Abstract
Given that Parkinson's disease broadly affects frontostriatal circuitry, it is not surprising that the disorder is associated with a reduction of working memory. We tested whether this reduction is due to diminished storage capacity or impaired ability to exclude task-irrelevant items. Twenty-one medication-withdrawn patients and 28 age-matched control subjects performed a visuospatial memory task while their electroencephalograms were recorded. The task required them to remember the orientations of red rectangles within the half of the screen that was cued while ignoring all green rectangles. Behavioural and electroencephalogram measures indicated that patients with Parkinson's disease were impaired at filtering out distracters, and that they were able to hold fewer items in memory than control subjects. The results support recent suggestions that the basal ganglia help control access to working memory.
Keywords: Parkinson's disease, visual working memory, event-related potentials, selective attention, dopamine
Introduction
Parkinson's disease is a progressive neurodegenerative disorder characterized by the loss of midbrain dopaminergic neurons and subsequent depletion of dopamine levels in the basal ganglia. Because the basal ganglia have extensive interconnections with the prefrontal cortex (Jellinger, 2001; Lewis et al., 2003), the motor symptoms of Parkinson's disease are often accompanied by cognitive deficits in planning, set shifting, reward learning or working memory capacity (Gabrieli et al., 1996; Zgaljardic et al., 2003; Owen, 2004).
Impairments of working memory have been documented for Parkinson's disease, but the nature of the deficit is ambiguous. We consider a study by Gabrieli and colleagues (1996) as an example: patients with Parkinson's disease were found to have only half the memory capacity of neurologically normal individuals in an ‘operation span’ task. Participants were presented with a series of arithmetic problems that alternated with individual words. At the end of the series, the participants were asked to recall the words in the order that they were given. These kinds of working memory tests have dual-task characteristics in that they require the person to simultaneously retain information (words) while carrying out processing (e.g. mental arithmetic). Thus, it is not clear whether the poor performance of patients with Parkinson's disease on such tasks is due to reduced storage capacity, inability to effectively process information or both (Cowan et al., 2005). Moreover, some studies have failed to document any deficit of working memory span in patients with Parkinson's disease (Cooper et al., 1991; Dalrymple-Alford et al., 1994).
Attentional filtering and storage capacity deficits
One possible cause of poor memory is a reduced ability to filter out irrelevant information. Given that people can hold only about four units of information in working memory (Luck and Vogel, 1997; Cowan, 2001), it is important not to let irrelevant items take up space. This general idea is supported by findings of Vogel et al. (2005a), who used EEG measures to show that effective attentional filtering is needed to ensure that working memory is filled with only relevant information.
In the Vogel et al. (2005a) study, after which the present experiment was modelled, subjects were required to remember the orientations of red rectangles on the cued side of a display. Trials in which there were two red and two blue rectangles (2-red–2-blue) in the attended half of the display were the most theoretically critical. These trials were compared with two other types that lacked distracters. One consisted of four (4-red) and the other of two (2-red), to-be-remembered, red rectangles on the attended side. The critical question was whether retention during the 2-red–2-blue trials would be more similar to the 2-red or the 4-red condition. If the participant can successfully filter out the blue distracters, then he or she needs to hold only two items in memory. If the subject cannot ignore distracters, then he or she has to retain four items.
From the scalp EEG recordings, an event-related potential was extracted that is known to reflect the amount of information held in visual working memory. It is referred to either as contralateral delay activity (CDA) (Vogel and Machizawa, 2004) or as sustained posterior contralateral negativity (Jolicoeur et al., 2008). These terms convey the fact that it is a surface-negative brain wave observed over the back part of the head, on the side opposite to the attended stimulus, and that it is sustained during the delay between the memory array and test array. Its cognitive correlates are quite specific: CDA amplitude is proportional to the number of items held in visual working memory (Vogel and Machizawa, 2004).
The young adult participants were split into two groups based on a behavioural estimate of working memory capacity. For high-capacity participants, CDAs were equivalent in the 2-red and the 2-red–2-blue condition, which implies that they were successful at keeping irrelevant distracters from their memory. For people with low capacity, however, CDAs were significantly larger when two red rectangles were presented with distracters than without. In fact, amplitudes in the 2-red–2-blue condition were comparable to those observed in the 4-red condition. The authors suggested that attentional filtering ability critically determines a person's working memory capacity. Individuals who perform well on memory span tests excel at filtering out irrelevant information; they may not have large memory spans, per se (see also Awh and Vogel, 2008).
The possibility that patients with basal ganglia disease might be especially vulnerable to filtering deficits is supported by a recent functional MRI study also involving healthy young adults by McNab and Klingberg (2008; see also Praamstra et al., 1998; Praamstra and Plat, 2001; Verleger et al., 2010). The main difference in this study, to that of Vogel et al. (2005a), was that on trials in which the items were not all of the same colour (analogous to the 2-red–2-blue condition) a precue indicated whether the non-standard items should be attended or ignored. Greater activation in the left and right-middle frontal gyri and the left basal ganglia (especially the globus pallidus) were observed when participants were cued to ignore the deviant items. This preparatory activity was more robust in participants with larger working memory capacity. Moreover, activity of the globus pallidus in response to the ‘ignore’ cue was inversely correlated with subsequent parietal lobe activation, which is known to be associated with retention of the memory array (Todd and Marois, 2004). By implication, the left globus pallidus is involved in filtering out distracters so that they do not usurp space in memory. Congruent with these results, several neuroimaging studies have shown that the basal ganglia are engaged during working memory tasks (Skeel et al., 2001; Lewis et al., 2004; Cools et al., 2008).
Independent of any filtering problems, it is possible that patients’ poor performance on working memory span tasks might be due to a reduction of storage capacity. If so, our CDA measures could detect such a deficit. As noted above, CDA varies in amplitude according to the number of items held in visual working memory, and its maximum amplitude is at a scalp site overlying posterior parietal cortex (Vogel and Machizawa, 2004). Recent evidence indicates that the maintenance of information in visual white matter occurs not in the frontal lobes but rather in the parieto-occipital region. For example, Postle et al. (2006) used transcranial magnetic pulse stimulation to show that the posterior parietal lobe is vulnerable to disruption during both retention and manipulation of items in working memory, whereas dorsolateral prefrontal cortex is sensitive only during the manipulation of information. The location of short-term storage of visual information within parietal cortex was identified more specifically in a functional MRI study by Todd and Marois (2004). These authors showed that activation within the intraparietal sulcus linearly increases with memory load, reaching an asymptote at about four objects.
Whether patients with Parkinson's disease have impairments in posterior parietal functioning that might lead to reduced storage capacity is unclear. Diffusion tensor imaging of white matter tracts does reveal a loss within the left parietal lobe of patients with Parkinson's disease (Matsui et al., 2007). However, other studies have found preserved parietal lobe function, at least in the context of tasks involving visuospatial orienting or sequential finger movements (Bennett et al., 1995; Hsieh et al., 1996; Samuel et al., 1997).
Rationale and predictions
In the present study, we examined whether patients’ poor performance on working memory span tasks is due to a reduced storage capacity per se, attentional filtering deficits or both. Medication-withdrawn patients with Parkinson's disease and age-matched control subjects were asked to remember the orientation of red rectangles on the cued side of a computer display while ignoring all green distracters. The initial display consisted of either two red, two red and two green, or four red rectangles on each side of the screen. After a short retention interval, the array was presented again. Participants then judged whether the orientation of any of the red rectangles on the attended side had changed slightly.
The number of relevant items held in and retrieved from working memory was estimated by a behavioural measure, K scores, defined as K = N*(H − FA), where N is the relevant set size, H is the hit rate and FA is the false alarm rate (Cowan, 2001). H reflects the proportion of changes between arrays correctly detected and FA reflects the proportion of unchanged arrays incorrectly judged to have changed. The formula arises through a process in which the participant knows the answer if the relevant item or items are in working memory and otherwise guesses whether there has been a change. This measure was more appropriate for our goals than reaction time, percentage correct, sensitivity (d') or other more familiar dependent variables. K scores have become standard in the field because they specifically estimate the number of relevant items held in working memory, independent of array size, while correcting for guessing and response bias. When array size is manipulated across a range of values (e.g. 1–7), the asymptotic value of K estimates the participant's memory capacity (Cowan, 2001; Cowan et al., 2005).
Because K scores estimate the amount of information retained in memory, they can be directly compared with our electrophysiological index of retention, CDA (Vogel et al., 2005a). These two measures provide distinct perspectives on the processes and representations of interest; whereas CDA amplitudes reflect the number of items stored in working memory irrespective of their task-relevance, K scores index the number of items stored in and then retrieved from working memory that were in fact task relevant.
If patients with Parkinson's disease have impaired attentional filtering, they would be expected to exhibit lower K scores and higher CDA amplitudes when distracters are present than when they are absent. By contrast, age-matched controls who have good memories should exhibit little difference in CDA amplitudes and K scores as a function of the presence versus absence of distracters, similar to the high-capacity young adults studied by Vogel and colleagues (2005a). If patients have reduced storage capacity per se, but no particular deficit in filtering, then the presence of the distracters should not matter for them any more than it does for controls. However, K scores and CDA amplitudes should be generally reduced, especially in the demanding 4-red condition.
Material and methods
Participants
Twenty-one patients with idiopathic Parkinson's disease and 28 age- and education-matched control subjects comprised the final sample (demographics are shown in Table 1). All participants reported having normal colour vision and normal or corrected-to-normal acuity. None exhibited evidence of dementia as assessed by the Mini-Mental State Examination [M = 28.5 for patients and M = 29.2 for controls (Folstein et al., 1975)]. Depression was evaluated by means of the Geriatric Depression Scale–short form (Sheikh and Yesavage, 1986). All controls and all but two patients scored under 10 on the 15-point scale (overall M = 2.3 for patients and M = 1.3 for controls). The two highest-scoring patients were above 10, the criterion for depression (Geriatric Depression Scale = 11 and 12). Five patients, including the two who scored highest on the Geriatric Depression Scale, as well as two of the control subjects, were taking duloxetine as an antidepressant at the time of the study.
Table 1.
Demographics and memory variables
| Age (years) | Gender |
Years of education | Hoehn and Yahr scale | Years of disease | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Male | Female | Mean (SD) | Mean (SD) | Mean (SD) | |
| Patients (n = 21) | 66.71 (9.83) | 13 | 8 | 14.29 (2.99) | 1.98 (0.58) | 6.7 (4.2) |
| Controls (n = 28) | 68.57 (6.77) | 12 | 16 | 14.59 (2.9) | – | – |
| Hit and false alarm rates |
||||||
|---|---|---|---|---|---|---|
| 2-red |
2-red-2-green |
4-red |
||||
| Hit | False alarm | Hit | False alarm | Hit | False alarm | |
| Patients (n = 21) | 0.84 | 0.25 | 0.79 | 0.29 | 0.62 | 0.39 |
| Controls (n = 28) | 0.93 | 0.24 | 0.91 | 0.27 | 0.73 | 0.44 |
| 2-red | 2-red-2-green | 4-red | Unnecessary storage | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| K scores | ||||
| Patients (n = 21) | 1.41 (0.47) | 1.26 (0.51) | 1.42 (0.71) | 0.16 (0.1) |
| Controls (n = 28) | 1.71 (0.22) | 1.63 (0.25) | 1.82 (0.59) | 0.08 (0.08) |
| CDA amplitudes | ||||
| Patients (n = 21) | −0.58 (0.43) | −0.82 (0.49) | −0.77 (0.62) | −0.25 (0.36) |
| Controls (n = 28) | −1.00 (0.37) | −1.12 (0.49) | −1.22 (0.51) | −0.13 (0.27) |
Descriptive data for participants' demographics (age, gender, years of education, patients' Hoehn and Yahr scale and years of disease) and memory performance indexed by hit and false alarm rates, K scores and CDA amplitudes across three trial types (2-red, 2-red-2-green and 4-red conditions).
Patients were free from other neurological disorders with three exceptions: the first had an old lacunar infarct in the left thalamus and another in the cerebellar vermis. The second had a history of epilepsy. At the time of his participation he was taking antiepileptic medication. The third had mild atrophy and diffuse atherosclerotic disease, but no well-defined abnormality was said to be visible on T1-weighted scans. The general pattern of results was essentially the same with or without these three patients; therefore, their data have been retained. The same was true for the seven participants who were taking antidepressants.
Sixteen patients were receiving the dopamine precursor levodopa as treatment. One patient was taking pramipexole (dopamine agonist) in addition to levodopa. The remaining five patients were receiving either azilect (a monoamine oxidase inhibitor) alone or in conjunction with trihexyphenidyl (an anticholinergic agent) or with pramipexole. In the morning of the experiment, patients skipped their initial dose of antiparkinsonian medication. The mean withdrawal period of 14 h (at least 11 h) would not be enough to achieve complete clearance. Rather, it was intended to enhance differences between experimental groups while minimizing the burden imposed on patients.
The patient's neurologist was contacted to obtain approval for this brief withdrawal as well as to confirm the diagnosis. The severity of the disease was reassessed prior to the start of the experiment using the Hoehn and Yahr scale (1967). Scores averaged 1.98 on the 5-point scale, with a range of 1 (mild unilateral tremor) to 3 (apparent balance problems). This indicates a mild to moderate stage in the progression of the disease (years of disease, M = 6.7 and SD = 4.2).
Control subjects reported neither a history of neurological problems nor any significant current psychiatric disorder. All participants gave their informed consent according to procedures approved by the ethics board at the University of Missouri–Columbia. Subjects were paid $15 per hour for their participation.
Stimuli and procedures
Stimulus arrays were presented within two 4 × 7.3° rectangular regions that were centred 3° to the left and right of a central fixation cross on a dark background (8.2 cd/m2), and were viewed at a distance of ∼70 cm. Arrays consisted of two or four coloured rectangles in each hemifield. Item positions were randomized across trials. Rectangles in red subtended 0.65 × 1.15° of visual angle and rectangles in green subtended 0.32 × 1.15°, with orientations selected randomly from a set of four possible values (vertical, horizontal, left-tilting 45° and right-tilting 45°).
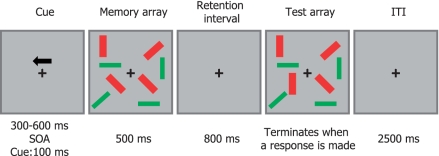
Each trial began with a 100 ms, arrow-shaped cue presented above the fixation cross (Fig. 1). This cue was followed at a 300 to 600 ms onset asynchrony by a 500 ms long memory array, then an 800 ms blank period with fixation cross and finally, the test array. The 800 ms blank period was intended to provide sufficient time for development of the CDA while extending beyond the limits of iconic memory (∼300–500 ms; Lu et al., 2005) and lateralized transients such as the visuospatial orienting component, N2pc (Luck and Hillyard, 1994). The test array ended as soon as the subject pressed a response key. Following a 2.5 s interval, the next trial commenced, starting with the arrow cue.
Figure 1.
Example of a 2-red–2-green condition in which the left hemifield was task relevant. SOA = stimulus onset asynchrony; ITI = inter-trial interval.
There were three different types of memory arrays: on 2-red–2-green trials, there were two relevant red rectangles interspersed with two green distracters on each side of the display. On 2-red trials, there were two red rectangles within each hemifield. Finally, on 4-red trials, four relevant red items were shown on each side of the screen. The three trial types were presented in random order in each block. On half of the trials, the memory and test arrays were identical, while on the other half the tilt of a single red rectangle within the to-be-remembered hemifield was altered for the test array. Participants responded by pressing one of two mouse keys to indicate whether such a change occurred. Participants used their preferred hand, which, in all cases, was the right hand. Accuracy was emphasized over speed, and participants were allowed to correct their response before the next trial began.
Participants were tested in a single session comprising two practice and 10 experimental blocks of 80 trials. All participants took part in the study in the morning beginning at 8:00 or 9:00 a.m. The experiment took about 1.5 h, and the whole session, including the training blocks and electrode attachment, lasted for about 4 h. Each trial block lasted for 6 min, including a 20 s break at the halfway point. Between blocks, participants were allowed to take as long a break as they wished. Subjects performed 200 trials of the 2-red, 200 of the 4-red and 400 of the 2-red–2-green variety.
Psychophysiological recordings
The EEGs were recorded using Ag/AgCl electrodes embedded in an elastic cap (Electrocap International, Eaton, OH) and filled with Grass electrode gel (Astro-Grass Instrument Co., Quincy, MA). Measurements were obtained at frontal, central, parietal, temporal and occipital electrode sites (F3, Fz, F4, C3, C4, P3, P4, T5, T6, O1 and O2). Scalp derivations employed a left-mastoid reference during acquisition. Bipolar recordings of horizontal eye movements (horizontal electro-oculograms) were obtained with electrodes placed ∼2 cm lateral to the outer canthus of each eye. Bipolar recordings of the vertical electro-oculograms were obtained with electrodes positioned above the right eyebrow and below the lower orbital rim to detect blinks and vertical eye movements. The EEG and electro-oculograms were amplified and filtered by a Grass model 12 amplifier with a band pass of 0.01–30 Hz. To reduce artefacts due to alpha waves (8–12 Hz) and tremor (4–8 Hz), the EEG data were low-pass filtered off-line using a 5 Hz cut-off. Analogue-to-digital conversion was performed at a rate of 500 Hz via a PC-compatible computer. Acquisition was carried out with Neuroscan software; preprocessing and waveform measurement were done with EEG lab (version 5.02). Horizontal and vertical electro-oculogram recordings were supplemented with infrared eye tracking for all elderly controls and the first 17 patients (Applied Science Laboratories Eye-Tracker, Model 504, Bedford, MA, USA).
Gaze control
Controlling the direction of the gaze was important in this experiment both to avoid contamination of lateralized event-related potentials and to ensure that the relevant and irrelevant sides of the memory display were encoded in separate hemispheres. The problem with encoding is that if participants moved their eyes after arrow onset and looked at, for example, the middle of the cued half of the display, then the CDA would not reflect the difference between the relevant and irrelevant halves of the bilateral display. Once encoding is completed, eye movements are of less concern because CDA still reflects lateralized representations within the original, recipient hemispheres (Eimer and Kiss, 2010).
The problem with contamination is that the back of the eyeballs has a negative charge. Consequently, if participants shift their gaze towards the cued half of the display, this negativity would propagate to contralateral scalp sites and would mimic the CDA. Although volume-conducted ocular potentials are extremely small at the back of the head (Mangun and Hillyard, 1991), they would compromise our ability to infer that negativity contralateral to the cued half of the display reflects working memory.
To minimize gaze shifts, two training blocks of 40 trials each were conducted prior to the main experiment. In these blocks, participants were given verbal feedback whenever loss of fixation (>30 μV, ∼2°) occurred during the arrow, memory array or retention interval. The data from subjects who were unable to adequately control their gaze in the main experimental blocks (fixation loss on >70% of trials) were rejected. For the other subjects, trials that were contaminated by horizontal gaze shifts (>20 μV) during the arrow cue or memory array were excluded from analysis. Supplementary analyses using a more conservative approach (strict 20 μV criterion during cue, memory array, as well as retention interval) yielded similar results.
To test the effectiveness of these training and data-cleansing procedures, the horizontal electro-oculogram data were signal averaged separately for left- and right-pointing cues. The Mean amplitudes during the interval used for CDA measurements averaged well below 3 µV for all control subjects (M = 0.19 μV, SD = 0.48 for attend left, and M = −0.34 μV, SD = 0.64 for attend right). The corresponding values for 20 of the patients were also well below 3 µV (M = 0.37 μV, SD = 0.65 for attend left, and M = −0.82 μV, SD = 0.58 for attend right). One patient had higher but acceptable values (3.77 µV for attend left and −4.70 µV for attend right). His data were retained in order to keep as many participants and trials as possible but the statistical outcome was the same with or without this patient.
Data analysis
The final sample comprised 21 patients and 28 neurologically normal participants; however, data from seven additional subjects were excluded from the analyses. Two patients and one control subject were rejected because they made horizontal eye movements on more than 70% of trials. Data were rejected from 2 patients and one control subject because their task performance was at chance level (∼50%). Finally, one control subject was eliminated due to problems with the EEG recording equipment.
Behavioural data analysis
As discussed earlier, our primary behavioural measure was the K score, which is derived from the hit rate (proportion of correct responses when a change was present) and false alarm rate (proportion of incorrect responses on no-change trials): K = N*(H − FA), where N is the number of relevant, to-be-stored items, H is the hit rate and FA is the false alarm rate. We report results using K scores based on the formula suggested by Cowan (2001), but the pattern of results regarding group differences was similar using either percentage of correct responses or K scores based on Pashler's (1988) formula.
When the number of relevant items is varied across a range of values (e.g. 1–7), healthy young adults exhibit an asymptotic value for K at about four items (Cowan, 2001). On the assumption that remembering the orientations of four simultaneously presented rectangles would be slightly beyond the ability of the majority of older adults, we used the K score in the 4-red condition to estimate working memory capacity for each of our participants.
Psychophysiological data analysis
Trials with both correct and incorrect responses were included (Vogel et al., 2005b) but analyses including only correct responses yielded generally similar results. Given that CDA amplitudes reflect how many items are held in working memory irrespective of their task relevance, the amplitudes from the incorrect trials should also be sensitive to the number of items kept in memory. It is important to retain incorrect trials, as participants may err because they hold irrelevant items in their working memory rather than relevant ones.
CDA at 600–1200 ms following memory array onset was measured at posterior parietal (P3, P4), posterior temporal (T5, T6) and occipital (O1, O2) electrode sites relative to a 700 ms pre-memory array baseline. The 600 to 1200 ms time window following onset of the memory array was selected a priori to assess the portion of the retention interval that is most specific to visual working memory. As noted above, this window was intended to avoid overlap with encoding, iconic memory and target onset transients, as well as retrieval processes during perception of the test array. Waveforms recorded at scalp sites on the same side as the attended array are subtracted from analogous waveforms on the opposite side to calculate CDAs (Vogel and Machizawa, 2004).
Behavioural and psychophysiological data were analysed using repeated measures ANOVAs, with group (Patient, Control) as a between-subject factor and trial type as the within-subject factor (2-red, 2-red–2-green, 4-red) along with the pairwise comparisons among the three trial types. Additional analyses were performed in which participants were categorized dichotomously according to their estimated memory capacity (high and low K scores, relative to an absolute cut-off). This planned analysis was motivated by the finding of Vogel and colleagues (2005a, discussed above) that healthy young adults with low but not high capacity exhibit impaired filtration of irrelevant information. An absolute cut-off was employed rather than a median split to allow comparison of patients and controls with similar mnemonic abilities.
Along with the repeated measures ANOVAs and the pairwise comparisons, intercorrelations among the main behavioural and psychophysiological measures are reported. All P-values vulnerable to sphericity violations were adjusted in accordance with the Greenhouse–Geisser epsilon value. An alpha level of 0.05 was adopted as the critical value, but marginally significant effects are reported when they are judged likely to be of interest to the reader.
Results
Behavioural data
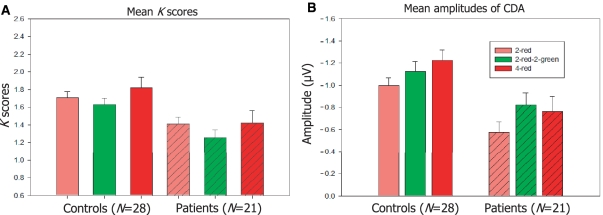
In the 2 × 3 factorial analysis of K scores there were significant main effects for both group [Patient, Control; F(1, 47) = 8.1, P = 0.007] and trial type [2-red, 2-red–2-green, 4-red; F(2, 94) = 7.0, P = 0.01], but the interaction was not significant [F(2, 94) = 0.6, P = 0.464]. Planned comparisons tested group differences between the theoretically critical 2-red–2-green and 2-red trials. Consistent with the assumption that patients with Parkinson's disease are more vulnerable to distraction, the interaction of group and trial type (2-red, 2-red–2-green) was significant [F(1, 47) = 7.7, P = 0.008]. As shown in Fig. 2, K scores in the 2-red–2-green condition were in fact lower than those in the 2-red condition for controls as well as patients (Fs > 30.5, Ps < 0.001), indicating that both groups had some difficulty ignoring distracters. K scores for the 2-red–2 green condition were also lower than those for the 4-red condition for both controls and patients [F(1, 27) = 6.1, P = 0.02 for controls and F(1, 20) = 4.4, P = 0.049 for patients] whereas the difference between 2-red and 4-red conditions failed to be significant for either group (Fs < 1.8, Ps > 0.201).
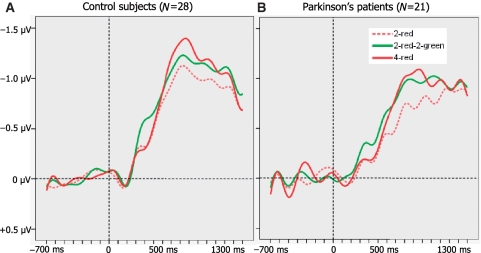
Figure 2.
(A) Mean K scores of control subjects and patients with Parkinson's disease (n = 28 and 21, respectively) as a function of trial type: K = N*(H−FA), where N is the relevant set size, H is the hit rate and FA is the false alarm rate. (B) Mean amplitudes of CDA at 600–1200 ms after memory array onset as a function of trial type for controls and patients. Error bars represent standard errors of the mean.
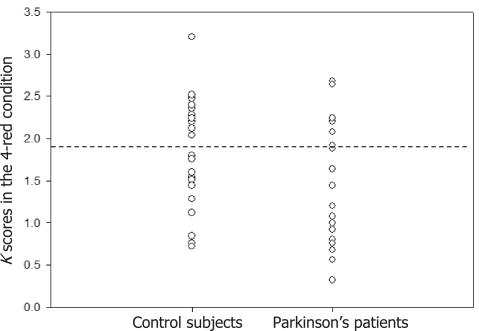
In the analyses that incorporated a breakdown by memory capacity, patients and controls were assigned to high and low subgroups based on an absolute cut-off (K = 1.92 items). The absolute cut-off was determined by looking for the natural discontinuity (Fig. 3) that was closest to the cut-off found to be meaningful by Vogel and colleagues in their study of healthy young adults (2005a). The proportions of patients and controls in the high-capacity category were 33% and 43%, respectively.
Figure 3.
K scores (number of relevant items held in and retrieved from working memory) in the 4-red condition for control subjects and patients with Parkinson's disease, respectively.
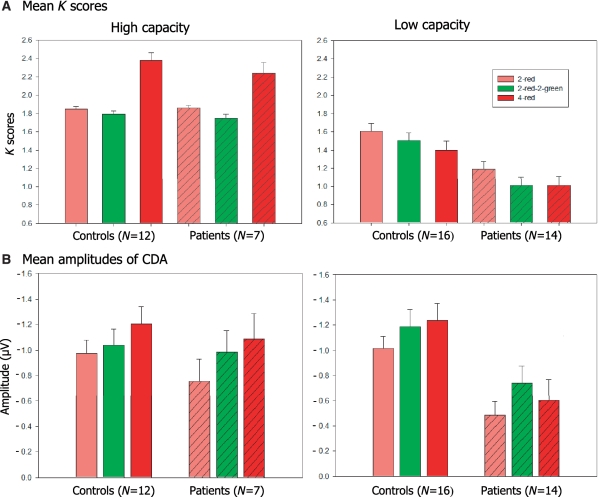
In the 2 × 3 analysis (Group, Trial Type) of patients and controls with high capacity, there was a significant effect for trial type [F(2, 34) = 55.8, P < 0.001; Fig. 4, upper-left panel] but not for Group [F(1, 17) = 0.07, P = 0.403]. Pairwise comparisons revealed that K scores for both controls and patients were lower on 2-red and 2-red–2-green trials than on 4-red trials (Fs > 12.1, Ps < 0.013). The critical comparisons between the 2-red–2-green and the 2-red trials also showed reliable differences for these subgroups of patients and controls [F(1, 11) = 5.9, P = 0.034 for controls and F(1, 6) = 37.6, P = 0.001 for patients]. Although the effect of distracters (2-red–2-green < 2-red; Fig. 4) was numerically small, both controls and patients with high capacity seemed to experience some difficulty in filtering out irrelevant information. However, the corresponding Group × Trial Type interaction did not achieve significance, as it had for the full sample of 49 participants (noted above).
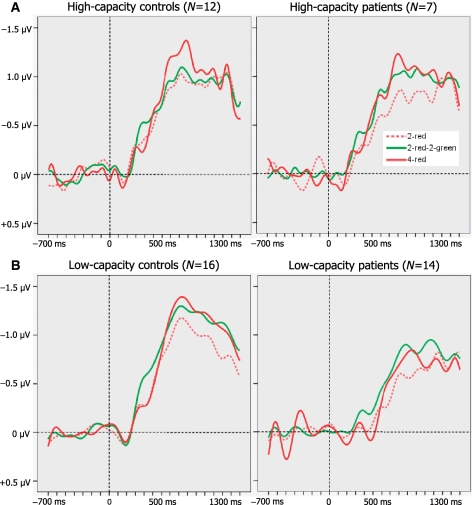
Figure 4.
(A) Mean K scores of high- and low-capacity controls and patients as a function of trial type: K = N*(H − FA), where N is the relevant set size, H is the hit rate and FA is the false alarm rate. (B) Mean amplitudes of CDA at 600–1200 ms after memory array onset as a function of trial type, for participants with estimated high and low overall storage capacity. Error bars represent standard errors of the mean.
In the 2 × 3 analysis of low-capacity participants, there were significant main effects of trial type [F(2, 56) = 14.1, P < 0.001] and group [F(1, 28) = 12.3, P = 0.002; Fig. 4, upper right panel]. Pairwise comparisons revealed that K scores for the 2-red–2-green condition were lower than those for the 2-red condition (Fs > 30.3, Ps < 0.001). The theoretically critical interaction between group and 2-red versus 2-red–2-green trials was significant [F(1, 28) = 4.8, P = 0.037]. This indicates that the disruptive effect of distracters in the 2-red–2-green condition was worse in patients.
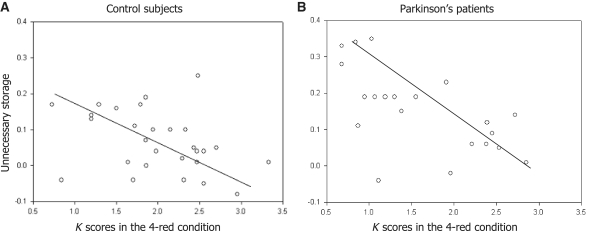
A categorical breakdown of participants by capacity was necessary to allow performance data to be directly compared with group-averaged CDA waveforms. However, the underlying relationship between capacity and impaired filtering was apparently not dichotomous. In the scatter plots shown in Fig. 5, the interfering effect of distracters is portrayed on the Y-axes as ‘unnecessary storage’, defined as the difference in K scores between 2-red and 2-red–2-green conditions. For the patients (n = 21), this quantity was negatively correlated with memory capacity as estimated by K scores in the 4-red condition (Pearson's correlation coefficient, r = −0.050, P = 0.023; Table 2). This association supports Vogel and colleagues’ (2005a) conclusion that apparent reductions in capacity might actually be due to usurpation of available space by irrelevant information. Our neurologically normal control subjects exhibited a similar relationship (r = −0.35, P = 0.07, n = 28; Table 2).
Figure 5.
Correlation between estimated capacity of visual working memory (K score in the 4-red condition) and unnecessary storage (K score difference: 2-red minus 2-red-2-green) for control subjects (A) and patients with Parkinson's disease (B).
Table 2.
Correlation matrix
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. K score for 2-red | – | |||||||
| 2. Unnecessary storage: K | −0.27 | – | ||||||
| 3. K score for 4-red | 0.84** | −0.50* | – | |||||
| 4. CDA for 2-red | 0.56** | 0.38 | 0.49* | – | ||||
| 5. Unnecessary storage: CDA | 0.03 | 0.14 | 0.04 | 0.19 | – | |||
| 6. CDA for 4-red | 0.50* | 0.32 | 0.47* | 0.60** | 0.37 | – | ||
| 7. Age | −0.64** | 0.31 | −0.69** | 0.25 | 0.23 | −0.47* | – | |
| 8. Hoehn and Yahr scale | −0.41 | 0.46* | −0.53* | 0.28 | 0.23 | 0.14 | 0.58** | – |
| 9. Years of disease | −0.18 | −0.20 | −0.19 | 0.10 | 0.16 | 0.06 | 0.13 | 0.31 |
| Controls | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| 1. K score for 2-red | – | |||||||
| 2. Unnecessary storage: K | −0.23 | – | ||||||
| 3. K score for 4-red | 0.82** | −0.35 | – | |||||
| 4. CDA for 2-red | 0.02 | 0.20 | 0.04 | – | ||||
| 5. Unnecessary storage: CDA | 0.31 | 0.05 | 0.15 | 0.24 | – | |||
| 6. CDA for 4-red | 0.03 | 0.07 | 0.06 | 0.79** | 0.30 | – | ||
| 7. Age | −0.38* | 0.24 | −0.41* | 0.20 | 0.29 | 0.22 | – |
Correlations between K scores (relevant items held in working memory) at 2-red and 4-red conditions, CDA amplitudes, age and stage of disease factors for patients with Parkinson's disease and Control subjects.
Unnecessary storage: K = the difference in K scores between 2-red and 2-red–2-green condition; Unnecessary storage: CDA = the difference in CDA amplitude between those two conditions. The negative signs for CDA amplitudes have been dropped so that larger values indicate more items stored in memory.
*P < 0.05, **P < 0.01.
In addition to problems with filtering out distracters, patients with Parkinson's disease might also have less storage space to start with. This possibility is supported by the finding that patients’ K scores were lower than those of controls for all trial types, even those without explicit distracters [in the analysis of all 49 participants, Fs(1, 47) > 8.9, Ps < 0.004 for 2-red and 2-red–2-green; F(1, 47) = 4.6, P = 0.037 for 4-red; Fig. 2, left panel]. In the separate analyses of participants with high and low capacity, there was a main effect of group (patient versus control) for the low-capacity subgroups, as noted earlier (P = 0.002), which was true for all three trial types [Fs(1, 28) > 7.4, Ps < 0.011].
Psychophysiological data
The pattern of results for retention-interval CDAs supported inferences drawn from behavioural data (Fig. 2 right panel and Fig. 6). Although some theoretically critical effects failed to reach significance, these electrophysiological data provide converging evidence concerning group differences and help determine whether behavioural effects are due to maintenance or retrieval processes.
Figure 6.
Grand averaged CDA waveforms time-locked to the onset of the memory array averaged across the posterior parietal, posterior temporal and occipital electrode sites for controls (A) and patients with Parkinson's disease (B) as a function of trial type. Negative voltage is plotted upwards.
In the 2 × 3 overall analysis there were significant main effects of trial type [F(2, 94) = 9.7, P < 0.001] and group [F(1, 47) = 9.6, P = 0.003], but no interaction [F(2, 94) = 1.3, P = 0.287]. Pairwise comparisons showed that CDA was larger in the 4-red than in the 2-red condition for controls [F(1, 27) = 14.7, P = 0.001; Figs 2 and 6], implying that these neurologically normal individuals were able to hold more items in memory when they were presented with a larger array size. Patients had smaller CDAs than controls for each of the three types of trials [Fs(1, 47) > 4.5, Ps < 0.039]. Amplitudes in the 2-red–2-green condition were significantly higher than in the 2-red condition for both healthy participants and patients with Parkinson's disease [F(1, 27) = 6.2, P = 0.019 for controls; F(1, 20) = 10.1, P = 0.005 for patients], implying that both groups experienced some difficulty in ignoring the green bars. Although the averaged waveforms shown in Figs 2 and 6 would seem to suggest that distracter effects were more severe in the patient group, this was not reflected in a reliable interaction of Group × Trial Type [2-red, 2-red–2-green; F(1, 47) = 1.9, P = 0.177].
In the analysis of high-capacity participants there was a significant main effect of trial type [F(2, 34) = 5.5, P = 0.013] but not of group [F(1, 17) = 0.5, P = 0.491]. For control subjects, pairwise comparisons showed that amplitudes in the 2-red–2-green condition were lower than in the 4-red condition [F(1, 11) = 8.1, P = 0.016] but comparable to the 2-red condition, suggesting good exclusion of distracters. As would be expected for an index of working memory, the size of the CDA was greater in the 4-red than in the 2-red condition [F(1, 11) = 8.9, P = 0.013]. For the patients with high capacity, CDA waveforms for the 2-red–2-green condition appeared to be more similar to the 4-red condition than to the 2-red condition, suggesting poor filtration of irrelevant information. However, neither this difference nor its interaction with group approached significance.
For the low-capacity subgroups, there were significant main effects of trial type [F(2, 56) = 5.8, P = 0.008] and group [F(1, 28) = 10.1, P = 0.004], but the interaction was not significant [F(2, 56) = 1, P = 0.365]. Pairwise comparisons showed that for both subgroups, CDA amplitudes in the 2-red–2-green condition were significantly higher than in the 2-red condition [F(1, 15) = 5.9, P = 0.028 for controls; F(1, 13) = 12.6, P = 0.004 for patients]. The critical interaction between group and 2-red versus 2-red–2-green was not significant [F(1, 28) = 0.7, P = 0.418]. Pairwise comparisons showed that the pattern of larger amplitudes in controls than patients was reliable across the three trial types [Fs(1, 28) > 5.4, Ps < 0.027], consistent with a difference in overall memory capacity. The CDA amplitudes were larger in the 4-red than in the 2-red condition for controls [F(1, 15) = 6.5, P = 0.022], but not for patients [F(1, 13) = 0.1, P = 0.336].
Relation to demographic variables
Controls and patients with Parkinson's disease did not differ with regard to demographic variables such as age, gender or years of education [Fs (1, 47) < 0.6, Ps > 0.43 for age and education, χ2(1) = 1.7, P = 0.187 for gender; Table 1]. Regarding the relationship between demographic and memory variables, both controls and patients showed a significant negative correlation between age and K scores for the 2-red and 4-red conditions (Pearson's correlation coefficients, rs > −0.38, Ps < 0.045 for 2-red and rs > −0.41, Ps < 0.032 for 4-red; Table 2). Congruently, CDA amplitudes in the 4-red condition decreased with increasing age for patients (r = −0.47, P = 0.032).
The unnecessary storage of distracters, as indexed by the difference in K scores between the 2-red and 2-red–2-green conditions, increased as a function of disease severity in the patient group, which was measured by the Hoehn and Yahr scale (1967; r = 0.46, P = 0.036). Although age was highly correlated with the disease severity (r = 0.58, P = 0.006), only the stage of disease was a significant predictor of unnecessary storage [t(46) = 3.6, P = 0.001], whereas both age and stage of disease were significant predictors of memory capacity, as indexed by K scores in the 4-red condition [t(46) = −4.1, P < 0.001 for age and t(46) = −3.3, P = 0.002 for the stage of the disease].
Discussion
In the present study we examined whether the poor performance of patients with Parkinson's disease in working memory span tasks is due to a reduced storage capacity per se, inability to filter out distracters or both. Participants were asked to remember the orientations of red rectangles on the cued side of a display while ignoring all green rectangles, and then to judge whether any relevant items changed. Behavioural and psychophysiological measures provided converging evidence that patients with Parkinson's disease had both impaired attentional filtering ability and reduced storage capacity.
Impaired attentional filtering
Both controls and patients had some difficulty ignoring distracters. They showed reduced K scores and enhanced CDA amplitudes when distracters were present. The interfering effect of distracters, however, was greater for the patient group as evidenced by a larger decline of K scores in the presence of distracters. This difference was also supported by patterns in the surface electrophysiological data, although the greater increase in patients’ CDA amplitudes failed to reach statistical significance due in part to tremor-induced noise in the EEG recordings. It should be kept in mind that the behavioural and psychophysiological measures are complementary: CDA amplitudes reflect how many items within the cued display are being stored in memory irrespective of their task-relevance, whereas K scores provide an index of the number of items stored in and then retrieved from working memory that were in fact task relevant. In this regard, patients’ enhanced CDAs and reduced K scores in the 2-red–2-green condition imply that they allocated some of their limited memory space to irrelevant information.
Considering subgroups separately, even the high-capacity controls experienced some difficulty in ignoring distracters. They showed a small but reliable fall in K scores when distracters were present. However, it is possible that the problem these individuals had with filtering might have occurred during retrieval and comparison with the test array, because their average CDA amplitudes for trials with two relevant items were the same regardless of whether distracters were or were not present. Hence, older adults who are neurologically normal and have good memories seem to be effective at blocking out distracters, at least during the encoding and maintenance phases. By contrast, control participants with low working memory capacity had reduced K scores and enhanced CDA amplitudes when distracters were present, a pattern indicating impaired filtration during encoding and maintenance.
For participants whose basal ganglia had been impaired by Parkinson's disease, the situation was different. Consider first the subgroup of patients identified as having a working memory of ample size. Their behavioural performance was as good as that of healthy participants who were mnemonically well endowed. However, the data for these patients indicated that they were probably impaired at keeping distracters from memory, since they showed reduced K scores and increased CDAs in the 2-red–2-green condition. For low-capacity patients, both behavioural and electrophysiological indices of unnecessary storage were highly significant.
Why should basal ganglia disease lead to an impaired ability to exclude irrelevant information from working memory? A plausible answer comes from the functional MRI study by McNab and Klingberg (2008) that was described earlier. Their study showed that subjects in whom pallidal activity greatly increased following the ‘ignore’ command were better able to filter out the irrelevant information. Similarly, our data from patients with Parkinson's disease showed that disease severity was a significant predictor of the unnecessary storage, which in turn exhibited a strong negative correlation with memory capacity. Regarding the present findings, it seems plausible that the loss of dopaminergic input to the basal ganglia in patients with Parkinson's disease leads to a diminished ability of the globus pallidus to regulate which items are loaded into working memory (O'Reilly and Frank, 2006).
An alternative interpretation based on the common Parkinson's disease symptom of bradyphrenia should be considered. Parkinsonian patients often demonstrate slowed thinking and slow responses to questions, but get the answers right if enough time is provided. In the present study, the memory array was presented only briefly (500 ms) in order to minimize eye movements and discourage strategic grouping of individual rectangles into meaningful units (Luck and Vogel, 1997). It is possible that patients with Parkinson's disease are generally able to filter out distracters but that they do so at a much slower rate than neurologically normal, older adults. This possibility is being examined in ongoing research.
Reduced storage capacity
In addition to problems with filtering, the data suggest that patients with Parkinson's disease have less space in working memory. Their K scores and CDA amplitudes were smaller than those of control subjects across trial types, even those without distracters. However, those patients categorized as having ample storage space were as good at retaining relevant items as similarly categorized control subjects. Furthermore, although CDA amplitudes were reliably larger for control subjects on trials with four than with two targets, this was not the case for patients, particularly those in the low-capacity subgroup (Fig. 4, upper right, and Fig. 7, lower right). Equivalence or even paradoxical reversal during 2-red and 4-red trials for low-capacity patients is congruent with previous findings in which parietal cortex activation was markedly reduced when neurologically normal subjects were presented with memory loads beyond their capacity, as if they were simply overwhelmed (Linden et al., 2003; Vogel and Machizawa, 2004).
Figure 7.
Grand averaged CDA waveforms as a function of trial type for high-capacity controls and patients (A, upper panel) and low-capacity controls and patients (B, lower panel).
A possible explanation of how basal ganglia disease might lead to reduced capacity comes from a study by Matsui and colleagues (2007) in which fractional anisotropy values of white matter were compared in patients with Parkinson's disease with and without impaired executive functions (e.g. Wisconsin Card Sorting Test scores). Abnormalities of left parietal white matter were observed in patients with impaired executive functions. On the assumption that the retention of information in visual working memory occurs within parietal cortex (Todd and Marois, 2004; Postle et al., 2006), pathological changes of the parietal lobe in our patients with Parkinson's disease may have led to reduced storage capacity.
Reduced parietal function might also be secondary to impairments in the basal ganglia. This view is supported by a study of Chang and co-workers (2007), in which brain activation during a high-memory-load condition was compared with that of a low-load condition in healthy young adults performing a modified Sternberg Task. Participants were asked to remember five successive numbers. In the high-load condition, the stimuli consisted of five different digits (e.g. 5 2 9 1 4), whereas in the low-load condition the same number was successively presented (e.g. 3 3 3 3 3). Memory load–dependent activation was observed in the basal ganglia, prefrontal and posterior parietal cortices during encoding and maintenance phases. What made this study special in comparison with other studies was that the authors used multiple regression approaches to determine which brain regions are functionally connected with the basal ganglia activations under high versus low memory load. They found enhanced connectivity between the left anterior caudate and ventrolateral prefrontal and posterior parietal cortices under the high memory load condition. Given that memory load was manipulated in the absence of any explicit distracters, this study suggests that basal ganglia control of visual working memory extends beyond blocking the entry of distracters (McNab and Klingberg, 2008).
Our conclusion that reduced storage capacity contributes to impaired performance in Parkinson's disease is asserted with some caution though, because of a limitation imposed by our use of bilateral displays. To successfully perform the task, participants needed to ignore the uncued side of the display. This would have imposed a filtering requirement even for those conditions in which no green rectangles were presented. Consequently, patients’ lower K scores and CDA amplitudes for the 2-red and 4-red conditions might actually have been due to an impaired ability to filter the to-be-ignored half of the display rather than to any reduction in storage capacity per se. As an example, consider the fact that patients’ CDA amplitudes were significantly increased when distracters were present. This indicates that patients with Parkinson's disease were in fact able to hold significantly more information than they did on 2-red trials, although some of that information was task irrelevant. In a planned study, we intend to test whether patients with Parkinson's disease indeed have reduced storage capacity in the absence of explicit distracters.
Funding
This work was supported by National Institute of Health grant 5 R01 HD-21338 to Dr Cowan and a Missouri University Interdisciplinary Center on Aging grant to Ms Lee.
Acknowledgements
We thank Ms Emily Evers for technical assistance and the patients for their willing participation.
Glossary
Abbreviations
- CDA
contralateral delay activity
References
- Awh E, Vogel EK. The bouncer in the brain. Nature Neurosci. 2008;11:5–6. doi: 10.1038/nn0108-5. [DOI] [PubMed] [Google Scholar]
- Bennett KM, Waterman C, Scarpa M, Castiello U. Covert visuospatial attentional mechanisms in Parkinson's disease. Brain. 1995;118:153–66. doi: 10.1093/brain/118.1.153. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–69. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–12. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114:1095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, et al. On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cognit Psychol. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW. A central executive deficit in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:360–7. doi: 10.1136/jnnp.57.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Kiss M. An electrophysiological measure of access to representations in visual working memory. Psychophysics. 2010;47:197–200. doi: 10.1111/j.1469-8986.2009.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:196–8. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Singh J, Stebbins GT, Goetz CG. Reduced working memory span in Parkinson's disease: evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology. 1996;10:322–32. [Google Scholar]
- Hsieh S, Hwang WJ, Tsai JJ, Tsai CY. Visuospatial orienting of attention in Parkinson's disease. Percept Mot Skills. 1996;82:1307–15. doi: 10.2466/pms.1996.82.3c.1307. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The pathology of Parkinson's disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- Jolicoeur P, Brisson B, Robitaille N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain research. 2008;1215:160–72. doi: 10.1016/j.brainres.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–60. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, et al. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage. 2003;20:1518–30. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Lu JL, Neuse J, Madigan S, Dosher BA. Fast decay of iconic memory in observers with mild cognitive impairments. Proc Natl Acad Sci. 2005;102:1797–802. doi: 10.1073/pnas.0408402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 1994;20:1000–14. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–81. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17:1057–74. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, et al. Wisconsin card sorting test in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:108–12. doi: 10.1111/j.1600-0404.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neurosci. 2008;11:103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44:369–78. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini PM, Alexander A, et al. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in prefrontal, but not posterior parietal cortex. J Cogn Neurosci. 2006;18:1712–22. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink MW. Reliance on external cues for movement initiation in Parkinson's disease. Evidence from movement-related potentials. Brain. 1998;121:167–77. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat FM. Failed suppression of direct visuomotor activation in Parkinson's disease. J Cogn Neurosci. 2001;13:31–43. doi: 10.1162/089892901564153. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements: a PET study. Brain. 1997;120:963–76. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention; pp. 165–73. [Google Scholar]
- Skeel RL, Crosson B, Nadeau SE, Algina J, Bauer RM, Fennell EB. Basal ganglia dysfunction, working memory, and sentence comprehension in patients with Parkinson's disease. Neuropsychologia. 2001;39:962–71. doi: 10.1016/s0028-3932(01)00026-4. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–4. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Verleger R, Hagenah J, Weiss M, Ewers T, Heberlein I, Pramstaller PP, et al. Responsiveness to distracting stimuli, though increased in Parkinson's disease, is decreased in asymptomatic PINK1 and Parkin mutation carriers. Neuropsychologia. 2010;48:467–76. doi: 10.1016/j.neuropsychologia.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–51. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005a;438:500–3. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Pushing around the locus of selection: evidence for the flexible selection hypothesis. J Cognit Neurosci. 2005b;17:1907–22. doi: 10.1162/089892905775008599. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PA. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cognit Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]