Scholar]
- 27.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992 March 1;42(3):473–480. doi: 10.1212/wnl.42.3.473. 1992. [DOI] [PubMed] [Google Scholar]
- 28.McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy bodies. Neurology. 1999;53:902–905. doi: 10.1212/wnl.53.5.902. [DOI] [PubMed] [Google Scholar]
- 29.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Annals of Neurology. 2005;57:480–488. doi: 10.1002/ana.20425. [DOI] [PubMed] [Google Scholar]
- 30.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures and senile changes in the cerebral gray matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 31.Grober E, Lipton RB, Katz M, Sliwinski M. Demographic Influences on Free and Cued Selective Reminding Performance in Older Persons. Journal of Clinical and Experimental Neuropsychology. 1998;20:221–226. doi: 10.1076/jcen.20.2.221.1177. [DOI] [PubMed] [Google Scholar]
- 32.Paivio A. Imagery and memory. The Cognitive Neurosciences. 1995:977–986. [Google Scholar]
- 33.Cherry KE, Hawley KS, Jackson EM, Volaufova J, Su LJ, Jazwinski SM. Pictorial superiority effects in oldest-old people. Memory. 2008;16(7):728–741. doi: 10.1080/09658210802215534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- 35.Tuokko H, Kristjansson E, Miller JA. The neuropsychological detection of dementia: An overview of the neuropsychological component of the Canadian Study of Health and Aging. Journal of Clinican and Experimental Neuropsychology. 1995;13:871–879. doi: 10.1080/01688639508405129. [DOI] [PubMed] [Google Scholar]
- 36.Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the Memory Impairment Screen. Neurology. 1999 January 1;52(2):231–238. doi: 10.1212/wnl.52.2.231. 1999. [DOI] [PubMed] [Google Scholar]
- 37.Rosen W. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- 38.Boustani M, Callahan C, Unverzagt F, et al. Implementing a screening and diagnosis program for dementia in primary care. Journal of General Internal Medicine. 2005;20(7):572–577. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pena-Casanova J, Gramunt-Fombuena N, Quinones-Ubeda S, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Rey-Osterrieth Complex Figure (Copy and Memory), and Free and Cued Selective Reminding Test. Arch Clin Neuropsychol. 2009 June 1;24(4):371–393. doi: 10.1093/arclin/acp041. 2009. [DOI] [PubMed] [Google Scholar]
- 40.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994 April 1;44(4):609–614. doi: 10.1212/wnl.44.4.609. 1994. [DOI] [PubMed] [Google Scholar]
- 41.Albert M, Moss M, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychology Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 42.Grober E. Nonlinguistic memory in aphasia. Cortex. 1984;20:67–73. doi: 10.1016/s0010-9452(84)80024-6. [DOI] [PubMed] [Google Scholar]
- 43.Freeman M, Leach L, Kaplan E, Winocur G, Schulman K, Delis D. Clock drawing: A neuropsychological analysis. Oxford University Press; 1994. [Google Scholar]
- 44.Dartigues JF, Commenges D, Letenneur D, et al. Cognitive predictors of dementia in elderly community residents. Neuroepidemiology. 1997;16(1):29–39. doi: 10.1159/000109668. [DOI] [PubMed] [Google Scholar]
- 45.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982-1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]