Abstract
Antibodies that immunoprecipitate 125I-α-dendrotoxin-labelled voltage-gated potassium channels extracted from mammalian brain tissue have been identified in patients with neuromyotonia, Morvan’s syndrome, limbic encephalitis and a few cases of adult-onset epilepsy. These conditions often improve following immunomodulatory therapies. However, the proportions of the different syndromes, the numbers with associated tumours and the relationships with potassium channel subunit antibody specificities have been unclear. We documented the clinical phenotype and tumour associations in 96 potassium channel antibody positive patients (titres >400 pM). Five had thymomas and one had an endometrial adenocarcinoma. To define the antibody specificities, we looked for binding of serum antibodies and their effects on potassium channel currents using human embryonic kidney cells expressing the potassium channel subunits. Surprisingly, only three of the patients had antibodies directed against the potassium channel subunits. By contrast, we found antibodies to three proteins that are complexed with 125I-α-dendrotoxin-labelled potassium channels in brain extracts: (i) contactin-associated protein-2 that is localized at the juxtaparanodes in myelinated axons; (ii) leucine-rich, glioma inactivated 1 protein that is most strongly expressed in the hippocampus; and (iii) Tag-1/contactin-2 that associates with contactin-associated protein-2. Antibodies to Kv1 subunits were found in three sera, to contactin-associated protein-2 in 19 sera, to leucine-rich, glioma inactivated 1 protein in 55 sera and to contactin-2 in five sera, four of which were also positive for the other antibodies. The remaining 18 sera were negative for potassium channel subunits and associated proteins by the methods employed. Of the 19 patients with contactin-associated protein-antibody-2, 10 had neuromyotonia or Morvan’s syndrome, compared with only 3 of the 55 leucine-rich, glioma inactivated 1 protein-antibody positive patients (P < 0.0001), who predominantly had limbic encephalitis. The responses to immunomodulatory therapies, defined by changes in modified Rankin scores, were good except in the patients with tumours, who all had contactin-associated-2 protein antibodies. This study confirms that the majority of patients with high potassium channel antibodies have limbic encephalitis without tumours. The identification of leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 as the major targets of potassium channel antibodies, and their associations with different clinical features, begins to explain the diversity of these syndromes; furthermore, detection of contactin-associated protein-2 antibodies should help identify the risk of an underlying tumour and a poor prognosis in future patients.
Keywords: autoimmune encephalitis, thymoma, epilepsy, neuromuscular junction, neuronal autoantibodies, Caspr2, Lgi1, VGKC
Introduction
Voltage-gated potassium channel (VGKC) antibodies have been reported in association with three main clinical syndromes: neuromyotonia, Morvan’s syndrome and limbic encephalitis (for review see Vincent, 2008). Neuromyotonia is a peripheral nerve hyperexcitability syndrome characterized by muscle cramps and stiffness (Hart et al., 2001). In Morvan’s syndrome, symptoms of neuromyotonia are associated with autonomic and central nervous system dysfunction, with frequent insomnia (Liguori et al., 2001; Josephs et al., 2004). By contrast, limbic encephalitis is characterized by amnesia, confusion, seizures and personality change or psychosis, with discrete hippocampal abnormalities on brain MRI, and in patients with VGKC-antibodies there is often an associated hyponatraemia (Vincent et al., 2004; Graus et al., 2008). In addition to these syndromes, VGKC-antibodies have also been identified in some patients with idiopathic epilepsy (McKnight et al., 2005) and recently in a form of late-onset dystonic epilepsy (Irani et al., 2008; Barajas et al., 2009). In most cases these syndromes have a subacute onset and respond well to immunomodulatory therapies (Buckley et al., 2001; Thieben et al., 2004; Vincent et al., 2004; Geschwind et al., 2008; Irani et al., 2008), although neuromyotonia may require only symptomatic treatments. Both neuromyotonia and Morvan’s syndrome can be associated with tumours, particularly thymomas, but tumours appear to be uncommon in typical VGKC-antibody associated limbic encephalitis or idiopathic epilepsy. However, there have been no systematic studies of large cohorts, except one where the clinical syndromes were not defined (Tan et al., 2008).
VGKC-antibodies are usually detected by immunoprecipitation of iodinated α-dendrotoxin (125I-α-DTX)-labelled VGKCs in digitonin-solubilized mammalian brain homogenates (Shillito et al., 1995; Hart et al., 1997; Thieben et al., 2004). The VGKC-antibody titres tend to be higher (>400 pM; normal <100 pM) in patients with CNS conditions compared to those with isolated neuromyotonia (generally between 100 and 1000 pM). α-DTX binds with high affinity to three different VGKC subunits, Kv1.1, 1.2 and 1.6. In neuromyotonia, early studies suggested that the antibodies were variably directed against these three Kv1 subunits (Hart et al., 1997), and the pathogenic role of purified Immunoglobulin G (IgG) was demonstrated by passive transfer to mice (Sinha et al., 1991; Shillito et al., 1995), and by application to neuronal cell line cultures or Kv1-transfected cells in vitro (Shillito et al., 1995; Nagado et al., 1999; Tomimitsu et al., 2004). In limbic encephalitis, immunohistological studies suggested antibodies mainly to Kv1.1 (Kleopa et al., 2006).
Our main aim was to investigate the specificity of the VGKC-antibodies for the different Kv1 subtypes and to relate these to the clinical phenotypes. In doing so, we also wished to describe the clinical phenotypes, tumour associations and treatment responses in the different syndromes. Surprisingly, only a few patients had antibodies binding to the Kv1 subunits themselves but most bound only to one of two proteins, leucine-rich glioma inactivated 1 (Lgi1) or contactin-associated protein-2 (Caspr2), that are part of the αDTX-labelled VGKC complexes used in the serological assay. Preliminary results have been reported in abstracts (Vincent, 2009; Irani et al., 2010). Five sera bound to contactin-2 itself but four of these sera also bound to Kv1, Caspr2 or Lgi1.
Materials and methods
Clinical material
Serum samples were stored at −20°C. The study was approved by the Oxfordshire Research Ethics Committee A (07/Q160X/28). To explore the antibody specificities, we initially used sera from patients with well-defined clinical syndromes (as previously described in Liguori et al., 2001; Vincent et al., 2004). Subsequently, to study the clinical phenotypes in a large cohort, we sent consent forms and questionnaires to neurologists, identified on the request forms, who had sent sera that were positive for VGKC-antibodies at >400 pM (high VGKC-antibodies) and that were representative of those received during the period 2003–08. To assess the effects of treatments, we asked the neurologists to provide modified Rankin scores (Graus et al., 2001) at onset and latest follow-up. From completed questionnaires and/or clinic letters, visits (S.R.I.) and/or telephone conversations, the clinical data were obtained for 96 patients.
Plasmid constructs
The cDNAs represented the human sequences, except for the β2, which was rat. cDNAs encoding full-length human Kv1.1, 1.2, 1.6 and rat β2 subunits (as in Kleopa et al., 2006) were cloned into pcDNA3.1-hygro (Invitrogen Ltd, CA, USA). The full-length human MuSK-EGFP construct has been described previously (Leite et al., 2008). Lgi1 was amplified by polymerase chain reaction from an image clone (IMAGE 4811956 from Geneservice, Cambridge, England) digested with Nhe1/Xho1 and subcloned into pcDNA 3.1 (+) (Invitrogen, UK). The vector pCR4-TOPO that contained the cDNA for Caspr2 (IMAGE: 7939625 from Geneservice) was digested with EcoRI. The fragment was subcloned into pcDNA3.1 (+) (Invitrogen) to give an untagged Caspr2 that expressed in mammalian cells. In order to tag Caspr2 with enhanced green fluorescent protein (EGFP), the pcDNA3.1 (+)-Caspr2 plasmid was digested with XhoI and XmaI. The fragment was subcloned into pEGFP-N1 (Clontech Laboratories, CA, USA). To express Lgi1 fused to the transmembrane region of Caspr2, the C-terminal cDNA sequence of Caspr2 (residues 1248–1331) was amplified by polymerase chain reaction with the primers GATCCTCGAGGGACAAGGCCAAGCTATAAGAAATG and ATCGTTTAAACTCAAATGAGCCATTCCTTTTTGC. This was cloned in frame with Lgi1 in pcDNA3.1+ with the restriction enzymes XhoI/PmeI. The stop codon was removed by site directed mutagenesis using the primers GTTGACTTAAGCGCAGGACTCGAGGGACAAGG and CCTTGTCCCTCGAGTCCTGCGCTTAAGTCAAC, to give a final product of Lgi1 attached to the transmembrane and cytoplasmic domains (residues 1248–1331) of Caspr2.
Transfection of human embryonic kidney cells
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal calf serum (TCS Cellworks Ltd, Buckingham, UK) and 100 units/ml each of penicillin G and streptomycin (Invitrogen) at 37°C in a 5% CO2 atmosphere. Cells were grown on six-well culture plates for immunoadsorption and toxin binding experiments, on 13 mm glass coverslips placed in six-well cell culture plates for microscopy, and in 175 cm2 flasks for protein extraction. Using polyethylenimine, cells were transiently co-transfected with plasmids containing Kv1.1, 1.2 and 1.6, EGFP, MuSK-EGFP, Caspr2-EGFP, Lgi1, contactin-2 cDNA (kind gift of Dr Karagogeos, University of Crete, Greece) or Lgi1 fused to Caspr2.
Electrophysiological studies on human embryonic kidney cells
For measuring VGKC currents, the HEK293 cells were co-transfected with Kv1.1 and EGFP, or Kv1.6 and EGFP, and plated onto glass coverslips for whole-cell patch clamp recordings (see Supplementary Methods for the details). The effects of patient plasmas on Kv1 currents were determined after acute application, or after incubation for 24 or 72h.
Binding of antibodies to transfected human embryonic kidney cells
Forty-eight hours after transfection, the immunofluorescent staining of the cells was performed. The coverslips were transferred to individual wells in 24-well culture plates and incubated at room temperature for 45 min with mouse anti-Kv1.1 extracellular domain antibody (1:100, Neuromab, California) or patient serum (1:20–1:100, as specified); these were diluted in Dulbecco’s modified Eagle’s medium buffered with HEPES [N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)] with added 1% bovine serum albumin to block non-specific binding. The cells were subsequently washed three times in Dulbecco’s modified Eagle’s medium/HEPES buffer and fixed with 3% formaldehyde in phosphate buffered saline at room temperature for 15 min, followed by further washing. They were then labelled for 45 min at room temperature with anti-mouse IgG or anti-human IgG Alexa Fluor 568-conjugated secondary antibody (Invitrogen-Molecular probes, Paisley, UK) at 1:750 in 1% bovine serum albumin/Dulbecco’s modified Eagle’s medium/HEPES buffer. For cell permeabilization, cells were initially incubated with fixative (as above) and subsequently all solutions contained 0.1% Triton-X-100. Rabbit anti-Kv1.1/1.2 and 1.6 antibodies (1:500, Alamone, Israel) were visualized with an anti-rabbit IgG Alexa Fluor 568-conjugated secondary antibody (Invitrogen-Molecular probes) on permeabilised cells. All coverslips were subsequently washed three times in phosphate buffered saline and mounted on slides in fluorescent mounting medium (DakoCytomation, Cambridge, UK) with DAPI (4′,6′-diamidino-2-phenlindoledichloride, 1 : 1000). They were visualized using a fluorescence microscope with a MacProbe v4.3 digital imaging system.
Immunoprecipitation assays for VGKCs, Kv1 subunits and Caspr2
This was performed as described earlier (Hart et al., 1997). VGKC complexes were extracted from rabbit brain membranes solubilized in 2% digitonin (Calbiochem, USA) in DTX-buffer (100 mM NaCl, 20 mM Tris, 5 mM KCl adjusted to pH 7.12) at 37°C for 20 min. The supernatant was diluted 1:2 with PTX (0.02 M phosphate buffer and 0.1% Triton-X-100, pH 7.2) and incubated with 106 cpm/ml of 125I-α-DTX (Perkin Elmer, USA). An amount of 50 µl volumes of the 125I-α-DTX-labelled extracts were incubated with 5 and 1 µl of each serum, the IgG-VGKC complexes were precipitated with anti-human IgG (The Binding Site, UK), and the pellets were washed and counted on a gamma counter (Cobra2, Perkin Elmer, USA).
To immunoprecipitate 125I-α-DTX-labelled Kv1s, cells were transfected with the cDNAs for the different subunits as above. At 48 h post-transfection, confluent 175 cm2 flasks of transfected cells were washed with phosphate buffered saline and lysed in 2% digitonin in DTX-buffer, with 1:100 protease inhibitor cocktail (Sigma-Aldrich, UK). Lysates were rotated for 1 h, spun (13 000 rpm for 5 min at 4°C) and labelled with 125I-α-DTX as above. An amount of 5 µl of human sera or commercial antibodies to Kv1.1, 1.2, 1.6 (Alomone Labs, USA) were added and the precipitations performed with goat anti-human IgG (RSR Ltd UK; we find that this antiserum can be used to immunoprecipitate human, mouse and rabbit IgG). The pellets were counted as above.
For immunoprecipitation of Caspr2, flasks of EGFP-Caspr2-transfected HEK293 cells were treated similarly and aliquots of the supernatant containing 100 fU of EGFP-Caspr2 were tested with 5 or 1µl of each serum. The complexes were immunoprecipitated with Protein A beads. For counting of the EGFP, the pellets were resuspended in 200 µl, transferred to a 96-well plate and analysed on a fluorescent plate reader (Gemini XS, Molecular Probes).
Indirect immunofluorescence on mouse tissue and live rat hippocampal neurons
Immunofluorescence detection of antibodies binding to fixed mouse cerebral and cerebellar sections, and sciatic nerve teased fibres was performed as described previously (Kleopa et al., 2006). Details on tissues and binding of the serum antibodies to live neurons in culture are provided in the Supplementary Methods and in Irani et al. (2010).
Results
VGKC-antibodies and clinical features in the large cohort
We obtained clinical information on 96 individuals who had not previously been studied and whose samples were representative of the >500 high VGKC-antibody positive sera identified since 2004. The majority were from the UK, with 14 from other European countries. The clinical features are summarized in Table 1. All of the 64 patients defined as limbic encephalitis (Bien and Elger, 2007) had amnesia and/or confusion and 59 (92%) had seizures, but only 40 (62%) had medial temporal lobe inflammation on MRI and only 38 (59%) had hyponatraemia, which has often been identified in VGKC-antibody associated limbic encephalitis (Vincent et al., 2004; Graus et al., 2008). All five patients with Morvan’s syndrome were male and had clinical neuromyotonia, confirmed on EMG, with dysautonomia, pain, usually in the feet and often burning in nature, and marked insomnia.
Table 1.
Clinical features in 96 patients with VGKC-antibodies >400 pM
| Limbic encephalitis (n = 64) | Morvan’s syndrome (n = 5) | Neuromyotonia (n = 11) | Epilepsy (n = 4) | Other disorders (n = 12) | |
|---|---|---|---|---|---|
| Age at onset, median (range) | 63 (19–83) | 64 (58–69) | 54 (39–74) | 65 (51–70) | 45 (17–83) |
| Male:female | 44:20 | 5:0 | 7:4 | 3:1 | 9:3 |
| Amnesia | 64 | 5 | 0 | 0 | 7 |
| Confusion/disorientation | 64 | 5 | 0 | 0 | 8 |
| Seizures | 59 | 3 | 0 | 4 | 2 |
| MRI medial temporal lobe high signal on T2 or FLAIR | 40 | 0 | ND | 0 | 0 |
| Movement disorder | 1 dyskinesia; 2 myoclonus | 0 | 0 | 0 | 2 |
| Hyponatraemia | 38 | 2 | 0 | 0 | 0 |
| Active tumour | 0 | 1 | 5 | 0 | 0 |
| Sleep disorder | 8 hypersomnia; 6 insomnia; 4 REMSBD; 3 sleep reversal | 5 insomnia | 1 insomnia | 0 | 2 |
| Ataxia | 6 | 0 | 1 | 1 | 0 |
| Pain | 3 | 5 | 4 | 0 | 1 |
| Hyperhidrosis | 6 | 2 | 4 | 0 | 0 |
| Any dysautonomia | 7 | 4 | 4 | 0 | 2 |
| Neuromyotonic discharges | 0 | 5 | 11 | 0 | 0 |
| Myasthenia gravis | 1 | 1 | 2 | 0 | 0 |
| Other features | 1 peripheral neuropathy | 4 peripheral neuropathies | 0 | See Supplementary Table 1 |
ND = not determined; REMSBD = rapid eye movement sleep behaviour disorder.
The distinction between the syndromes was not absolute. A third of the patients with limbic encephalitis had sleep disorders, including six with insomnia, which are more typically associated with Morvan’s syndrome. Four of the patients with neuromyotonia had pain or dysautonomia but they had no CNS involvement and were generally less severely affected than the patients with Morvan’s syndrome; we used the diagnosis of neuromyotonia provided by the neurologists. Four patients had epilepsy only and twelve patients had presentations that could not be defined as limbic encephalitis, Morvan’s syndrome, neuromyotonia or epilepsy (summarized in Supplementary Table 1).
Nine patients had past histories of various malignancies, including two prostate, one breast, one non-small cell lung cancer and one melanoma, but all of these appeared to be inactive or stable for >5 years. Active tumours were present in only six of the patients; five thymomas and one endometrial adenocarcinoma. Four patients had a history of myasthenia gravis (one with limbic encephalitis who subsequently also developed neuromyotonia and neuromyelitis optica, one with Morvan’s syndrome, two with neuromyotonia).
Most VGKC-antibodies bind to associated VGKC-complex proteins and not directly to Kv1 subunits
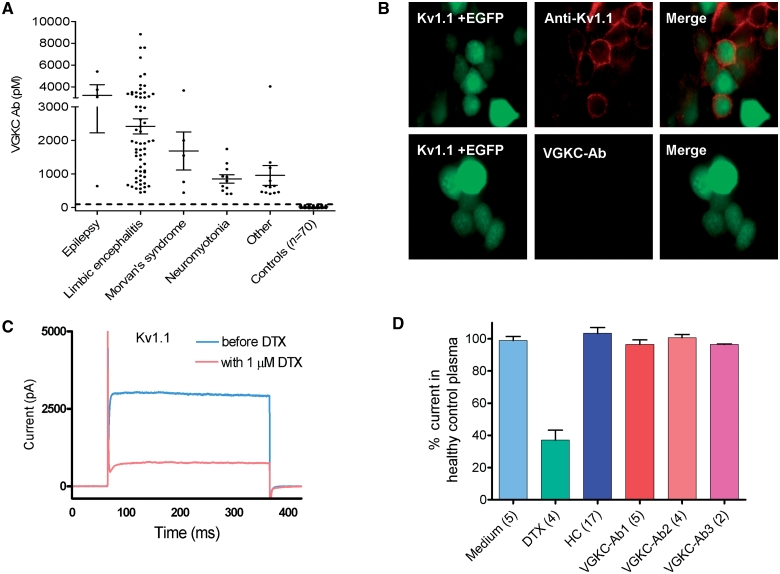
Figure 1A shows the VGKC-antibody titres in the 96 patients. To define the antibody targets, we expressed the individual α-DTX-binding Kv1 subunits (Kv1.1, 1.2 and 1.6) in HEK293 cells, and compared binding of patients’ serum IgG antibodies with that of commercial polyclonal antibodies to the individual subunits. For these studies we used sera from previously characterized patients with known limbic encephalitis or Morvan’s syndrome (VGKC-antibodies >400 pM). Although commercial Kv1.1 antibodies, for instance, bound to the Kv1.1-transfected cells, none of the patients’ IgG showed detectable binding (e.g. Fig. 1B). In addition, we measured voltage-dependent currents in Kv1.1- and Kv1.6-transfected HEK293 cells. As expected, there was a marked reduction in Kv1 currents with the specific VGKC blocker α-DTX (Fig. 1C), but no effect following application of VGKC-antibody positive sera, either acutely (Fig. 1D) or after 24 or 72 h of incubation; similar results were obtained with Kv1.6 (all results are summarized in Table 2).
Figure 1.
VGKC-antibodies immunoprecipitate VGKCs from brain extracts but do not bind directly to Kv1 subunits. (A) VGKC-antibody levels (pM), measured by immunoprecipitation of 125I-α-DTX-VGKCs from rabbit brain extracts, in 96 patients, divided according to the clinical diagnoses (Table 1), and from 70 healthy or other disease controls. The cut-off (100 pM) represents the mean plus three standard deviations of the healthy control values. (B) Binding of antibodies to the surface of HEK293 cells transfected with cDNA for the Kv1.1 subunit; the cells were co-transfected with EGFP to identify those cells taking up the cDNA. Positive staining was found with a rabbit antibody raised against the extracellular domain of Kv1.1 (anti-Kv1.1), but VGKC-antibody positive sera did not show any staining (lower panels). Magnification × 1000 (C) Voltage-dependent currents measured in Kv1.1 transfected HEK293 cells were substantially reduced by the neurotoxin, α-DTX (1 µM). (D) Sera (1:100 diluted) from six healthy individuals (total 17 cells recorded) or from three individual patients with VGKC-antibodies (n = number of cells examined for each patient) had no effect on voltage-dependent currents in Kv1.1 transfected HEK293 cells (Table 2). Ab = antibody; HC = healthy control.
Table 2.
Summary of effects of VGKC-antibody positive patients’ plasma antibodies on α-DTX-sensitive currents
| Peak current amplitude (pA) in incubation medium alone | + 1 µm α-DTX percentage of medium alone (number of recordings) | Duration of incubation in plasma (1 : 100) | Current amplitudes from recordings in six healthy individuals’ plasmas (number of recordings)a | Current amplitude averaged from recordings in VGKC-antibody patients’ plasmas, as percentage of controls (number of recordings)a | |
|---|---|---|---|---|---|
| Kv1.1 | 3286 ± 211 (73) | 37 ± 6% (4) | Acute incubation | 103 ± 4% (17) | 98.6 ± 1% (15) |
| ND | 24 h | 100 ± 12.7% (45) | 97 ± 10% (55) | ||
| ND | 72 h | 100 ± 13 % (34) | 127 ± 18% (43) | ||
| Kv1.6 | 9594 ± 898 (18) | 9 ± 1% (3) | Acute | 101 ± 3% (17) | 95.8 ± 3% (14) |
| ND | 24 h | 100 ± 11% (29) | 103 ± 11% (39) | ||
| ND | 72 h | 100 ± 8% (47) | 92 ± 13% (92) |
Results are presented as mean ± SEMs of the pretreatment amplitudes for the acute incubations, or of the mean of the healthy plasma recordings for the 24 and 72 h incubations; ND = not determined.
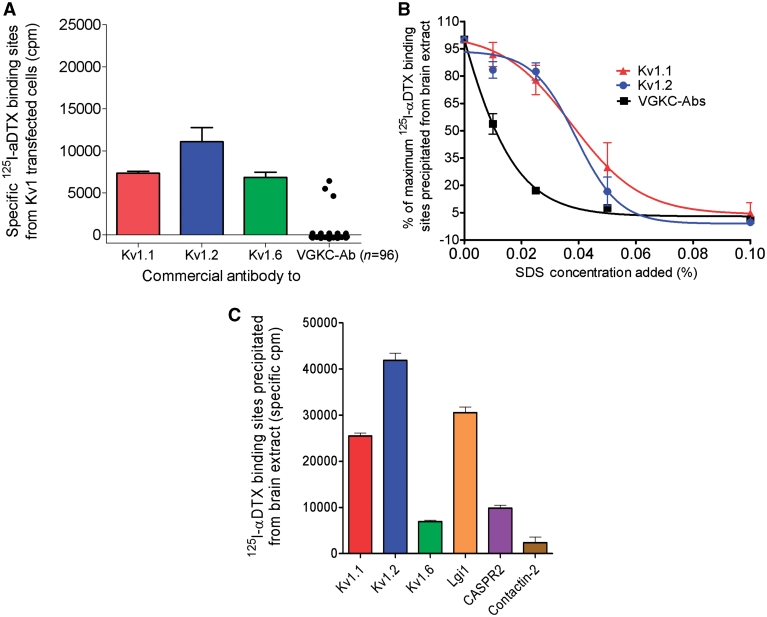
Finally, to reproduce the immunoassays used for testing the patients’ sera, which use 125I-α-DTX-labelled digitonin extracts of rabbit brain tissue, we expressed Kv1.1, 1.2, 1.6 and β2 subunits together in HEK293 cells, made a 2% digitonin extract, added 50 000 cpm of 125I-α-DTX to each 50 µl aliquot, and compared immunoprecipitations by the commercial antibodies to the individual subunits with that by the patients’ serum antibodies. All three Kv1 subunits were well expressed and bound by their respective antibodies, but only three of the VGKC-antibody positive sera immunoprecipitated the 125I-α-DTX -labelled VGKCs (Fig. 2A).
Figure 2.
VGKCs are not the targets for the VGKC-antibodies. (A) A digitonin-extract was prepared of HEK293 cells that had been cotransfected with cDNAs for Kv1.1, 1.2, 1.6 and β2. The extract was labelled with 125I-α-DTX. Rabbit antibodies to each of the Kv1 subunits, but only three of the 96 VGKC-antibody positive sera, immunoprecipitated the 125I-α-DTX-labelled Kv1 complexes, showing that 93/96 of the patients’ antibodies did not bind the Kv1s at detectable levels. (B) The effect of a harsher detergent on VGKC-complexes. Addition of increasing amounts of sodium dodecyl sulphate showed that immunoprecipitation by the patients’ antibodies was reduced at very low concentrations of sodium dodecyl sulphate, in comparison with immunoprecipitation by the antibodies to Kv1.1 or 1.2, suggesting that the patients’ binding sites were easily dissociated from the 125I-α-DTX-labelled Kv1s. (C) Immunoprecipitation of 125I-α-DTX-labelled VGKCs extracted from brain tissue with antibodies directed against individual Kv1 subunits, Lgi1, Caspr2 and contactin-2. Each of the antibodies immunoprecipitated significant but variable quantities of 125I-α-DTX labelled rabbit brain Kv1s indicating that Lgi1, Caspr2 and contactin-2 were complexed with the Kv1s. SDS = sodium dodecyl sulphate.
From these results, we concluded that the majority of VGKC-antibody sera did not bind directly to Kv1 subunits but might bind to non-Kv1 proteins that remain complexed with the Kv1s in the rabbit brain digitonin extracts; digitonin is a mild detergent that does not dissociate all protein complexes. To see whether we could dissociate the sites for the patients’ antibodies from the Kv1s, we added increasing amounts of the harsh detergent sodium dodecyl sulphate to the 125I-α-DTX-labelled VGKCs in rabbit brain extracts (Fig. 2B). Immunoprecipitation of VGKCs by the patients’ antibodies was sensitive to low concentrations (e.g. 0.025%) of sodium dodecyl sulphate, which do not usually affect the binding of antibodies directly to their targets (e.g. antibodies to acetylcholine receptors or MuSK in myasthenia, unpublished results), whereas the commercial antibodies to Kv1.1 or 1.2, as expected, were able to immunoprecipitate the 125I-α-DTX -labelled VGKCs at these sodium dodecyl sulphate concentrations (Fig. 2B).
We then tested the brain extract with commercial antibodies directed against proteins reported to associate with Kv1s: Lgi1, Caspr2 and contactin-2 (Poliak et al., 1999, 2003; Schulte et al., 2006). The antibodies to these proteins precipitated approximately 60, 20 and ∼5% of the total number of 125I-α-DTX-binding sites (Fig. 2C), respectively. Other commercial antibodies to proteins such as PSD95, PSD93 and ADAM22 did not precipitate appreciable amounts of the 125I-α-DTX -VGKCs (data not shown).
Caspr2 is a target for VGKC-antibodies
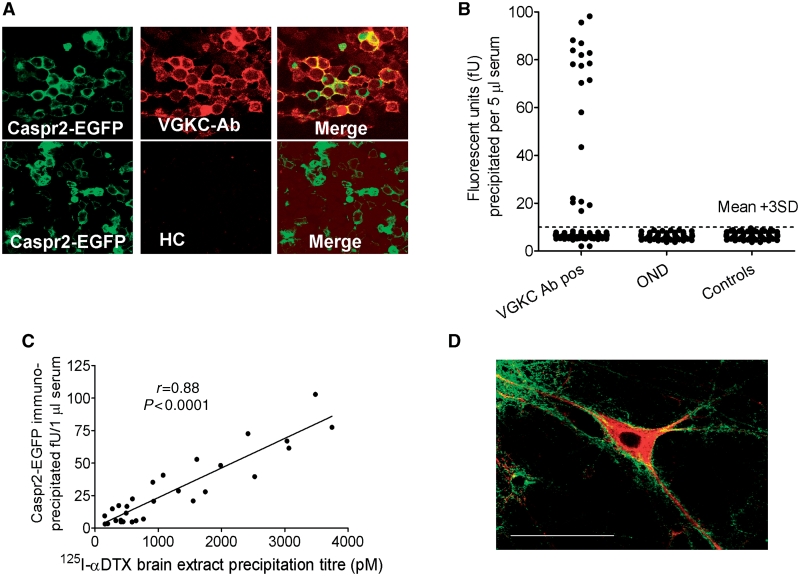
Caspr2 co-localizes with Kv1.1 and 1.2 at neural juxtaparanodes, is essential for VGKC clustering in vivo (Poliak et al., 2003), and has a large extracellular sequence. In order to detect antibodies to Caspr2, we expressed full-length human Caspr2 in HEK293 cells, tagging the protein by introducing EGFP at the intracellular C-terminus. Nineteen of the VGKC-antibody positive sera (20%) at 1 : 100 dilution bound to the surface of these cells, and not to cells transfected with control vectors (data not shown); control sera did not bind Caspr2-transfected HEK293 cells (Fig. 3A). The advantage of using EGFP-tagged Caspr2 is that the EGFP can be used to quantify the antibodies after immunoprecipitation from extracts of the transfected cells (Fig. 3B). There was a strong positive correlation between VGKC-antibody titre and Caspr2-EGFP-antibody titre (Fig. 3C), and immunoabsorption against Caspr2-transfected cells, but not Kv1-transfected cells, abolished precipitation of 125I-α-DTX-VGKCs (Supplementary Fig. 1A). Caspr2 positive sera also bound strongly in a punctate manner to the surface of cultured hippocampal neurons (Fig. 3D).
Figure 3.
Caspr2-antibodies in VGKC-antibody positive patients. (A) HEK293 cells were transfected with Caspr2-EGFP. A VGKC-antibody positive serum (1:100) bound to the surface of the Caspr2-EGFP-expressing cells (green), whereas healthy serum (HC) did not bind. Strong binding (red) was found in 19 of the 96 VGKC-antibody positive sera. (B) Immunoprecipitation of Caspr2-EGFP from digitonin extracts of the transfected cells by 5 µl of serum was also positive in 19 of the 96 sera, compared with sera from other neurological disease controls (OND; multiple sclerosis, myasthenia with thymomas, non-VGKC-antibody associated encephalopathies) or healthy individuals (controls). (C) There was a highly positive correlation between the binding to Caspr2-EGFP and binding to 125I-α-DTX-labelled Kv1s. For these data, the Caspr2-EGFP immunoprecipitation was performed with 1 µl of each serum to obtain more quantitative results. (D) Caspr2-antibody positive serum IgGs (detected with fluorescein-anti-human IgG, green) also bound strongly to the surface of live cultured hippocampal neurons, 18 days in culture; the neurons were subsequently fixed, permeabilized and stained for MAP2 (red). The bar represents 25 µm.
Lgi1 is a target for VGKC-antibodies
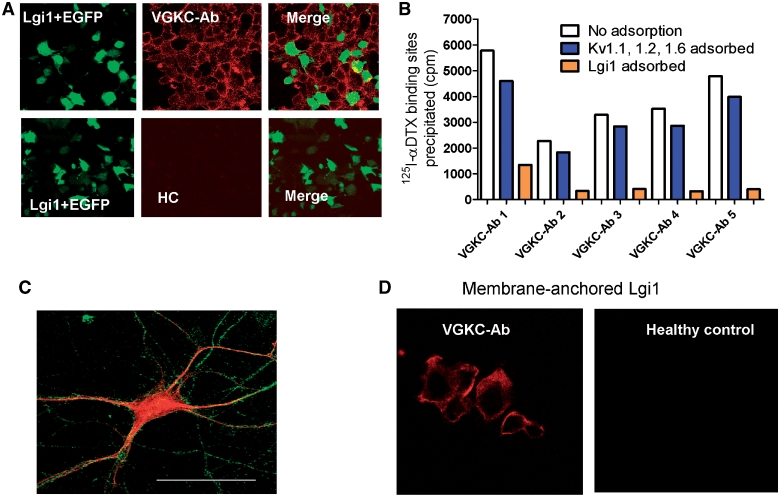
Lgi1 associates specifically with Kv1.1 subunits in CNS presynaptic terminals (Schulte et al., 2006), and is a secreted protein that can associate with neuronal and non-neuronal cell membranes (Sireol-Piquer et al., 2006). To determine whether VGKC-antibodies bound to Lgi1, we expressed full-length Lgi1 in HEK293 cells. We found that 46 of the 96 VGKC-antibody positive sera (48%), and none of 100 disease or healthy control sera, bound to the surface of unpermeabilized Lgi1-transfected cells (Fig. 4A). However, these sera also bound to cells in the same cultures that were not transfected (Fig. 4A), although not to cells from different cultures that were transfected with other vectors including Kv1s, Caspr2 or a neuromuscular junction antigen, MuSK (data not shown). Binding to the non-transfected cells was explained by the fact that Lgi1 could be detected in the medium of Lgi1-transfected cells (data not shown), consistent with its reported secretion (Fukata et al., 2006; Sireol-Piquer et al., 2006; Zhou et al., 2009) and this medium, but not that from other HEK293 supernatants, could transfer to untransfected HEK293 cells the ability to bind VGKC-antibodies (Supplementary Fig. 3).
Figure 4.
Lgi1-antibodies are present in many VGKC-antibody positive patients. (A) HEK293 cells were co-transfected with cDNAs for Lgi1 and EGFP (green) and incubated with patient sera (1:20). A representative image of binding of a limbic encephalitis VGKC-antibody positive serum to the cells, detected with anti-human IgG (red). Note that the red stain is found not only on the Lgi1/EGFP transfected cells, but also on the surface of non-transfected cells. Similar results were found in 46 of the 96 patient sera, but were not observed with healthy control sera (HC). (B) Immunoadsorption of the VGKC-antibody positive sera against Lgi1-transfected cells, but not against Kv1-transfected cells, substantially reduced immunoprecipitation of 125I-α-DTX–VGKC complexes from rabbit brain extracts. (C) Lgi1-positive sera binding to a live hippocampal neuron detected with fluorescein-anti-human IgG (green); the neurons were subsequently fixed, permeabilized and stained for MAP2 (red). The bar represents 25 µm. (D) To confirm that Lgi1 was the target for the antibody binding shown in A, cDNA for Lgi1 was fused to cDNA for the transmembrane domain and cytoplasmic tail of Caspr2. This construct expressed well in HEK293 cells and provided a direct assay for Lgi1 antibodies. Nine additional sera (one illustrated here) bound to these cells; but there was no binding of healthy control sera or Caspr2-antibody positive sera.
To confirm that the Lgi1 binding was related to VGKC-antibodies, as defined by immunoprecipitation of 125I-α-DTX-VGKCs, we showed that immunoabsorption against Lgi1-transfected cell cultures substantially reduced the ability of sera to precipitate 125I-α-DTX-VGKCs from the rabbit brain extract, whereas immunoabsorption against cells expressing Kv1.1/1.2/1.6 did not (Fig. 4B). Sera with Lgi1 antibodies bound to the surface of cultured hippocampal neurons (Fig. 4C), although the binding was relatively weak. Finally, we established a direct assay for Lgi1 antibodies by expressing Lgi1 fused on to the C terminal/transmembrane domain of Caspr2 so that it was anchored to the membrane. Lgi1-antibody positive sera bound directly to the surface of the transfected cells (Fig. 4D), and using this improved assay we were able to detect Lgi1-antibodies in a further nine sera, making a total of 55 Lgi1-antibody positives (57% overall).
Contactin-2 is a target for VGKC-antibodies
Only five patients had antibodies to contactin-2 (Supplementary Fig. 2). Two were also Caspr2-antibody positive. Of the other three, one had Lgi1-antibodies, one had Kv1.2 antibodies and one had no other VGKC-complex antibody detected.
VGKC-antibodies show similar localization to their respective antigens in mouse brain tissue
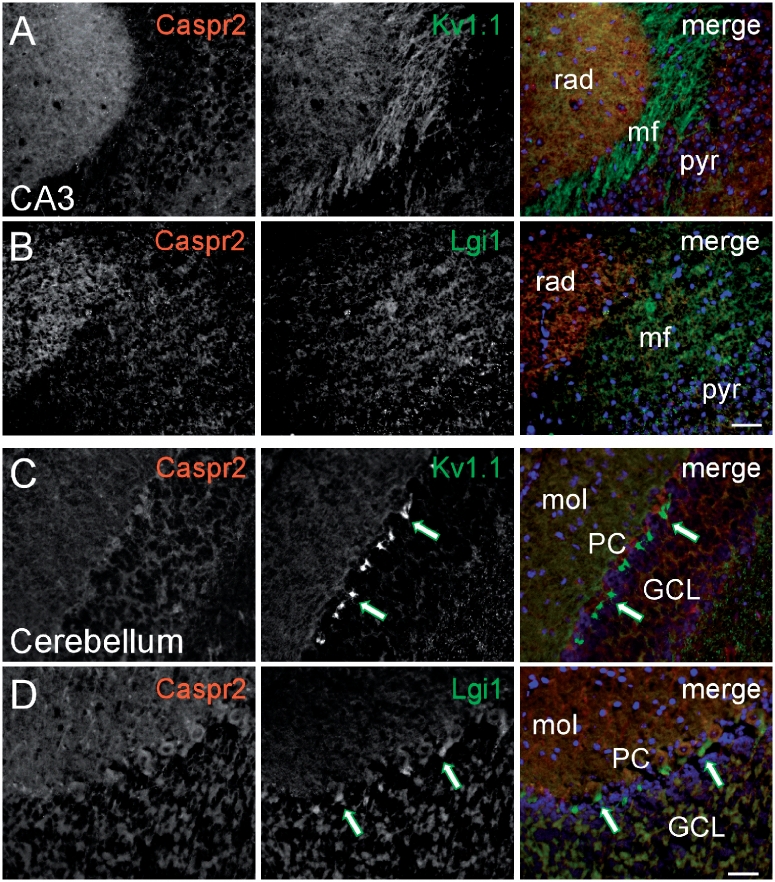
To explore further the relationships between the two main antibody targets, the potassium channels and the clinical syndromes, we first examined the relative distribution of Lgi1, Caspr2 and Kv1.1 in the hippocampus and cerebellum of mouse brains. Lgi1 was expressed strongly in the mossy fibre layer of the CA3-hippocampal subfield (Fig. 5B) as well as in the cerebellar pinceau (Fig. 5D), in a manner similar to Kv1.1 (Fig. 5A and C). By contrast, Caspr2 was expressed more prominently in the stratum radiatum of CA3 (Fig. 5A and B) and molecular and granule cell layers of the cerebellum (Fig. 5C and D), but not in the mossy fibre layer of CA3 or in the cerebellar pinceau.
Figure 5.
Expression pattern of Caspr2 and Lgi1 in the CNS. Black and white images of fixed hippocampal (A and B) and cerebellar (C and D) sections double stained with specific antibodies to Caspr2 (red), Lgi1 (green) and Kv1.1 (green) as indicated in the merged images in the right column where cell nuclei are stained with DAPI (blue). In the CA3 area of the hippocampus, Caspr2 (A and B) is strongly expressed in the stratum radiatum (rad), whereas Kv1.1 (A) and Lgi1 (B) are most prominent in the mossy fibre layer (mf) where Caspr2 is almost absent. In the cerebellum, Caspr2 (C and D) is expressed in the molecular (mol) and granule cell layers (GCL), but not in the pinceau (green arrows) where Kv1.1 (C) as well as Lgi1 (D) are strongly expressed. Scale bar = 20 μm. pyr = pyramidal cell layer; PC = Purkinje cell layer.
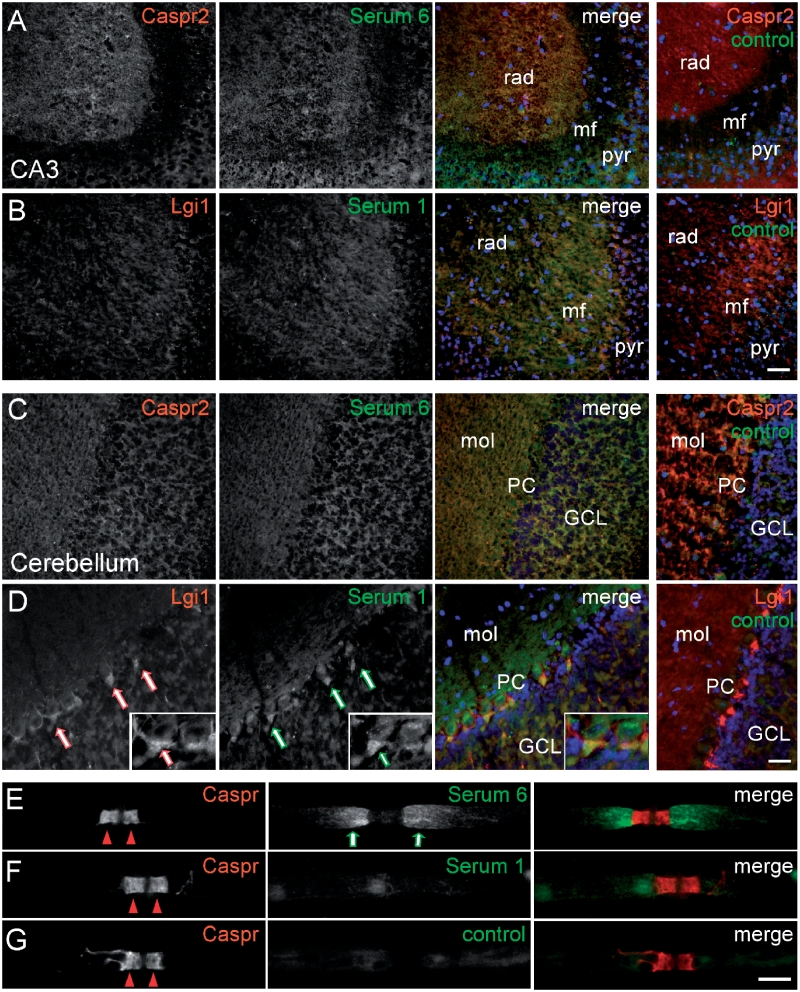
Fourteen sera (10 VGKC-antibody positive; four healthy individuals) were applied to these slices with the observer (K.K.) blind to their antibody status or clinical syndrome. Six sera (all Lgi1-antibody positive) bound with similar localization to that of antibodies to Lgi1, whereas two (both Caspr2-antibody positive) showed strong binding that was similar to that of antibodies to Caspr2 in both the hippocampus and cerebellum, as well as at the juxtaparanodes of teased sciatic nerve fibres (Fig. 6 and data not shown), which are known to express strongly Caspr2 (Poliak et al., 1999) but not significantly Lgi1 (data not shown). The binding of the antibodies in Caspr2-immunoreactive sera was abolished in different neural tissues in Caspr2 knockout mice, including areas where Caspr2 is dissociated from potassium channel expression (Supplementary Fig. 2), confirming their specificity for Caspr2. The remaining six sera (two VGKC-antibody positive and four controls) did not show clear localization with either Lgi1 or Caspr2.
Figure 6.
Binding patterns of Caspr2 and Lgi1 immunoreactive sera in the CNS. Images of fixed hippocampal (A and B) and cerebellar (C and D) sections, or sciatic nerve teased fibres (E–G), immunostained with sera (green) from representative patients with limbic encephalitis/Morvan’s syndrome or healthy controls (far right column in A–D and G) as indicated, combined with specific antibodies (red) to Caspr2 (A and C), Lgi1 (B and D) or Caspr (which localizes to the paranode, E–G). For the limbic encephalitis/Morvan’s syndrome, sera in A–D channels are shown separately as black and white images, as well as merged images to demonstrate reactivity of the sera, while for the control sera only merged images are shown. Cell nuclei are stained with DAPI (blue). In the CA3 area of the hippocampus Serum 6 (A) binds strongly to the stratum radiatum (rad) where Caspr2 is prominently expressed (A) but not to the mossy fibre layer (mf) where Caspr2 is almost absent. In contrast, Serum 1 (B) binds to the mf more than the rad, colocalizing with Lgi1. In the cerebellum, Serum 6 (C) colocalizes with Caspr2 in the molecular (mol) and granule cell layers (GCL), but does not bind to the pinceau where Lgi1 is strongly expressed (red arrows in D) but Caspr2 is absent. In contrast to Serum 6, Serum 1 shows strong binding to the pinceau (green arrows in D) colocalizing with Lgi1 (insets in D show the pinceau at higher magnification). Control sera show no specific binding in CA3 or cerebellum (A–D). In E–G staining of sciatic nerve teased fibres with antibodies to the paranodal marker Caspr (distinct from Caspr2) and patient serum, shows that Caspr2-reactive Serum 6 strongly labels the juxtaparanodes that are known to express Caspr2 but not significantly Lgi1 (green arrows in E), whereas Lgi1-reactive Serum 1 (F), as well as a control serum (G), shows no specific binding. Scale bars: A–D = 20 μm, E–G = 10 μm. Serum 1 was from a typical Lgi1-antibody positive limbic encephalitis patient and Serum 6 was from a Caspr2-antibody positive Morvan’s syndrome patient. Pyr = pyramidal cell layer; PC = Purkinje cell layer.
Clinical significance of Lgi1 and Caspr2 antibodies
In Table 3, we compare the clinical features of all the Lgi1-antibody and Caspr2-antibody patients. Among the 55 patients with Lgi1-antibodies, 49 developed amnesia, confusion, neuropsychiatric disturbance and/or seizures typical of limbic encephalitis. Thirty-one had medial temporal lobe high signal on MRI and 34 had serum hyponatraemia (<135 mM). One had epilepsy only, two had Morvan’s syndrome, only one had isolated neuromyotonia and two had other disorders (Supplementary Table 1). None had active tumours, as determined by whole body MRI/PET/CT imaging.
Table 3.
Clinical features and diagnoses in patients with Lgi1 or Caspr2 antibodies
| Lgi1 (n = 55) | Caspr2 (n = 19) | Caspr2 versus Lgi1 (uncorrected P-values)* | |
|---|---|---|---|
| Male:female | 37 : 18 | 16 : 3 | NS |
| Amnesia | 47 | 10 | 0.002 |
| Confusion/disorientation | 41 | 8 | 0.009 |
| Seizures | 49 | 10 | 0.0004 |
| MRI medial temporal lobe high signal | 31 | 5 | 0.03 |
| Hyponatraemia | 34 | 2 | <0.0001 |
| Neuromyotonia | 2 | 10 | <0.0001 |
| Pain | 6 | 7 | 0.031 |
| Insomnia | 4 | 6 | 0.017 |
| Other sleep disorder | 12 | 0 | 0.028 |
| Any dysautonomia | 8 | 6 | NS |
| Active tumour (thymoma) | 0 | 6 (5) | 0.0002 |
| Weight loss | 2 | 6 | 0.003 |
| Death | 1 | 4 | 0.016 |
| Final diagnosis | |||
| Limbic encephalitis | 49 | 7 | <0.0001 |
| Morvan's syndrome | 2 | 3 | NS |
| Neuromyotonia only | 1 | 7 | 0.0002 |
| Epilepsy only | 1 | 2 | NS |
| Other | 2 | 0 | NS |
| Morvan’s syndrome or neuromyotonia | 3 | 10 | <0.0001 |
*The proportions of patients with each antibody who had particular clinical features were compared by Fishers’ exact test and have not been corrected for multiple comparisons. NS = not significant.
By contrast, although around half of the total number of 19 Caspr2-antibody patients had amnesia, confusion and neuropsychiatric features, which were very frequent in Lgi1-antibody positive patients, seizures were less common and many had neuromyotonia, neuropathic pain, insomnia, dysautonomia and weight loss, which were infrequent in Lgi1-antibody patients. All of the six active tumours (five B2 and B3 thymomas, one endometrial adenocarcinoma) were in Caspr2-antibody patients. The differences in the proportions of these features between the two groups are shown in Table 3, although some would not reach significance after correction for multiple comparisons, due to the small numbers of Caspr2-antibody positive patients and the diversity of their clinical syndromes.
The patients without detectable antibodies to Lgi1, Caspr2, contactin-2 or Kv1.2, included seven with limbic encephalitis, one with epilepsy only, one with neuromyotonia, and nine of the 12 with undefined neurological syndromes (Supplementary Table 1).
Response to treatments and outcomes
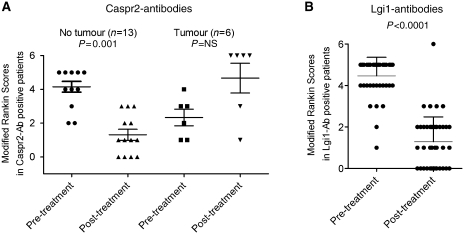
We used the modified Rankin scores at the time of diagnosis and following immunomodulatory therapies to evaluate treatment responses. Although the treatment schedules and follow-up times were highly variable, the modified Rankin scores were reduced by immunotherapies in the Caspr2-antibody patients, except in the six with tumours (Fig. 7A), four of whom died (Table 3). They were substantially reduced in the Lgi1-antibody patients (Fig. 7B), and only one patient died (from an unrelated course).
Figure 7.
Clinical scores before and after treatments in patients with Caspr2- or Lgi1-antibodies. (A) Modified Rankin scores pre- and post-immunotherapies in patients with Caspr2 antibodies with or without tumours. Many of the patients without tumours improved but those with tumours often deteriorated, and four died (modified Rankin scores = 6). (B) Modified Rankin scores in patients with Lgi1-antibodies before and after immunotherapies. The modified Rankin scores were significantly reduced following treatments. Only one Lgi1-antibody positive patient died (modified Rankin scores = 6), and their death was unrelated to the clinical syndrome. The Modified Rankin Scores (0 = asymptomatic patient; 1 = symptoms do not interfere with lifestyle; 2 = symptoms lead to some restriction of lifestyle but do not prevent totally independent existence; 3 = symptoms significantly interfere with lifestyle or prevent totally independent existence; 4 = symptoms clearly prevent independent existence, although patient does not need constant attention day and night; 5 = severe disability, with patient totally dependent and requiring constant attention day and night; 6 = death due to the condition) ranges from 0 (normal) to 6 (death) (Graus et al., 2001).
Discussion
VGKC-antibodies define neurological conditions that are usually immunotherapy-responsive. As a result, these antibodies have become part of the investigation of patients with unexplained subacute onset of epilepsy, memory or cognitive problems, or peripheral nerve hyperexcitability syndromes, but it has been unclear how the antibodies could cause such a range of different clinical presentations. Here, we show that the majority of VGKC-antibodies of high titre are not directed towards the Kv1 subunits themselves but to two proteins, Lgi1 and Caspr2, that are closely associated with VGKCs in brain tissue and remain complexed with the VGKCs in 2% digitonin extracts. Lgi1 antibodies were found almost exclusively in patients with limbic encephalitis or epilepsy, all without tumours, whereas antibodies to Caspr2 were found in patients with limbic encephalitis, Morvan’s syndrome or neuromyotonia, often with thymomas. It is interesting that mutations in genes encoding both these proteins are found in hereditary epilepsy and other disorders (reviewed by Morante-Redolat et al., 2002; Kumar and Christian, 2009), reflecting the fact that genetic and autoimmune conditions often target the same proteins.
An important aim of this work was to study the clinical phenotypes of the patients in parallel with their antibody specificities. We studied 96 patients with high titres of VGKC-antibodies, representative of those referred to us for testing. The clinical follow-ups confirm earlier observations in much smaller studies (Thieben et al., 2004; Vincent et al., 2004; Graus et al., 2008) that many VGKC-antibody positive patients (with titres >400 pM) have CNS disease, that tumours are rare, and that most respond well to immunotherapies. The majority of the patients (67%) had limbic encephalitis with typical clinical presentations; the presence of VGKC-antibodies confirmed this diagnosis in the 41% whose MRIs were normal (Bien and Elger, 2007). Strikingly, there were no detectable active tumours in the Lgi1-antibody positive patients despite a median follow-up of over 3 years. Only five patients were identified as having Morvan’s syndrome, illustrating the rarity of this syndrome; their condition was characterized by pain, often burning in nature, as well as the typical features of neuromyotonia, autonomic disturbance and insomnia. Eleven patients were diagnosed by their neurologists as having neuromyotonia only, but four of these had pain, autonomic dysfunction or insomnia, features that could be ascribed to Morvan’s syndrome, suggesting considerable overlap between the two syndromes.
Previous immunohistological data on a small number of high titre VGKC-antibody positive limbic encephalitis and Morvan’s syndrome sera suggested co-localization with Kv1.1 or 1.2, or occasionally Kv1.6 (Buckley et al., 2001; Liguori et al., 2001; Ances et al., 2005; Antozzi et al., 2005; Kleopa et al., 2006). Having identified the true targets for the antibodies in these patients, it appears that these results were confounded by the very similar localization of Lgi1 and Kv1.1, particularly in the hippocampus, and of Caspr2 and Kv1.2 (Kv1.2 not shown here but can be seen in Kleopa et al., 2006), both in the hippocampus and cerebellum. Moreover, the precipitation of 125I-α-DTX-VGKCs with antibodies to Lgi1 that we show here, confirms earlier experimental data that the two are closely associated (Schulte et al., 2006). Whereas Caspr2 is strongly associated with Kv1.1 and Kv1.2 at juxtaparanodes (Poliak et al., 2003), Lgi1 expression in peripheral nerves is weak but present (Ogawa et al., 2010; KA Kleopa, unpublished data).
It is important to recognize that the antibodies to Lgi1 and Caspr2 were first measured by binding to the surface of unpermeabilized cells, and therefore the antibodies are directed towards ‘cell-surface antigens’ on neurons, defining their pathogenic potential (Vincent et al., 2006; Graus et al., 2010), which was supported by the antibody binding to live hippocampal cultures. At present we do not have an explanation for the 19% of patients in whom we could not identify any VGKC-complex antigen; their lower VGKC-antibody titres (Supplementary Figure 3) suggests that improvements in our antibody assays may reduce the number of sera with undefined target antigens. Conversely, it is highly likely that some patients with limbic encephalitis or Morvan’s syndrome, previously negative for VGKC antibodies, will prove to have Lgi1 or Caspr2 antibodies. Further work is clearly needed.
Lgi1 is an appropriate target for antibodies in limbic encephalitis and epilepsy, and Lgi1 antibodies have recently been confirmed by others (Lai et al., 2010). Mutations in Lgi1 are associated with a distinct form of temporal lobe epilepsy without peripheral nerve dysfunction (Morante-Redolat et al., 2002). It is notable that 91% of the Lgi1-antibody positive patients had limbic encephalitis, consistent with Lgi1’s hippocampal localization, and only one had peripheral symptoms. When we looked for Lgi1 antibodies in the sera, we were initially confused because the patients’ serum IgG bound to all of the Lgi1-transfected human embryonic kidney cells, rather than just to those that were transfected. This was explained by the fact that Lgi1 is secreted from neurons and can then bind to cell surfaces, as shown in neuroblastoma and COS7 cells (Sirerol-Piquer et al., 2006) and now demonstrated in HEK293 cells. Lgi1 binds ADAM22 on CNS postsynaptic membranes (Fukata et al., 2006; Ogawa et al., 2010) but we could not detect ADAM22 in our human embryonic kidney cell cultures (data not shown); further Lgi1 receptors are being identified and might be responsible for our findings (Sagane et al., 2008). Since, we were unable to use EGFP-tagged Lgi1 in immunoprecipitation experiments (data not shown), perhaps because the EGFP-tag interfered with its conformation, to confirm the target of the VGKC-antibodies, we expressed Lgi1 fused to the C-terminal and transmembrane domain of Caspr2; this has provided a more direct and sensitive method for detection of patients’ antibodies and will now be developed for routine use. An EGFP construct will also be made so that the serum antibodies can be quantified by immunoprecipitation.
Caspr2 is a more conventional antigenic target. It is a membrane protein with a large extracellular sequence consisting of multiple well-defined domains (Poliak et al., 1999), and is essential for the co-localization of Kv1.1 and 1.2 at the juxtaparanodes of the nodes of Ranvier. Caspr2-knockout mice show dispersed, non-clustered Kv1 neural expression (Poliak et al., 2003), similar to that seen in one study of patients with Caspr2-mutations (Strauss et al., 2006). It is likely that binding of Caspr2 antibodies results in down-regulation of Caspr2/Kv1.1/1.2 complexes on the peripheral nerve axon, leading to neuromyotonia, neuropathic pain and autonomic dysfunction. How these antibodies cause insomnia, one of the defining features of Morvan’s syndrome, is not clear but mutations in CNTNAP2 (which encodes Caspr2) are associated with epilepsy and cognitive decline as well as peripheral nerve involvement (Strauss et al., 2006), and have also been identified in various forms of schizophrenia, epilepsy and autism spectrum disorders (reviewed in Kumar and Christian, 2009). Although in this cohort, only three of the five patients with Morvan’s syndrome had Caspr2-antibodies, in a further nine patients with Morvan’s syndrome and VGKC-antibodies, the majority had Caspr2-antibodies and six had thymomas (Vincent, 2009 and unpublished observations); it will be interesting to look for Caspr2 expression in these tumours.
Earlier studies suggested that the Kv1s were the target antigens in neuromyotonia (Arimura et al., 1997; Hart et al., 1997), but most of those sera had lower VGKC-antibody titres compared with those studied here. The targets for sera with lower VGKC antibodies should now be re-explored, but from our initial findings (unpublished observations) we anticipate that Kv1s, Caspr2 and contactin-2 will individually, or in combinations, prove to be targets in many patients with VGKC-antibody positive neuromyotonia, and also may be positive in some that are currently negative for VGKC-antibodies.
In the last few years, antibodies to ion channels and a growing number of receptors have been identified in patients with acute or subacute onset of CNS syndromes (Dalmau and Rosenfeld 2008; Vincent et al., 2008; Graus et al., 2010). Our results help explain the diversity of clinical syndromes associated with VGKC-antibodies and suggest that proteins complexed with these receptors and ion channels may also prove to be targets for autoantibodies in patients with these and other autoimmune CNS disorders.
Funding
Much of the preliminary work was funded by a grant from the Dana Foundation. The research described here was supported by a fellowship from the National Institute of Health Research (NIHR), Department of Health, UK (to S.R.I.); Wellcome Trust funded OXION studentship (to S.K.A.); Oxford Biomedical Research Centre (to P.W.); Cyprus Telethon and Research Promotion Foundation (to K.A.K.); European Federation of Neurological Societies (EFNS) fellowship (to L.Z.); Israel Science Foundation and Hanna Hertz Professorial Chair for Multiple Sclerosis and Neuroscience (to E.P.), and Epilepsy Research UK (to B.L.).
Conflict of interest: A.V. and the Department of Clinical Neurology in Oxford receive royalties and payments for antibody assays. A patent has been filed by the University of Oxford claiming Caspr2, Lgi1 and Contactin-2 as targets for autoantibodies.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors thank Dr Domna Karagogeos (University of Crete, Greece), for the gift of plasmid encoding contactin-2. The authors are very grateful to the patients, and to their neurologists who provided the clinical data: Drs A. Grubenac, M. Lawden, J. Ponsford (Birmingham), Drs K. Sieradzan, G. Smith (Bristol), Dr D. Chan (Brighton), Dr T. Hughes (Cardiff), Dr M. Lee (Cambridge), Dr T. Lynch (Dublin), Dr C. Mumford, Prof. R. Will (Edinburgh), Drs J. Greene, R. Metcalfe, C. O’Leary, R. Petty, (Glasgow), Dr N. Davies (Hereford), Dr J. Bowen (Lincoln), Drs I. Hart, B. Lecky, S. Wong (Liverpool), Prof. M. Rossor, Drs J. Rees, P. Rudge, J. Schott, R. Simister, S. Smith, T. Andrews, O. Cockerell, M. Craner, D. Soon, R. deSilva, A. Everitt, J. Gibbs, R. Hadden, P. Jarman, M. Johnson, (London), Drs J. Ealing, D. Gow (Manchester), Dr P. Maddison (Nottingham), Drs J. Adcock, Y. Hart, D. Hilton-Jones, M. Jackson, K. Nithi, J. Palace, K. Talbot, M. Turner, S. Wimalaratna (Oxford), Dr P. Tidswell (Preston), Drs M. Hadjivassilou, B. Sharrack (Sheffield), Drs G. Burke, I. Galea, A. Manson (Southampton), Dr C. Everett (Southend), Drs R. Chalmers, R. Walters (Swansea), Drs C. Mazia, V. Salutto (Argentina), Drs S. Reddel, A. Michell, Prof. E. Somerville (Australia), Drs Bensa, C. Clerc (France), Drs C.G. Bien, F. Hochberg, H. Baezner, S. Zierz, B. Schoser (Germany), Dr A. Kelemen (Hungary), Dr Sostarka (Croatia) Drs R. Liguori, M. Spinazzi, G. Martino, G. Milano, R. Gentile M.-C. Vigliani (Italy), Drs D. Ezpeleta, D. Genis (Spain), Dr P. Lalive (Switzerland) and Prof. R. Karabudak (Turkey). The anti-contactin-2 monoclonal antibody developed by Dr David R Soll was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. The monoclonal antibody against the extracellular domain of Kv1.1 was developed by and/or obtained from the UC Davis/NINDS/NIMH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Pharmacology, School of Medicine, University of California, Davis, CA 95616.
Glossary
Abbreviations
- Caspr2
contactin-associated protein-2
- DTX
dendrotoxin
- EGFP
enhanced green fluorescent protein
- HEK
human embryonic kidney
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- 125I-α-DTX
iodinated alpha-dendrotoxin
- IgG
immunoglobulin G
- Lgi1
leucine-rich, glioma inactivated 1 protein
- VGKC
voltage-gated potassium channel
References
- Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antozzi C, Frassoni C, Vincent A, Regondi MC, Andreetta F, Bernasconi P, et al. Sequential antibodies to potassium channels and glutamic acid decarboxylase in neuromyotonia. Neurology. 2005;64:1290–3. doi: 10.1212/01.WNL.0000156945.39471.2C. [DOI] [PubMed] [Google Scholar]
- Arimura K, Watanabe O, Kitajima I, Suehara M, Minato S, Sonoda Y, et al. Antibodies to potassium channels of PC12 in serum of Isaacs' syndrome: western blot and immunohistochemical studies. Muscle Nerve. 1997;20:299–305. doi: 10.1002/(SICI)1097-4598(199703)20:3<299::AID-MUS6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Barajas RF, Eric Collins D, Cha S, Geschwind MD. Adult-onset drug-refractory seizure disorder associated with anti-voltage-gated potassium-channel antibody. Epilepsia. 2009;51:473–7. doi: 10.1111/j.1528-1167.2009.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG, Elger CE. Limbic encephalitis: a cause of temporal lobe epilepsy with onset in adult life. Epilepsy Behav. 2007;10:529–38. doi: 10.1016/j.yebeh.2007.03.011. (Review) [DOI] [PubMed] [Google Scholar]
- Buckley C, Oger J, Clover L, Tuzun E, Carpenter K, Jackson M, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–8. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- Caleo M. Epilepsy: synapses stuck in childhood. Nat Med. 2009;15:1126–7. doi: 10.1038/nm1009-1126. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327–40. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313:1792–5. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- Geschwind MD, Tan KM, Lennon VA, Barajas RF, Jr, Haman A, Klein CJ, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol. 2008;65:1341–6. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Keime-Guibert F, Rene R, Benyahia B, Ribalta T, Ascaso C, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- Graus F, Saiz A. Dalmau J.Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509–17. doi: 10.1007/s00415-009-5431-9. [DOI] [PubMed] [Google Scholar]
- Graus F, Saiz A, Lai M, Bruna J, López F, Sabater L, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology. 2008;71:930–6. doi: 10.1212/01.wnl.0000325917.48466.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart IK, Waters C, Vincent A, Newland C, Beeson D, Pongs O, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol. 1997;41:238–46. doi: 10.1002/ana.410410215. [DOI] [PubMed] [Google Scholar]
- Irani SR, Buckley C, Vincent A, Cockerell OC, Rudge P, Johnson MR, et al. Immunotherapy-responsive seizure-like episodes with potassium channel antibodies. Neurology. 2008;71:1647–8. doi: 10.1212/01.wnl.0000326572.93762.51. [DOI] [PubMed] [Google Scholar]
- Irani SR, Waters P, Kleopa KA, Lang B, Vincent A. Antibodies to components of the voltage-gated potassium channel - associated complex: LGI1 and CASPR2 as antigenic targets in limbic encephalitis, Morvan’s and neuromyotonia. Neurology. 2010 (in press) (Abstract) [Google Scholar]
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-Methyl-D-Aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–67. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol. 2004;21:440–5. doi: 10.1097/00004691-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129:1570–84. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–97. doi: 10.1007/s11910-009-0029-2. (Review) [DOI] [PubMed] [Google Scholar]
- Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010 doi: 10.1016/S1474-4422(10)70137-X. [Epub 28 June 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain. 2008;131:1940–52. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al. Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124:2417–26. doi: 10.1093/brain/124.12.2417. [DOI] [PubMed] [Google Scholar]
- McKnight K, Jiang Y, Hart Y, Cavey A, Wroe S, Blank M, et al. Serum antibodies in epilepsy and seizure-associated disorders. Neurology. 2005;65:1730–6. doi: 10.1212/01.wnl.0000187129.66353.13. [DOI] [PubMed] [Google Scholar]
- Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel alpha and beta subunits in rat hippocampal formation. J Neurosci. 2001;21:5973–83. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, Saenz A, Poza JJ, Galan J, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–28. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- Nagado T, Arimura K, Sonoda Y, Kurono A, Horikiri Y, Kameyama A, et al. Potassium current suppression in patients with peripheral nerve hyperexcitability. Brain. 1999;122(Pt 11):2057–66. doi: 10.1093/brain/122.11.2057. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, et al. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–47. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–60. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagane K, Ishihama Y, Sugimoto H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int J Biol Sci. 2008;4:387–96. doi: 10.7150/ijbs.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte U, Thumfart JO, Klocker N, Sailer CA, Bildl W, Biniossek M, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49:697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38:714–22. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- Sinha S, Newsom-Davis J, Mills K, Byrne N, Lang B, Vincent A. Autoimmune aetiology for acquired neuromyotonia (Isaacs' syndrome) Lancet. 1991;338:75–7. doi: 10.1016/0140-6736(91)90073-x. [DOI] [PubMed] [Google Scholar]
- Sirerol-Piquer MS, Ayerdi-Izquierdo A, Morante-Redolat JM, Herranz-Perez V, Favell K, Barker PA, et al. The epilepsy gene LGI1 encodes a secreted glycoprotein that binds to the cell surface. Hum Mol Genet. 2006;15:3436–45. doi: 10.1093/hmg/ddl421. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–7. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–90. doi: 10.1212/01.wnl.0000312275.04260.a0. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62:1177–82. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- Tomimitsu H, Arimura K, Nagado T, Watanabe O, Otsuka R, Kurono A, et al. Mechanism of action of voltage-gated K+ channel antibodies in acquired neuromyotonia. Ann Neurol. 2004;56:440–4. doi: 10.1002/ana.20221. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Vincent A. Autoimmune channelopathies: John Newsom-Davis's work and legacy. A summary of the Newsom-Davis Memorial Lecture 2008. J Neuroimmunol. 2008;201–202:245–9. doi: 10.1016/j.jneuroim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- Vincent A, Lang B, Kleopa KA. Autoimmune channelopathies and related neurological disorders. Neuron. 2006;52:123–38. doi: 10.1016/j.neuron.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Vincent A. Antibodies to contactin-associated protein 2 (Caspr2) in thymoma and Morvans Syndrome. Ann Neurol. 66(Suppl. 13):S3. (Abstract) [Google Scholar]
- Zhou YD, Lee S, Jin Z, Wright M, Smith SE, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–14. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.