Abstract
Central venous and arterial catheters are a major source of thrombo-embolic disease in children. We hypothesized that guided high mechanical index (MI) impulses from diagnostic three-dimensional (3D) ultrasound during an intravenous microbubble infusion could dissolve these thrombi. An in vitro system simulating intra-catheter thrombi was created and then treated with guided high MI impulses from 3D ultrasound, utilizing low MI microbubble sensitive imaging pulse sequence schemes to detect the microbubbles (Perflutren Lipid Microsphere, Definity®, Lantheus). Ten aged thrombi over 24 hours old were tested using 3D ultrasound coupled with a continuous diluted microbubble infusion (Group A), and ten with 3D ultrasound alone (Group B). Mean thrombus age was 28.6 hours (range 26.6–30.3). Groups A exhibited a 55 ± 19 % reduction in venous thrombus size, compared to 31±10 % for Group B (p=0.008). Feasibility testing was performed in 4 pigs, establishing a model to further investigate the efficacy. Sonothrombolysis of aged intra-catheter venous thrombi can be achieved with commercially available microbubbles and guided high MI ultrasound from a diagnostic 3D transducer.
Keywords: Pediatric, Sonothrombolysis, Microbubble, Three-dimensional echocardiography
INTRODUCTION
Central venous and arterial catheters are a major source of thrombo-embolic disease in children.1 Central venous catheters (CVC) are widely used in the care of pediatric patients for intensive care, chemotherapy, transfusion therapy, acquisition of blood samples, and to facilitate supportive care including nutrition and hydration. The presence of an indwelling CVC is the single most important risk factor for childhood venous thrombo-embolism (VTE),1–3 with approximately 60% of childhood VTE associated with a CVC. VTE is a growing health problem in children, and is associated with significant acute mortality and chronic morbidity.1 There has been a dramatic increase in the diagnosis of VTE at children’s hospitals in the United States.5 The current estimated incidence of VTE is 4.2/1,000 pediatric hospitalizations.4 Furthermore, acute VTE is associated with an estimated two-fold increased risk of in-hospital death.4 Chronic consequences are recurrence of VTE and post-thrombotic syndrome. Children may be impaired for longer periods of time with these complications as compared to adults.
Optimal therapy for pediatric CVC-associated thrombus or VTE remains poorly defined. There are several management issues unique to children. Removal of the CVC is often fraught with problems because many patients require long-term access and insertion of a new CVC may further increase thrombo-embolic risk.6 The decisions on CVC removal after a thrombotic event must be balanced against factors such as difficulty of venous access, and need for further treatment and blood sampling. Pediatric CVC-associated thrombus and VTE warrant rapid thrombus resolution in most cases, and early implementation of thrombolysis is beneficial.7 However, use of thrombolytic drugs in children is controversial due to concerns of major hemorrhage. Reported rates of major bleeding with thrombolytic drugs in children are as high as 40%.5 Due to the absence of large, well-designed clinical trials assessing the use of thrombolytic drugs in children, therapeutic regimens have been extrapolated from adult guidelines. There are important differences in the fibrinolytic system in children that influence the response to thrombolytic agents, and the pathophysiologic mechanisms of thrombosis are quite different from those in adults.8 Finally, thrombo-prophylaxis with heparin has been shown in several trials to have no benefit and in general is not recommended in children.6
Ultrasound has been investigated as an adjunct to pharmacologic thrombolysis, as well as an independent treatment for vascular thrombosis.9–12 Guided high mechanical index (MI) impulses from a diagnostic ultrasound system during intravenous microbubble infusion have the potential to dissolve intravascular thrombi (termed sonothrombolysis) without the need for fibrinolytic therapy. Cavitation and resultant shear forces are one of the proposed mechanisms for thrombus dissolution by ultrasound.13 We have previously studied the feasibility of treating deeply located acute intravascular thrombi with ultrasound and intravenous microbubbles.14–15 The potential utility of these therapies has not been previously tested using CVC, where thrombi are older and more stasis-related than arterial thrombi. However, ultrasound appears to dissolve thrombi by a mechanical shearing effect related to cavitation, and thus may be more effective than lytic agents in aged thrombi. Our hypothesis, therefore, was that sonothrombolysis might be an alternative method of treating CVC related thrombi.
METHODS
To test our hypothesis, we developed an in vitro system simulating intra-catheter thrombi (Figures 1 and 2). Our objective was to test the feasibility and success of sonothrombolysis with guided high MI impulses from a three-dimensional (3D) ultrasound transducer; and utilizing low MI microbubble sensitive imaging pulse sequence schemes to detect the microbubbles. The study was approved by the Institutional Animal Care and Use Committee and was in compliance with the Standards in the Guide for the Care and Use of Laboratory Animals.
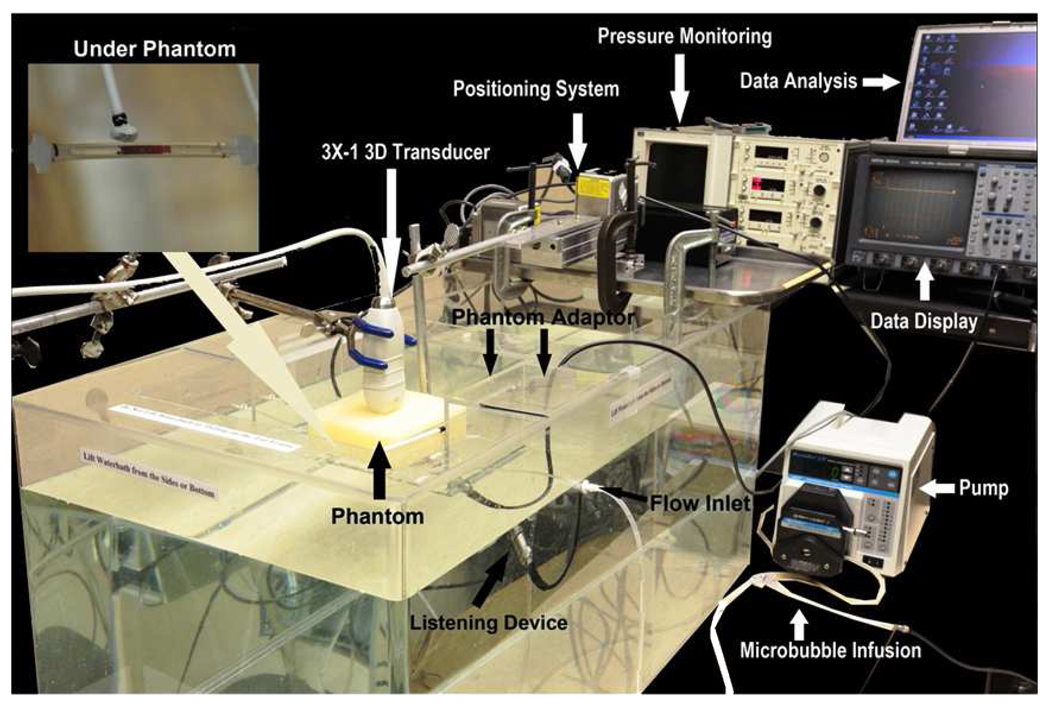
Figure 1.
Photograph of the in vitro flow system developed for 3D ultrasound and microbubble treatments. Inset shows a sample placed for testing underneath the tissue-mimicking phantom. 3D=Three-dimensional
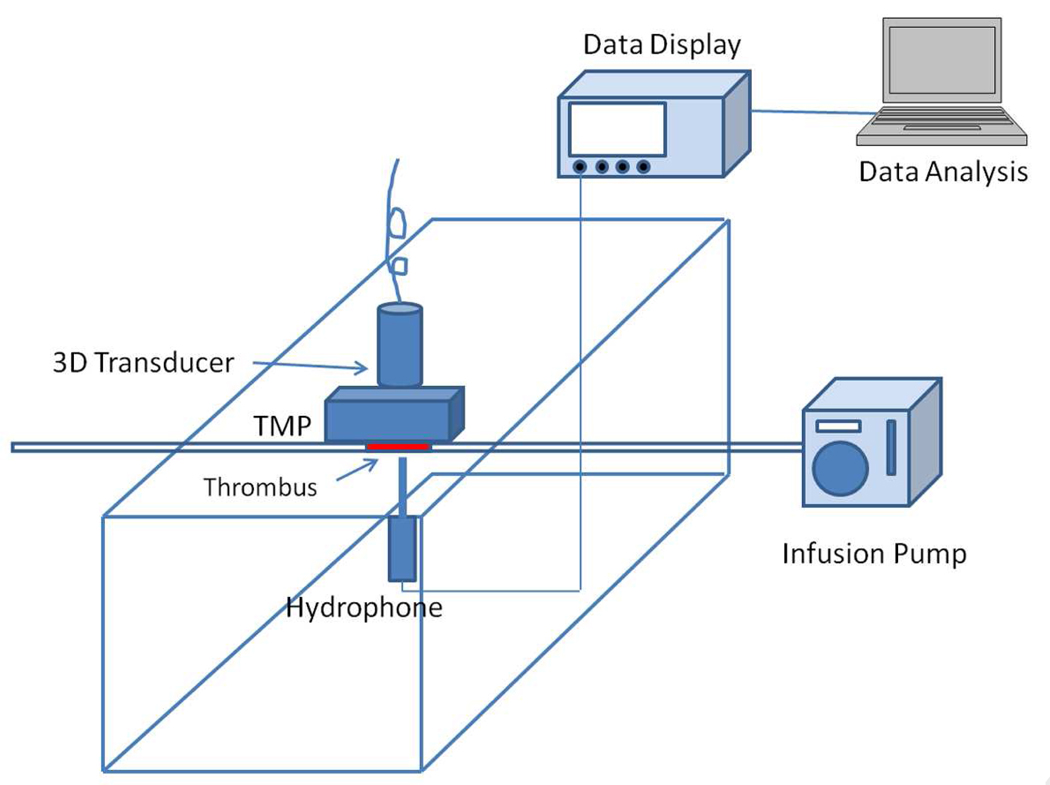
Figure 2.
Schematic diagram of the in vitro study set up. TMP=Tissue Mimicking Phantom
Venous blood samples were drawn from healthy pigs to prepare thrombi aged >24 hours within a silastic tubing (Dow Corning, Midland, MI) of length 6 cm, internal diameter 2.64 mm, and wall thickness 1.12 mm. The blood samples were not anticoagulated. Each tubing had a layer of Transpore surgical tape (3M Healthcare, St. Paul, MN) pasted to the central aspect of its inner wall with the adhesive surface exposed. A plastic connector was attached to one end of the tubing for stability of the unit on a flat surface during weighing as well as during facilitation of thrombus formation. The tubing with the connector was weighed as a unit on a balance with 0.001g readability (Adam Equipment Inc., Milton Keynes, United Kingdom). After withdrawal of venous blood from the pig, 0.1 ml of the blood sample was introduced into the center of the tubing in a contiguous manner. The sample adhered to the surface of the tape as a film <1.5 cm in length, facilitating red cell deposition on its rough surface and serum formation above. After more than 24 hrs, the tubing with the adherent thrombus within it was weighed, and then connected to the in vitro flow system. A five-centimeter thick tissue-mimicking phantom was placed over the tubing, to simulate the typical distance between a diagnostic transducer and the superior vena cava in a pediatric patient.
A diagnostic 3D ultrasound (iE33, Philips Ultrasound systems, Andover, MA) with an X3-1 matrix array transducer of 1.6 MHz frequency was used at an angle of 90 degrees with respect to the thrombus (Figure 3). The live 3D volume of coverage had a focused beam, which covered a 30 by 60 degree field at a depth of 5 cm. During treatment, the transducer was fixed with the aid of a holder so that the center of its three-dimensional beam matrix (90 degrees with respect to the thrombus) permitted the highest peak negative pressure to reach the thrombus underneath. The spatial distribution of peak negative pressures during the high MI impulses from the 3D transducer was measured through the five-centimeter standoff using a calibrated 0.5 mm diameter needle hydrophone (Precision Acoustics Ltd, Dorchester, United Kingdom). The hydrophone was moved to different locations by a computer-controlled three-axis positioning system with 1.0 mm increment.
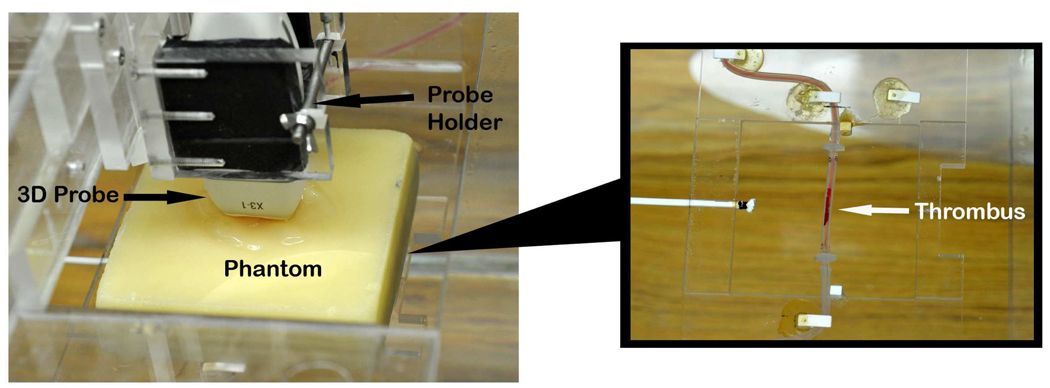
Figure 3.
The 3D ultrasound transducer with probe holder placed above the tissue-mimicking phantom (left panel). The right panel shows a sample placed for testing underneath the tissue-mimicking phantom. 3D=Three-dimensional
For treatment of 10 samples (Group A), brief high MI>1.0 impulses (10 seconds on; 10 seconds off) were delivered to cavitate microbubbles within the region of interest during the continuous infusion of 0.5% diluted Perflutren Lipid Microsphere (Definity®, Lantheus Medical Imaging, N. Billerica, MA). The diluted microbubble solution was connected to a saline infusion pump flowing at 1 ml/min. The number of microbubbles passing through the site of the thrombus was calculated to be 6 × 107 microbubbles per minute. Group B consisted of 10 samples tested with 3D ultrasound alone during a normal saline infusion at the same (1 ml/min) flow rate. After treatment, each sample with its connector was dried using 30-minute air blow and weighed on the same balance as above. In this way an accurate thrombus weight was obtained before and after sonothrombolytic treatment of each sample.
Establishment of in vivo model for feasibility
To demonstrate feasibility in vivo, we designed and tested the application in a porcine model of chronic indwelling CVC. The design and testing was accomplished in three steps as outlined below.
Step 1. Creation of a Chronic Model of CVC System
After the animal was placed under isoflurane general anesthesia and preparation of the surgical site following aseptic precautions, an incision was made over the jugular furrow of the neck and continued through the subcutaneous tissue and cutaneous coli muscle. The jugular veins were exposed with blunt dissection, and a short incision was made in the skin of the dorsal interscapular region. A trocar was be used to tunnel subcutaneously from the ventral incision to the dorsal region of the chest wall. A 10F single lumen CVC (long-term carbothane hemodialysis catheter, 35 cm, Bard Inc., Covington, GA) was advanced through the subcutaneous tunnel for placement via surgical cut down in the superior vena cava. The jugular vein was canulated using the modified Seldinger technique, and the CVC was positioned with its tip is in the proximal superior vena cava. A check angiogram through the CVC was used to guide accurate position of the tip. The vessel incision was closed with 6-0 prolene and the CVC was anchored in the vessel using 4-0 prolene. The ventral neck incision was closed in layers. The exteriorized portion of the CVC was secured to the skin using non-absorbable sutures; and covered by a protective canvas pouch that was sutured to the dorsum of the animal. The dorsal exteriorizing site was closed with 1-0 prolene. The animal was recovered from anesthesia with continuous monitoring. The CVC system was flushed with normal saline every 24 hours until Step 2.
Step 2. Facilitation of Thrombus Formation in the CVC
The animal returned to the lab 5 days after CVC placement. Under general anesthesia, femoral arterial and venous sheaths (7 Fr) were percutaneously placed for vascular access and hemodynamic monitoring. Further to checking the CVC system with a normal saline flush, 0.5 ml of blood was drawn into the CVC to facilitate thrombus formation within its tip over the next 24 hours.
Step 3. Sonothrombolytic Treatment of CVC
Each animal was randomized to receive one of the two treatments for 40 minutes: 3D ultrasound with microbubble infusion or 3D ultrasound with 0.9% saline infusion. Two end points were chosen as indices of recanalization; comparison of (1) rate of increase in the drip rate through the CVC during treatment, and (2) histopathologic assessment of the residual thrombus present in the explanted CVC.
Prior to treatment, the CVC tip was localized using fluoroscopy and subclavicular high frequency 2D ultrasound. The fluid infusion pump was kept at a fixed height of 90 inches and the change in the drip rate/min was continuously monitored during treatment using a digital stopwatch. Following treatment, the animal was euthanized and histopathologic examination of the CVC performed by a pathologist (SJR).
Statistical Analysis
For the in vitro study, non-paired t test was used for comparison between groups. Statistical analysis was performed using MS Excel (Microsoft Inc., Redmond, WA) and Minitab 15 (Minitab Inc, State College, PA). A p value of <0.05 represented significance.
RESULTS
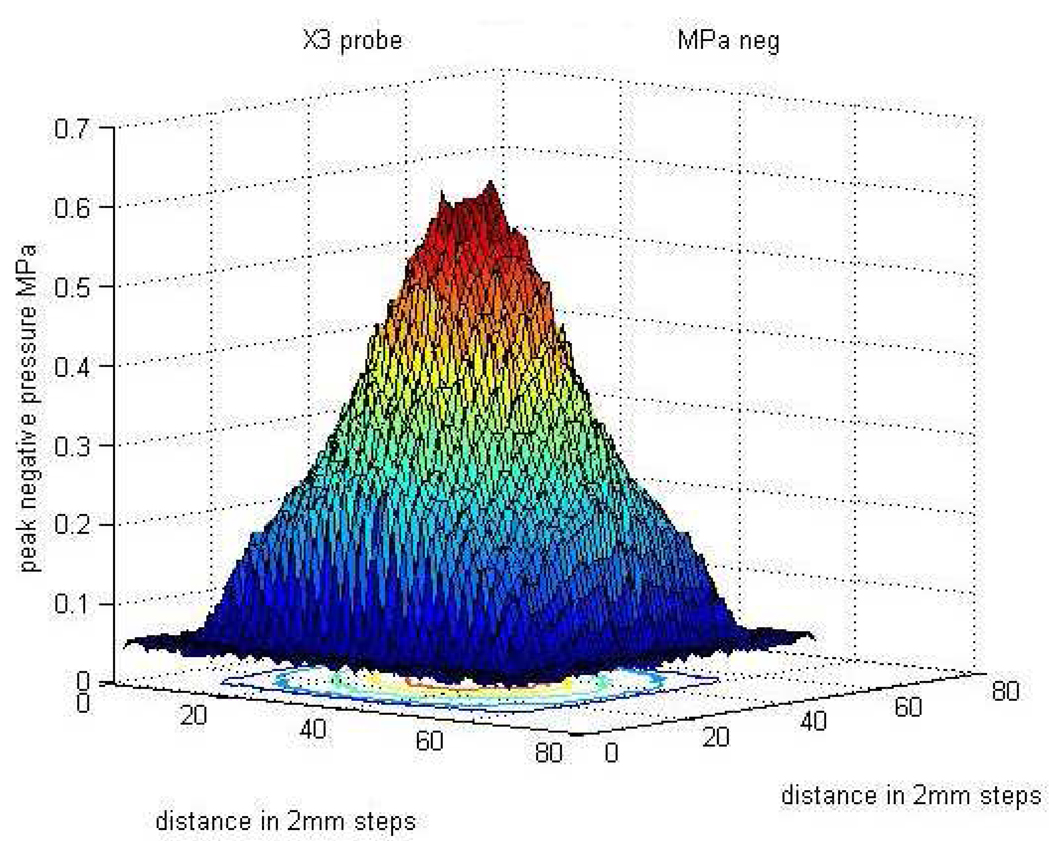
The 3D peak negative pressure field measured in our laboratory during high MI impulses passing through the five-centimeter thick tissue-mimicking phantom is displayed in Figure 4. Note that the beam is cone-shaped with significant drops in peak negative pressures near the edges of the 3D field.
Figure 4.
Schematic display of the 3D pressure spectrum at the focal distance of the transducer. The hydrophonic measurements of peak negative pressure emanated in high MI mode were made through the tissue-mimicking phantom (shown in the Y-axis). 3D=Three-dimensional
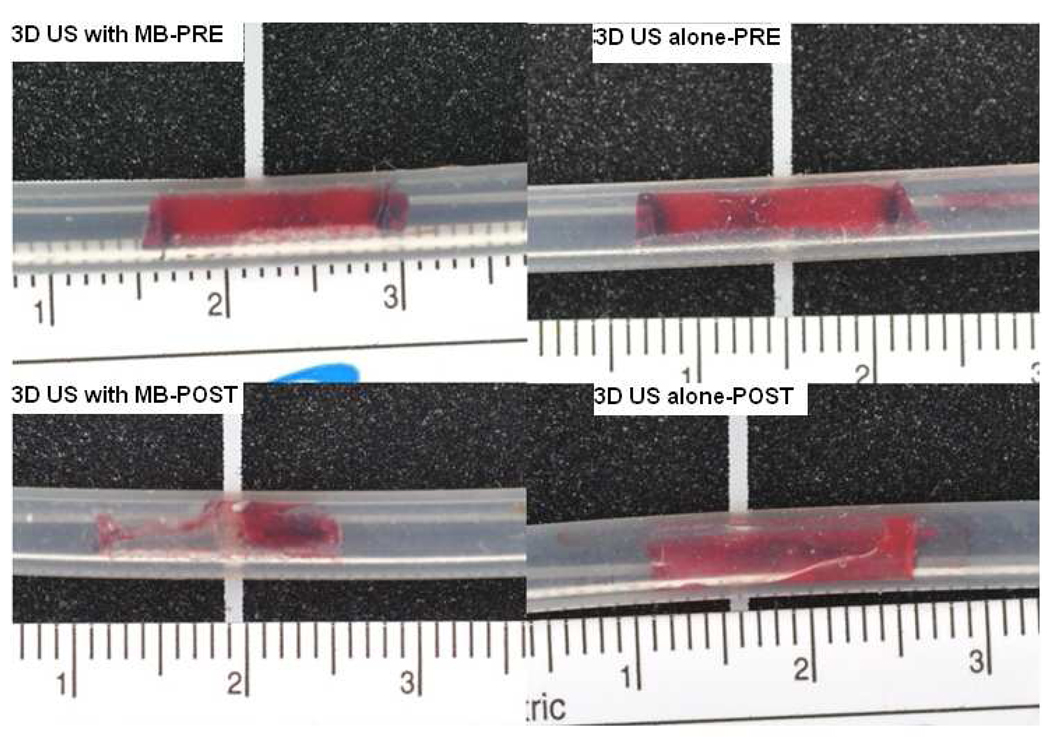
Mean venous thrombus age was 28.6 hours (range 26.6–30.3 hours). Mean thrombus weight was 42.1± 4.6 mg in Group A and 42.7 ± 3.6 mg in Group B. Mean thrombus weight after treatment was 19.1± 8 mg in Group A and 29 ± 5.6 mg in Group B. The mean change in weight was 23 ± 8.5 mg in Group A and 13.7± 3.7 mg in group B. The 10-minute ultrasound and microbubble treatment resulted in a 55 ± 19 % reduction in venous thrombus weight in the 3D ultrasound and microbubble treated group as compared to 31±10 % for Group B (p=0.008). Figure 5 depicts a representative sample of the venous thrombus dissolution achieved with the microbubbles and intermittent 3D high MI impulses.
Figure 5.
Photographs of thrombi in two samples before and after treatment. Upper panel shows images acquired before treatment (3D ultrasound+ microbubbles on the left side; ultrasound alone on the right). Lower panel shows the same samples after treatment (3D ultrasound+ microbubbles on the left side; ultrasound alone on the right).
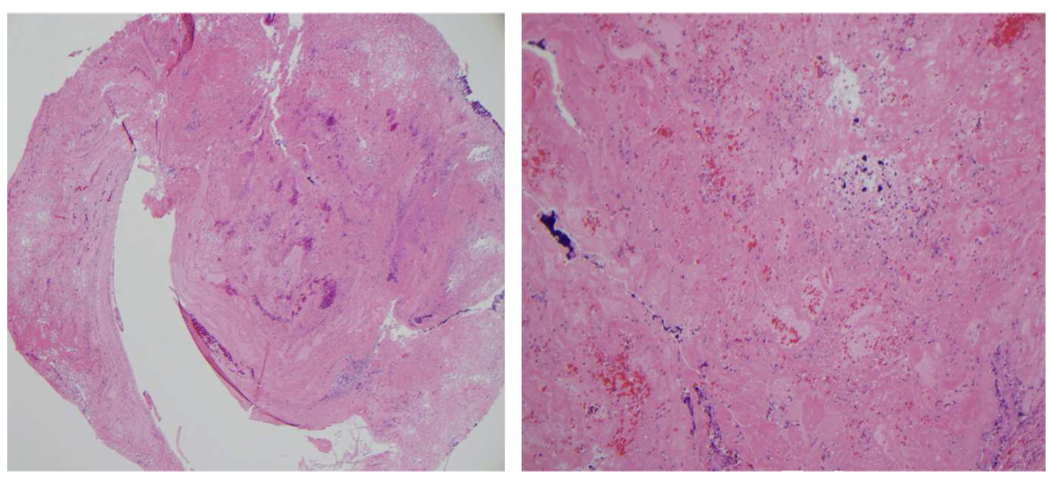
In vivo feasibility testing was successfully performed in 4 pigs, thereby establishing a model to investigate the efficacy of sonothrombolysis in this setting. Histopathologic examination of the explanted CVC from the animal and in a sample of intra-tubing thrombus (as in the in vitro study) showed that the thrombus composition was similar. The thrombus composition was fibrin-platelet mixture with a higher percentage of the mixture seen in the in vivo specimen, (Figure 6) and the remainder being red blood cells and partially degenerated leukocytes.
Figure 6.
Histopathologic examination of the residual thrombus in cross sectional specimen of a CVC explanted from an animal is shown. Left (Hematoxylin and Eosin X 40) and right panels (Hematoxylin and Eosin X 200) demonstrate a mixture of fibrin with foci of granular platelets that composed the great majority of the thrombus (50–75%). In addition, scattered erythrocytes and partially degenerated leukocytes are seen.
DISCUSSION
The main finding of the present study is that successful dissolution of aged venous thrombi is achievable using microbubbles coupled with intermittent 3D high MI impulses. This study is the first to demonstrate the effectiveness of this technique in older venous thrombi, which form on chronic indwelling catheters or grafts, as well as in deep venous thrombosis. The type of microbubble used in this study is already commercially available, and thus there is a high likelihood that this technique may be feasible in children.
Sonothrombolysis is an emerging therapeutic application in which microbubbles are used to enhance the effectiveness of tissue plasminogen activator and the rate of thrombolysis. Since the first report by Tachibana et al9 that sonicated human serum albumin microbubbles enhanced in vitro thrombolysis with combined application of urokinase and ultrasound, other studies have examined thrombus-dissolving effects of ultrasound with and without thrombolytic agents.10, 16–17 Microbubble enhanced thrombus dissolution with low frequency ultrasound, in the absence of fibrinolytic agents, has been demonstrated in an in vivo iliofemoral occlusion model.16,18 It was shown in previous work from our institution that brief high MI impulses generated from a diagnostic ultrasound transducer can successfully recanalize acute intravascular thrombi during a continuous microbubble infusion.15 These studies have demonstrated that systemic administration of microbubbles may augment the cavitation effect of ultrasound in dissolving acute (four to six hour old) thrombi. In the current study, a high degree of effectiveness was seen with older venous thrombi, indicating that dissolution of these more chronic thrombi is also possible, even without the aid of a lytic agent or systemic anticoagulation. We believe that microbubble-enhanced sonothrombolysis occurs by a process of dissolution of micro fragments from the outer surface of such aged non-occlusive thrombi, which is different from fibrin destabilization that occurs in acute thrombi. We have shown passage of microbubbles through micro channels within six-hour-old thrombi and resultant promotion of lysis.15 However, in the setting of older venous thrombi as in the present study, micro channels are less likely to be present because of fibrin cross-linking and platelet related contraction of the thrombus. We were unable to show evidence of embolization in vitro, which may be related to the small size of the thrombi created in the study. Establishment of our in vivo model will enable further studies to examine the efficacy of 3D ultrasound and microbubbles in improving flow through thrombosed CVC.
The body of previous work in this field attests to the safety of sonothrombolysis with intravenous microbubbles, which is an important consideration in children. In addition, the lack of reported systemic and local complications with this technique is particularly appealing, especially as compared to riskier therapies like thrombolytic drugs. Recent guidelines discourage the routine use of thrombolytic drugs for treatment of pediatric VTE, except in life, organ or limb-threatening scenarios. Sonothrombolysis may be particularly advantageous in infants and children because of the proximity of vascular structures to the chest wall with less intervening tissue to limit attenuation of externally applied ultrasound impulses. Because of decreased attenuation of ultrasound, there is less likelihood of reduction in the peak negative pressure and resultant mechanical effects in the region of interest. The advantage of a 3D field is that nearly all angles of interrogation would be expected to insonify the CVC associated thrombus. A unique feature about microbubble-enhanced sonothrombolysis of CVC associated thrombi is that microbubbles can be infused through the CVC, which is not typically completely occluded. A high concentration of microbubbles is achievable in the region of interest, i.e. near the thrombus insonified in the 3D field. Thus, targeted 3D ultrasound delivery is feasible and thrombolysis occurs due to an outer shearing process caused by microbubbles.
VTE in children may occur in an extremity, systemic venous system, abdominal viscera or cerebral sinuses. The superior (superior vena cava and innominate vein) and inferior (inferior vena cava) systemic venous systems are the most common CVC locations and can be suitably imaged from standard suprasternal and subcostal pediatric ultrasound windows. Delivery of targeted ultrasound to CVC in these locations is therefore very likely achievable without significant attenuation of energy and may contribute to improved results.
Limitations
The sizes of the dissolution products of sonothrombolysis were not examined in this study. Animal studies have shown that downstream emboli have not been observed with ultrasound and microbubble mediated thrombus dissolution of arteriovenous graft occlusions.19 The example in Figure 5 indicates that the dissolution of these aged thrombi appears to be a shearing away from the outer surface, similar to peeling of an onion. This “micro-shearing” phenomenon would not be expected to be associated with any clinically relevant emboli, but we do recognize that large thrombi were not tested and pulmonary embolization was not systematically examined in the present study. Further in vivo investigations, with possible incorporation of sensitive radiotracer scanning techniques may be necessary to examine the effects of distal embolization after ultrasound and microbubble treatment.
We speculate that the CVC related thrombi being variable in their age could pose a potential disadvantage to the application of sonothrombolysis in the clinical setting. Further studies are therefore required to assess the success of this technique in the setting of very old thrombi (aged several days or weeks), and the most optimal ultrasound views for positioning a diagnostic ultrasound transducer to reach a CVC associated thrombus. Finally, evaluation of the possibility of soft tissue injury in neonates and very small infants due to bioeffects associated with hgh MI impulses from the transducer requires further study.
CONCLUSIONS
In conclusion, guided high MI ultrasound from a diagnostic 3D transducer during a microbubble infusion has potential for non-invasive sonothrombolysis of aged venous thrombi attached to catheters. The unique feature of sonothrombolysis in this setting is that because the CVC are typically not completely occluded, microbubbles can be infused through the CVC and thereby a high concentration is achievable in the region of interest. Unlike other clinical applications where only small concentrations of systemically administered microbubbles reach the site of thrombus due to low flow, the treatment of CVC thrombi may be very feasible because the microbubbles can still be administered directly into the thrombosed region in the majority of cases. Progress in the development of this modality could result in successful treatment of CVC associated thrombi, and thrombi related to palliative systemic venous or systemic to pulmonary artery shunts in children and young adults with congenital heart disease.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (NIH/NIBIB R01 EB009050-01). The authors thank Stacey L Therrien for assistance with preparation of the manuscript.
Abbreviations
- MI
Mechanical index
- 3D
Three dimensional
- CVC
Central venous catheter
- VTE
Venous thrombo-embolism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM. Venous thrombosis in children. J Thromb Haemost. 2003;1(7):1443–1455. doi: 10.1046/j.1538-7836.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 2.Monagle P, Adams M, Mahoney M, Ali K, Barnard D, Bernstein M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Journeycake JM, Buchanan GR. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J Clin Oncol. 2006;24:4575–4580. doi: 10.1200/JCO.2005.05.5343. [DOI] [PubMed] [Google Scholar]
- 4.Vu LT, Nobuhara KK, Lee H, Farmer DL. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg. 2008;43:1095–1099. doi: 10.1016/j.jpedsurg.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 6.Shivakumar SP, Anderson DR, Couban S. Catheter-associated thrombosis in patients with malignancy. J Clin Oncol. 2009;27:4858–4864. doi: 10.1200/JCO.2009.22.6126. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg NA, Durham JD, Knapp-Clevenger R, Manco-Johnson MJ. A thrombolytic regimen for high-risk deep venous thrombosis may substantially reduce the risk of postthrombotic syndrome in children. Blood. 2007;110:45–53. doi: 10.1182/blood-2006-12-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albisetti M. Thrombolytic therapy in children. Thromb Res. 2006;118:95–105. doi: 10.1016/j.thromres.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92 doi: 10.1161/01.cir.92.5.1148. 1148-50.12. [DOI] [PubMed] [Google Scholar]
- 10.Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J. 1996;132:964–968. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Dhond MR, Nguyen TT, Dolan C, Pulido G, Bommer WJ. Ultrasound-enhanced thrombolysis at 20 kHz with air-filled and perfluorocarbon-filled contrast bispheres. J Am Soc Echocardiogr. 2000;13:1025–1029. doi: 10.1067/mje.2000.107006. [DOI] [PubMed] [Google Scholar]
- 12.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Prokop AF, Soltani A, Roy RA. Cavitational mechanisms in ultrasoundaccelerated fibrinolysis. Ultrasound Med Biol. 2007;33:924–933. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20:1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- 15.Xie F, Lof J, Everbach C, He A, Bennett RM, Matsunaga T, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc Imaging. 2009;2:511–518. doi: 10.1016/j.jcmg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li S, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug: recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 17.Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, et al. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 18.Nishioka T, Luo H, Fishbein MC, Cercek B, Forrester JS, Kim CJ, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: an in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561–568. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui JM, Xie F, Johanning J, Lof J, Cory B, He Amming, et al. Treatment of deeply located acute intravascular thrombi with therapeutic ultrasound guided by diagnostic ultrasound and intravenous microbubbles. J Ultrasound Med. 2006;25:1161–1168. doi: 10.7863/jum.2006.25.9.1161. [DOI] [PubMed] [Google Scholar]