Abstract
Focal seizures appear to start abruptly and unpredictably when recorded from volumes of brain probed by clinical intracranial electroencephalograms. To investigate the spatiotemporal scale of focal epilepsy, wide-bandwidth electrophysiological recordings were obtained using clinical macro- and research microelectrodes in patients with epilepsy and control subjects with intractable facial pain. Seizure-like events not detectable on clinical macroelectrodes were observed on isolated microelectrodes. These ‘microseizures’ were sparsely distributed, more frequent in brain regions that generated seizures, and sporadically evolved into large-scale clinical seizures. Rare microseizures observed in control patients suggest that this phenomenon is ubiquitous, but their density distinguishes normal from epileptic brain. Epileptogenesis may involve the creation of these topographically fractured microdomains and ictogenesis (seizure generation), the dynamics of their interaction and spread.
Keywords: epilepsy, seizure, intracranial EEG, microseizure, microcircuit, seizure generation, ictogenesis, epileptogenesis
Introduction
Partial epilepsy is the most common pharmacologically resistant seizure disorder (Engel et al., 2003). Although the established electrophysiological signature of partial epilepsy is focal seizures, little is known about the spatial and temporal scales that define the neuronal assemblies underlying this emergent pathological oscillation. For decades, epilepsy surgery has utilized intracranial EEG recorded over a narrow bandwidth (1–100 Hz) from large (∼1–10 mm diameter), widely spaced (5–10 mm) electrodes (Engel et al., 2007). This practice, however, is largely based upon tradition and the limits of sensor technology when intracranial EEG was first recorded, rather than our knowledge of the human brain. These technological limitations often frustrate epileptologists looking for discrete, functional ‘lesions’ to remove during epilepsy surgery, because seizures arising from the neocortex often appear to start abruptly from large regions of brain (Quesney, 2000). Other applications awaiting better definition of the neurophysiological generators of seizures are seizure prediction (Lehnertz, 2005; Mormann et al., 2007), whose controversial performance may be due in part to the poor temporal and spatial resolution of clinical intracranial EEG, and implantable anti-epileptic devices (Sun et al., 2008), whose efficacy might be improved with better targeting and understanding of seizure generators. To date the emergence of spontaneous focal seizures in humans has not been thoroughly investigated at high temporal sampling rates on sub-millimetre spatial scales.
Wide-bandwidth local field potential recordings using microelectrodes (diameter < 100 μm) in epileptic human hippocampus and neocortex have identified several new classes of electrographic activity localized to sub-millimetre-scale tissue volumes, inaccessible to standard clinical intracranial EEG technology. Pathological high-frequency oscillations have been localized to microdomains (< 1 mm3) in human epileptic hippocampus (Bragin et al., 2002b; Worrell et al., 2008). Penetrating microelectrode arrays embedded directly into human epileptic neocortex reveal microperiodic epileptiform discharges (Schevon et al., 2008) and high-frequency oscillations (Schevon et al., 2009) confined to 200-µm-diameter tissue regions. There is debate regarding the significance of microperiodic epileptiform discharges, however, because they have morphology and temporal behaviour similar to what is reported after cortical injury (Ebersole and Pedley, 2003), and they have not been established as a specific electrophysiological marker for epileptic tissue.
Work from Goldensohn et al. (1975) in the 1960s describes microepileptiform discharges obtained from a glass pipette electrode on the surface of cat cortex treated with a focal injection of penicillin. They demonstrated focal evolving microepileptiform discharges after penicillin injection on single electrodes in an array of electrodes spaced 2 mm apart with no reflection of the discharges on adjacent electrodes. The magnitudes of the recorded potentials were as large as 3 mV and were largest in the superficial cortical layers when depth profiles were measured.
Material and methods
To investigate the spatial and temporal scales underlying the genesis of focal seizures, we obtained prolonged local field potential recordings from brain regions generating spontaneous seizures (ictal onset zone) and brain regions not generating seizures (non-ictal onset zone) in patients with epilepsy, and from control brain of patients without epilepsy undergoing similar electrode implantation for experimental treatment of intractable facial pain (Lima and Fregni, 2008). Data were acquired on a DC capable 320 channel system, sampled at 32 kHz, with a dynamic range of ± 132 mV at 1 µV resolution (Neuralynx, Inc.). Raw data were converted to a lossless compressed format yielding average compression ratios of 10% from 32 bit samples (Brinkmann et al., 2009). Continuous, long-term recordings were obtained from a total of 780 clinical macroelectrodes (1–10 mm2, 10 mm spacing) and 756 microelectrodes (10−3 mm2, 0.5–1 mm spacing) in 14 patients with epilepsy and two patients with intractable facial pain and no history of seizures. Microwire electrodes were incorporated into standard clinical macroelectrodes in a hybrid arrangement (see the online Supplementary Material). Candidate seizure events on clinical macro- and research microelectrodes were identified with an automated seizure detector applied to all the data (12.5 terabytes) using an adaptive signal line-length feature (Gardner et al., 2007), and thresholded for hypersensitive detection (Supplementary data). All candidate seizure events were confirmed or rejected by expert visual review, based on the electrographical features of seizures (Schiller et al., 1998; Lee et al., 2000; Worrell et al., 2004; Bragin et al., 2007) that include (i) paroxysmal change arising from background intracranial EEG activity; (ii) temporal and spectral evolution of the seizure discharge; and (iii) discrete termination of the seizure discharge. In addition, focal periodic or quasi-periodic epileptiform discharges were detected on isolated clinical macro- and research microelectrodes. Even when present on clinical macroelectrodes, this electrographic pattern was not associated with clinical seizure activity, and therefore was not labelled as seizure when occurring on either macro- or microelectrode arrays. We labelled these events as ‘micro periodic epileptiform discharges’ when they occurred on isolated microelectrodes (Schevon et al., 2008). Electrographic seizure-like discharges isolated to single microelectrodes that demonstrated temporal and spectral evolution commonly associated with clinical macroelectrode seizures were labelled ‘microseizures’.
Results
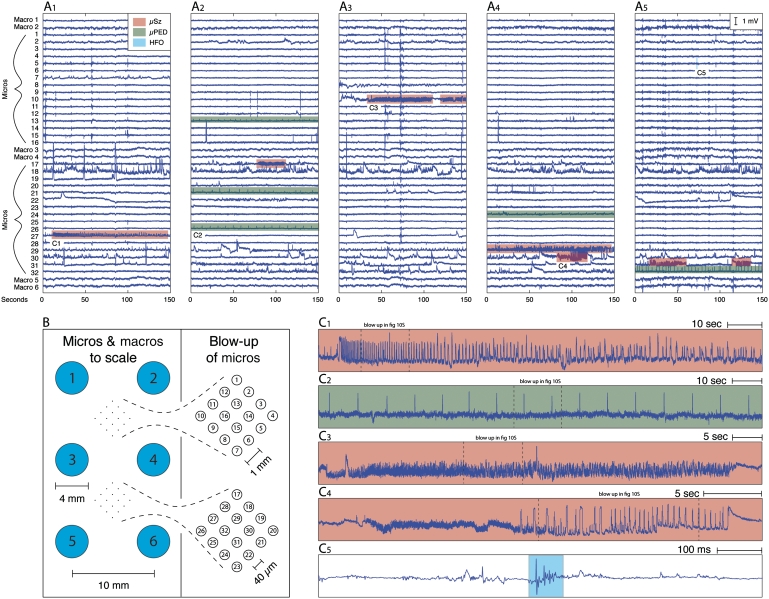
A total of 66 clinical macroelectrode seizures were verified from the 780 clinical macroelectrodes during the 2256 h of intracranial EEG. Seizures were identified from the macroelectrode recordings in all 14 patients with epilepsy, and none were seen in the two control patients. Of the 14 patients with epilepsy, five patients had exclusively neocortical onset seizures, seven patients had exclusively mesial temporal (amygdalohippocampal) onset seizures and two patients had independent neocortical and mesial temporal onset seizures (Supplementary Table 1). Epileptiform spikes and seizures recorded on clinical macroelectrodes were simultaneously observed on adjacent microelectrode arrays in all cases. Frequent interictal epileptiform activity, however, was recorded on isolated microelectrodes and was not detected on neighbouring micro- or macroelectrodes (Fig. 1A and B). Automated seizure detection identified 75 200 candidate events isolated to individual microelectrodes. Subsequent visual review verified 7088 seizure-like events isolated to single microelectrodes. Epileptiform discharges spatially isolated to single microelectrodes (Fig. 1C1–5) included microperiodic epileptiform discharges (Fig. 1C2), high-frequency oscillations (Fig. 1C5) and microseizure discharges (Fig. 1C1,3–4). The microseizure discharges were characterized by a paroxysmal local field oscillation (∼1–500 Hz) and temporal and spectral evolution commonly seen with macroelectrode seizures. Most often these events were stereotyped discharges with monotonically decreasing frequency and increasing local field potential amplitude (Fig. 1C1,4), though other patterns of spectral evolution were also observed (Supplementary Fig. 7). Both microseizures and microperiodic epileptiform discharges were clinically silent. Microseizures were electrophysiologically distinct from the microperiodic epileptiform discharge events, and the microperiodic epileptiform discharges had none of the spectral hallmarks of seizures recorded from clinical macroelectrodes or microseizures.
Figure 1.
Sub-millimetre scale epileptiform activity in human partial epilepsy includes microseizures (µSz), microperiodic epileptiform discharge (µPED) and high-frequency oscillations (HFO). Panels A1–A5 each show representative examples of interictal intracranial EEG recorded (150 s) from a hybrid subdural grid (B) containing clinical macro- and microelectrode arrays in a patient with partial epilepsy. Microseizures (red), microperiodic epileptiform discharges (green) and high-frequency oscillations (blue) are highlighted in panels A1–5, and examples shown on an expanded scale in panels C1–5. The microseizures (C1,3,4), microperiodic epileptiform discharges (C2) and high-frequency oscillations (C5) shown here are isolated to single microelectrodes and not detected on adjacent microelectrodes or macroelectrodes. Additional details and expanded views are in the Supplementary Material.
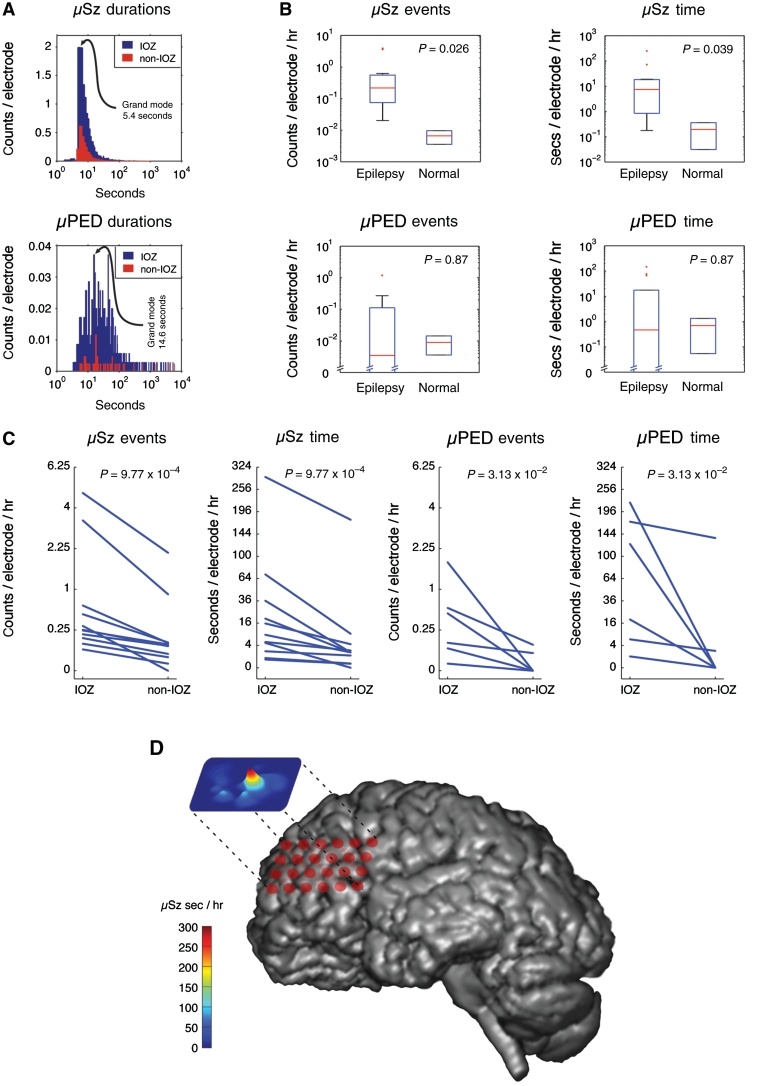
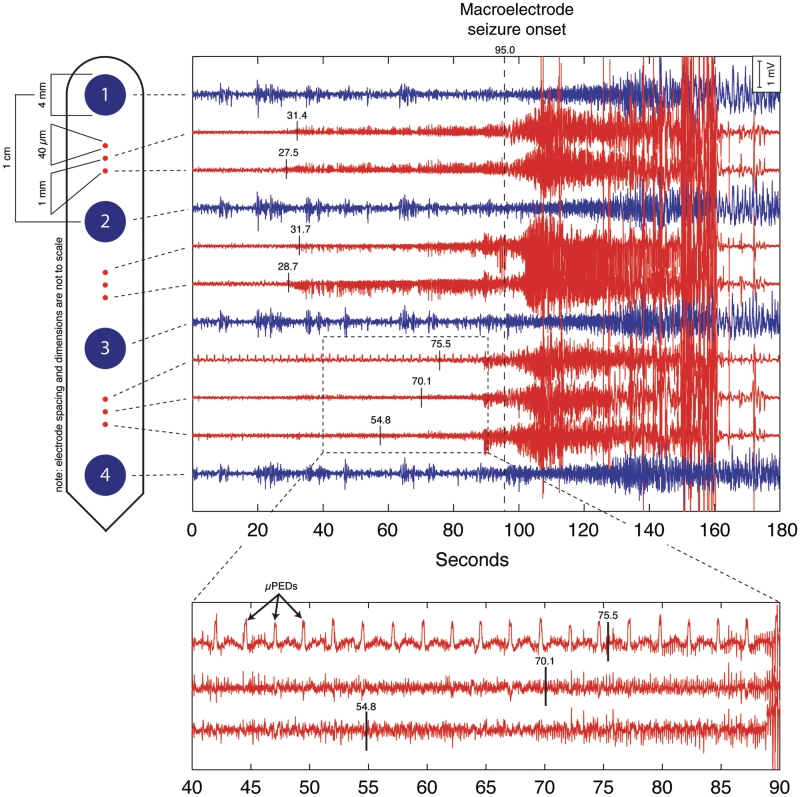
There was significant variability in the number, duration and morphology of epileptiform activity isolated to individual microelectrodes (Figs 1 and 2). The number of microseizures per electrode-hour (P = 0.026) and their average duration (P = 0.039) were increased in patients with epilepsy compared with the two control subjects. No difference, however, was observed in the number or duration of microperiodic epileptiform discharge events when comparing epileptic and control brain across all microelectrodes. Of the 14 patients with partial epilepsy, 11 (78.6%) had microelectrode arrays implanted in both the ictal onset zone [determined by the macroelectrode seizure onset (Litt, 2002)] and the non-ictal onset zone outside the brain regions generating seizures (Fig. 2C and D). In these patients, independent microseizures and microperiodic epileptiform discharges were identified in non-contiguous, localized microdomains. In patients with epilepsy, the number per electrode-hour and duration of microseizures (P = 9.77 × 10−4) and microperiodic epileptiform discharges (P = 3.13 × 10−2) were increased in the ictal onset zone compared with the non-ictal onset zone. Unlike the characteristic pattern of spatial evolution seen with macroelectrode seizures, microseizures and microperiodic epileptiform discharges largely remained spatially stable and localized to isolated microdomains not detected by clinical macroelectrodes or adjacent microelectrodes. In 13 of the 66 macroelectrode seizures (19.7%), microseizures or microperiodic epileptiform discharge activity began prior to and continued into the clinical seizures (Fig. 3 and Supplementary Fig. 6). Macroelectrode seizure onsets appeared simultaneously on several macroelectrodes, while microseizures and microperiodic epileptiform discharges were asynchronous and spatially dispersed across individual, non-adjacent microelectrodes.
Figure 2.
Characterization of microseizures (µSz) and microperiodic epileptiform discharges (µPED). (A) The distribution of microseizures and microperiodic epileptiform discharge event durations in the ictal onset zone (IOZ) compared with the non-ictal onset zone (non-IOZ) brain regions (including control brain). The modal duration of microseizures was 5.4 s compared with 14.6 s for the microperiodic epileptiform discharge events, with rare microperiodic epileptiform discharges extending beyond 1000 s. (B) The microseizure events were more frequent (P = 0.026) and of longer duration (P = 0.039) in patients with epilepsy compared with control subjects without epilepsy. However, microperiodic epileptiform discharge events did not show a significant difference in frequency of occurrence or duration in patients with epilepsy compared with controls. (C) Eleven of the 14 patients with epilepsy had microelectrode arrays implanted into both ictal onset zone and non-ictal onset zone brain regions. In these patients both microseizures and microperiodic epileptiform discharges were more frequent and longer in duration in the ictal onset zone compared with the non-ictal onset zone. (D) Co-registration of volume-rendered MRI and subdural hybrid grid from a representative patient with neocortical epilepsy (Patient 6; Supplementary Table 1). The microseizure event map is projected above the MRI and red corresponds to the regions of high microseizure activity. The region of high microseizure activity co-localized to the ictal onset zone determined by the location of macroelectrode seizures.
Figure 3.
Seizure generation is preceded by a build-up of seizure-like activity on the microelectrodes. Top: hybrid subdural strip containing clinical macroelectrodes (blue) and microelectrodes (red). Top right: transition from non-seizure activity, interictal state, to seizure. The onset of seizure activity recorded by the clinical macroelectrode is at 95 s. The macroelectrode seizure is preceded by microseizure activity beginning at ∼27.5 s. Independent, asynchronous microseizure activity is recorded on microelectrodes well before the seizure becomes apparent on the clinical macroelectrodes. Bottom: expanded time scale of the top figure. The expanded tracing (centred ∼65 s) shows an ongoing microperiodic epileptiform discharge that started 10 min prior to the onset of seizure. Prior to the onset of seizure there is increased microelectrode epileptiform spiking evident on the two lower tracings. (Recording with a common distant scalp reference for microelectrodes and macroelectrodes. Two microelectrodes were not recording and are not displayed.)
Based on macroelectrode intracranial EEG, 11 of the 14 patients with epilepsy were determined to be good candidates for surgical treatment and underwent focal neocortical resection (n = 6) or anterior temporal lobectomy (n = 6) (one patient is counted in both groups). The pathology of resected tissue included cortical dysplasia, oligodendroglioma, astrocytoma, non-specific and subpial gliosis and mesial temporal sclerosis (Supplementary Table 1).
We studied patients with neocortical and mesial temporal partial epilepsy with a range of tissue pathologies (Supplementary Table 1) and control patients without a history of seizures. Note that only primary motor and premotor cortices were sampled from the control patients, whereas the epileptic patients were sampled from other cortical regions as well. Independent of tissue pathology, asynchronous microseizures and microperiodic epileptiform discharges were observed on microelectrodes over wide regions of brain and their spatial density, rate and duration were increased within the ictal onset zone. The normalized ratio of microseizure counts was 2.02 times higher within the ictal onset zone compared to non-ictal onset zone regions. Additionally, microseizures were recorded on a limited number of microelectrodes, with 90% of all microseizure events recorded from 18.8% of microelectrodes and 90% of all time within microseizures (total microseizure duration) isolated to 0.6% of all microelectrodes. These results suggest that human epileptic brain is topographically fractured (i.e. composed of non-contiguous, sparsely distributed microdomains (<1 mm diameter) generating pathological local field potential oscillations) and that increased microseizure and microperiodic epileptiform discharges are electrophysiological signatures of epileptic networks (Fig. 2).
Discussion
The findings described here suggest that clinical seizures begin from sub-millimetre scale epileptiform activity that spreads to neighbouring regions before a sufficient population of synchronously firing cells is recruited to be detectable on the macroelectrodes.
The lack of statistical specificity of microperiodic epileptiform discharges for epileptic brain, and their similarity to activity described after local tissue trauma, suggests that the mechanism of seizure generation involving microperiodic epileptiform discharges may be different than for microseizures, and perhaps responsible for generating post-traumatic seizures.
In previous studies, microelectrode recordings capable of probing epileptic microdomains were limited to isolated regions (<1 cm diameter) of hippocampus or neocortex, and included patients with a limited range of tissue pathologies, particularly mesial temporal sclerosis and non-specific gliosis (Bragin et al., 2002b; Schevon et al., 2008, 2009; Worrell et al., 2008). Because these studies were limited to patients with epilepsy, the specificity of interictal microdomain discharges to epileptic brain could not be explored. Additionally, the spatiotemporal and spectral evolution of microdomain discharges in relation to seizures recorded on clinical macroelectrodes could not be evaluated because sufficient simultaneous recordings were not available. In this study, microseizures were observed in all patients with epilepsy and were increased in the ictal onset zone. This suggests that microseizures are pathological and probably ubiquitous within the ictal onset zone, because the microelectrode arrays dramatically undersample the volume of tissue under an implanted grid. Although the observation of microperiodic epileptiform discharges in control brain could be associated with electrode-related tissue damage, rare microseizures were also observed in control brain, suggesting that potentially pathological microdomain activity can be present in normal brain. It is unlikely that either microseizures or microperiodic epileptiform discharges arise solely from tissue damage, because microseizure and microperiodic epileptiform discharge rate and duration were increased in the ictal onset zone and ∼20% of clinical seizures were preceded by evolving microdomain activity.
In a rat model of epilepsy created by intra-hippocampal kainic acid injection (Bragin et al., 2000), pathological high-frequency oscillations emerged in microdomains (<1 mm3) weeks to months before spontaneous seizures developed. Epileptogenesis was proposed to be initiated by local cellular injury, resulting in small clusters of pathologically interconnected neurons. Bragin et al. (2000) hypothesize that pathologically interconnected neurons generate hypersynchronous discharges that kindle the brain through the creation of new pathological microdomains, and the emergence of an interacting network of pathologically interconnected neuron clusters. The results presented here, from human epileptic brain, are consistent with this hypothesis. The fact that they are independent of tissue pathology suggests that the topographically fractured functional organization may underly the process of epileptogenesis.
The progression of normal brain tissue to epileptic tissue capable of generating spontaneous seizures (epileptogenesis) may reflect a continuum of increasing density or connectivity of pathological microdomains. Similarly, the transition from normal brain activity to seizure (ictogenesis) may involve the interaction and spread of pathological microdomain activity (Fig. 4). In this model of seizure generation, the earliest local field potential oscillations of seizures are multifocal, asynchronous microseizures and microperiodic epileptiform discharges that recruit surrounding microdomains until a critical volume or network of tissue progresses into a large-scale seizure. Our observation of both microseizures and microperiodic epileptiform discharges in normal brain supports the hypothesis that epileptiform activity can occur in non-epileptic tissue but is controlled by homoeostatic mechanisms or is insufficient in spatial or temporal density to initiate a seizure. That focal seizures arise in normal individuals after exposure to conditions such as hyperglycaemia, electrolyte abnormalities and toxic exposures strengthens the hypothesis that an individual's ‘seizure threshold’ may be a function, in part, of the volume of tissue generating microseizures and microperiodic epileptiform discharges.
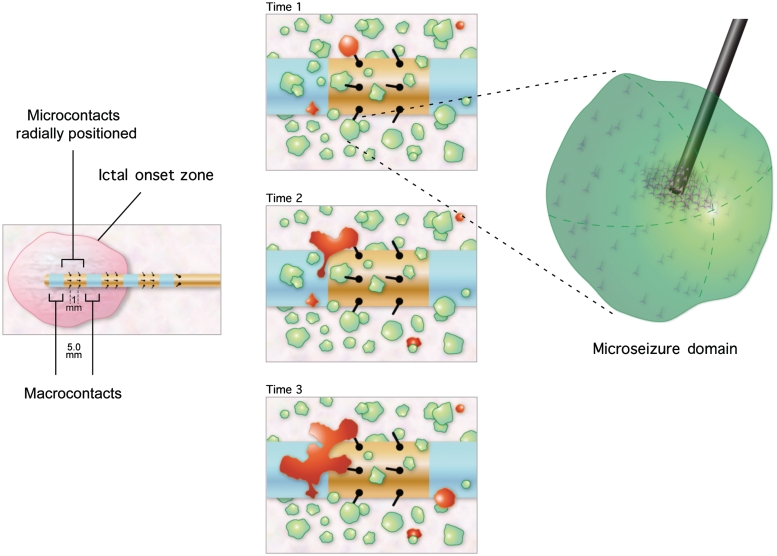
Figure 4.
Hypothetical model of the ictal onset zone composed of sparse, non-contiguous, pathological microdomains that are characterized by the ability to generate microseizures, microperiodic epileptiform discharges and high-frequency oscillations. Left: the macroscale ictal onset zone determined by the region generating seizures detected on clinical macroelectrodes (blue). The arrays of microelectrodes are positioned between macroelectrodes and record multi-neuronal unit activity and local field potential oscillations. Centre: three successive times (Time 1, Time 2, and Time 3) and spatial evolution of sub-millimetre (<1 mm3) microdomains that generate microseizures. The clinical ictal onset zone contains microdomain islands (red) generating microseizures that are initially detected on the microelectrodes, but not the clinical macroelectrodes. As the volume of tissue involved in the microseizure discharge increases (Time 3), the seizures are detected on the clinical macroelectrode. Right: enlarged view of the sub-millimetre domains of pathologically interconnected neurons generating microseizures.
Although these results implicate pathological microdomains in epileptogenesis and ictogenesis, the anatomical and cellular substrate of epileptic microdomains cannot be elucidated from our data. We can suggest that the microseizures and microperiodic epileptiform discharges we recorded from our neocortical microwires are probably generated in superficial cortical layers given their proximity to the pial layer and the density of synaptic currents known to occur there. We cannot speak to the origins of these events from our hybrid depth electrodes that are typically implanted in the three-layered archicortex of the mesial temporal structures.
The extracellular local field potentials recorded by microwires is primarily a manifestation of the co-operative activity of the local neuronal population. Until recently, the local field potential was thought to exclusively reflect the summation of post-synaptic currents because of their relatively slow dynamics. This is the reason that extracellularly recorded action potentials—with the fast Na+ current being the largest contributor—are detected only if the microwire is close to the cell. The amplitude of the extracellular action potential falls off rapidly with distance, and the events are unlikely to constructively sum because of their brief duration. However, it has been recognized that there are additional sources of local field potentials not associated with synaptic currents and they can be significant [reviewed in Buzsaki et al. (2003)]. They include Ca2+-mediated action potentials generated in dendrites (Wong et al., 1979), slow long-lasting calcium-mediated potassium currents, voltage-dependent intrinsic oscillations in neurons (Leung and Yim, 1991) and currents related to glia–neuron interactions (Tian et al., 2005). Understanding the cellular mechanisms underlying the generation of microseizures and microperiodic epileptiform discharges is important and a focus of our current research but is beyond the scope of the work described here.
In support of epileptiform activity inherently originating at the microscale, previous work from our group and others (Schevon et al., 2008; Worrell et al., 2008) has demonstrated in situ epileptiform activity on scales as small as 1 mm3 or less; similar dimensions have been observed in animal models (Bragin et al., 2002a; Supplementary Fig. 6). Recent work in resected human epileptogenic cortex demonstrated runs of epileptiform spikes in 0.5 mm in vitro slices, the approximate width of a human cortical column (Mountcastle, 1978). This spontaneous activity bears morphologic similarity to the microseizures and microperiodic epileptiform discharges described in this work. Furthermore, the in vitro activity was necessarily generated by highly localized neuronal networks, and was dependent on gap junction connections between neurons, suggesting a non-synaptic generator of some forms of epileptiform activity (Roopun et al., 2010). Also, realistic computational models of single cortical columns have been shown to be capable of generating a rich array of physiological and epileptiform discharges (Traub et al., 2005).
A potential concern regarding these phenomena is that, because of their frequent restriction to single microwires, they are a form of artefact. There are several observations that make this unlikely however.
On occasions we see spread to adjacent microwires (Figs 1A4, 3 and Supplementary Fig. 6). Such spread has also been shown by others with more tightly placed microwires e.g. ∼400 µm apart (Schevon et al., 2008).
As can be seen by example in Fig. 1A2, we observe microperiodic epileptiform discharges occurring simultaneously, yet asynchronously, with different periods on separate microwires effectively excluding an exogenous source of this artefact. Also arguing against exogenous artefact sources is the fact that microseizures occur asynchronously on independent microwire channels.
Between events we record ‘morphologically conventional’ EEG on these channels making electrode damage, high impedance and channel-restricted electronic failure quite unlikely. Furthermore, we have recorded from a saline–gelatin solution simultaneously with a patient recording, thus exposing the electrodes and electronics to all the same noises present in the patient recording milieu. No microseizures or microperiodic epileptiform discharges were detected over 4 days of recording in the saline–gelatin, while these events were detected in the patient.
These signals localize the clinically determined ictal onset zone, a finding whose most conservative probability estimate of occurring by chance was <0.03, as shown in Fig. 2.
These phenomena display morphologic and spectral structures that are not seen as common sources of artefact in electrophysiologic recording such as 60 Hz line noise and movement artefact. We do see these types of artefact and they are easily identified visually or algorithmically and excluded from the analysis.
Multiple investigators have hypothesized that the functional cortical column may play a fundamental role in the initiation and propagation of seizures (Ebersole and Levine, 1975; Gabor et al., 1979; Reichenthal and Hocherman, 1979). In this study, we observe microscale electrophysiology whose spatial extent is consistent with the scale of cortical columns. Cortical columns have sufficient recurrent excitatory inter-connections (Ayala et al., 1973) to provide the cortical substrate for pathologically interconnected neuron clusters. Therefore, we hypothesize that relatively sparse pathological cortical columns are the anatomical substrate of focal neocortical epilepsy, ‘the sick column hypothesis’. Although archicortex does not exhibit columnar organization, pathologically interconnected neurons could serve as the ‘sick column’ substrate in these structures.
The observation that focal seizures begin on spatial and temporal scales not probed by current clinical intracranial EEG systems may explain the difficulty in identifying a focal discrete region of seizure onset, the apparent random nature of seizure occurrence and the limited success of first-generation responsive stimulation devices that attempt to detect and abort seizures. Microseizures could provide interictal biomarkers of epileptic tissue, possibly improving the efficacy of epilepsy surgery. They may also illuminate the process of ictogenesis, and thereby open new therapeutic windows for seizure warning and preventive stimulation devices.
Funding
National Institutes of Health R01-NS063039 (to G.A.W.), R01-NS48598 (to B.L.) and NS041811 (to B.L.); Mayo Clinic Discovery Translation Grant; Minnesota Partnership for Biotechnology and Medical Genomics; and CURE Foundation.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We appreciate the technical support provided by Cindy Nelson, Karla Crockett and Steve Goerss.
References
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- Bragin A, Claeys P, Vonck K, Van Roost D, Wilson C, Boon P, et al. Analysis of initial slow waves (ISWs) at the seizure onset in patients with drug resistant temporal lobe epilepsy. Epilepsia. 2007;48:1883–94. doi: 10.1111/j.1528-1167.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel JJ. Local generation of fast ripples in epileptic brain. J Neurosci. 2002a;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel JJ. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–52. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel JJ. Interictal high-frequency oscillations (80-500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002b;52:407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Brinkmann BH, Bower MR, Stengel KA, Worrell GA, Stead M. Large-scale electrophysiology: acquisition, compression, encryption, and storage of big data. J Neurosci Methods. 2009;180:185–92. doi: 10.1016/j.jneumeth.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Traub RD, Pedley TA. The cellular basis of EEG activity. In: Ebersole JS, Pedley TA, editors. Current practice of clinical electroencephalography. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- Ebersole JS, Levine RA. Abnormal neuronal responses during evolution of a penicillin epileptic focus in cat visual cortex. J Neurophysiol. 1975;38:250–6. doi: 10.1152/jn.1975.38.2.250. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Pedley TA. Current practice of clinical electroencephalography. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- Engel J, Pedley TA, Aicardi J, Dichter MA, Moshé S. Epilepsy: a comprehensive textbook. Philadelphia PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Gabor AJ, Scobey RP, Wehrli CJ. Relationship of epileptogenicity to cortical organization. J Neurophysiol. 1979;42:1609–25. doi: 10.1152/jn.1979.42.6.1609. [DOI] [PubMed] [Google Scholar]
- Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–43. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldensohn ES. Initiation and propagation of epileptogenic foci. In: JK Penry and DD Daly, editors. Advances in neurology. New York: Raven Press; 1975. [PubMed] [Google Scholar]
- Goldensohn ES. Structural lesions of the frontal lobe. Manifestations, classification, and prognosis. Adv Neurol. 1992;57:435–47. [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Lehnertz K, Litt B. The first international Collaborative Workshop on seizure prediction: summary and data description. Clin Neurophysiol. 2005;116:493–505. doi: 10.1016/j.clinph.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Leung LW, Yim CY. Intrinsic membrane potential oscillations in hippocampal neurons in vitro. Brain Res. 1991;553:261–74. doi: 10.1016/0006-8993(91)90834-i. [DOI] [PubMed] [Google Scholar]
- Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- Litt B, Lehnertz K. Seizure prediction and the preseizure period. Curr Opin Neurol. 2002;15:173–7. doi: 10.1097/00019052-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–33. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. An organization principle for cerebral function: the unit module and the distributed system. In: H Petsche and JR Hughes, editors. The mindful brain. Cambridge, MA: MIT Press; 1978. [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 (Pt 4):701–22. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Quesney LF. Intracranial EEG investigation in neocortical epilepsy. Adv Neurol. 2000;84:253–74. [PubMed] [Google Scholar]
- Reichenthal E, Hocherman S. A critical epileptic area in the cat's cortex and its relation to the cortical columns. Electroencephalogr Clin Neurophysiol. 1979;47:147–52. doi: 10.1016/0013-4694(79)90216-5. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Simonotto JD, Pierce ML, Jenkins A, Nicholson C, Schofield IS, et al. A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex. Proc Natl Acad Sci USA. 2010;107:338–43. doi: 10.1073/pnas.0912652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Waziri A, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–30. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–59. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39:380–8. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ, Wharen RE. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5:68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–81. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, Lebeau FE, Buhl EH, Garner H, et al. Transient depression of excitatory synapses on interneurons contributes to epileptiform bursts during gamma oscillations in the mouse hippocampal slice. J Neurophysiol. 2005;94:1225–35. doi: 10.1152/jn.00069.2005. [DOI] [PubMed] [Google Scholar]
- Wong RK, Prince DA, Basbaum AI. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci USA. 1979;76:986–90. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.