Abstract
Screening a phage-display single-chain antibody library for binding to the breast cancer cell line PM-1 an antibody, scFv173, recognising activated leukocyte cell adhesion molecule (ALCAM, CD166) was isolated and its binding profile was characterized. Positive ALCAM immunohistochemical staining of frozen human tumour sections was observed. No ALCAM staining was observed in the majority of tested normal human tissues (nine of ten). Flow cytometry analyses revealed binding to 22 of 26 cancer cell lines of various origins and no binding to normal blood and bone marrow cells. Antibody binding inhibited invasion of the breast cancer cell line MDA-MB-231 by 50% in an in vitro Matrigel-coated membrane invasion assay. Reduced growth of tumours in nude mice was observed in an in vivo model in which the mice were injected subcutaneously with colorectal carcinoma HCT 116 cells and treated with scFv173 when compared to control. In summary, we have characterized a novel fully human scFv antibody recognising ALCAM on cancer cells and in tumour tissues that reduces cancer cell invasion and tumour growth in accordance with the hypothesised role for ALCAM in cell growth and migration control.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0892-3) contains supplementary material, which is available to authorized users.
Keywords: Single-chain antibody, CD166, ALCAM, Matrigel invasion, In vivo tumour growth
Introduction
An antibody molecule has great potential in diagnosis and treatment of diseases. Currently 22 monoclonal antibodies have been approved for therapeutic use in the United States [1] and more than 200 are in clinical development [2]. Nine of these have been approved for use in cancer therapy [2]. Development of protein engineering technology has made it possible to tailor-make antibody molecules with size, pharmacokinetics, immunogenicity, specificity, valence and effector function for the intended application [3]. Only a small fraction of the antibodies evaluated in clinical studies are antibody fragments, and of the 54 fragments currently studied 19 are single-chain fragment V (scFv) antibodies [1].
In the present study a human antibody phage library was screened to identify breast cancer-specific scFv antibodies. The breast cancer cell line PM-1 was used as target cell line for selection of antibodies recognising natural folded epitopes on the cell surface. Here we report the characterisation of one scFv (scFv173) antibody found to bind to the cell adhesion molecule CD166/ALCAM; a transmembrane glycoprotein member of the immunoglobulin superfamily. The extracellular part of ALCAM contains five immunoglobulin (Ig) domains followed by a transmembrane region and a short cytoplasmic tail. ALCAM form heterodimers with CD6 [4] or ALCAM-ALCAM homodimers [5] and is broadly expressed in human normal tissue and in a number of cancer cell lines. Altered ALCAM expression has been reported in different human tumour types such as melanoma and breast cancer [6]. The scFv173 antibody does not stain normal human tissues except for weak staining of one of four normal breast samples. The scFv 173 antibody inhibited breast cancer cell invasion in Matrigel invasion assay, and showed no effect on cell growth or cell to matrix-protein adhesion. In a mouse model, treatment with the scFv173 antibody reduced tumour growth of the human colorectal carcinoma cell line HCT 116.
Methods and materials
Cells
The human breast carcinoma cell line PM-1 was established in our laboratory (Department of Tumor Biology, Institute for Cancer Research) in 1992 and originates from pleural fluid aspirated from a breast cancer patient with advanced disease treated at the Norwegian Radium Hospital.
Human carcinoma cell lines BÜX [7], OHS [8], FEMX [9], EKVX [10], PC-MM1 (kindly provided by Klaus Pantel, Germany), OSA (kindly provided by Dr. A Thomas Look, St Jude’s hospital, Memphis, USA), SF-295 and U-138MG [11], MA11 [12] and D54MG [13] were cultured in RPMI-1640 medium (BioWhittaker, Verviers, Belgium) supplemented with 10% FCS (Gibco BRL, Paisley, UK), 2 mM l-glutamine (BioWhittaker, Verviers, Belgium) and 10 mM HEPES (BioWhittaker, Verviers, Belgium). Other cell lines, BT-474, T-47D, MCF-7, MDA-MB-231, SK-BR-3, HT-29, HCT 116, HTB-182, H146, SK-MEL-28, SW900, HeLa, OVCAR-3, SKOV-3, DU145, LNCaP and LL 86 were obtained from The American Type Culture Collection (ATCC) and cultivated as described above. Bone marrow cells were aspirated from the iliac crest of healthy adult donors with their informed consent and the approval of the Regional Ethics Committee (REK # S-90128).
Materials
The monoclonal IgG anti-ALCAM antibody MAB656 was purchased from RnD Systems (Abingdon, UK). Laminin, fibronectin and BSA were all from Sigma–Aldrich (St Louis, MO), gambogic acid (GA) from Biomol International (PA), N-hydroxysuccinimidyl-6-biotinamido hexanoate from Vector Laboratories (CA), and FITC-conjugated Streptavidin from Amersham Biosciences (GE Healthcare, Oslo, Norway). Human tumour samples (REK #2.2006.2342) and murine normal tissue were from Oslo University Hospital Radiumhospitalet, and normal human tissue purchased from Biochain (CA) or TriStar Technology Group (MD). Buffers, streptavidin–HRP and 3′,3′-diaminobenzidine (DAB) substrate used for immunochemistry were all from DAKO (Denmark). The scFv antibodies 173 and 141 were generated in collaboration with Affitech AS, Oslo, Norway as described below. Other chemicals were purchased from Merck (Germany).
Cell-based antibody selection (CBAS)
Antibody candidates recognising CD166/ALCAM were isolated from a human recombinant antibody library [14] using a cell-based screening method described elsewhere [15]. The candidates were analysed, and the most promising clones were improved in soluble scFv format for increased characteristics, resulting in the generation of scFv173 and scFv141.
Expression and purification of scFv in E. coli
ScFvs clones 141 and 173 were expressed for screening, analysis and purification in the secretion vector pHOG21 [16] and also purified according the methods described there.
For micro-scale expression, clones were expressed in 96 deep well format (100 μl volume) overnight at 30°C with 0.1 mM IPTG using the pHOG 21 system. The next day the plates were centrifuged (10 min at 3,220×g, 4°C) and the supernatants containing the expressed scFv were used in further experiments without further purification.
Biotinylation
Biotinylation was performed according to Warren et al [17]. After diluting the scFv in PBS to 800 μg/ml, fivefold molar excess of N-hydroxysuccinimidyl-6-biotinamido hexanoate (1 mg/ml in DMSO) was added, followed by 0.5 M sodium borate (pH 8) to a final concentration of 0.1 M. The mixture was incubated for 2 h at 37°C. The reaction was blocked by addition of 1 M Tris (pH 8) to 0.2 M final concentration and BSA was added to 0.1%. Free biotin was removed by dialysis overnight against 50 mmol/L Tris–HCl, 150 mmol/L NaCl, 0.5 g/L NaN3 (pH 7.8). The protein concentration was measured using the BCA assay (Pierce, Thermo Fisher Scientific, IL).
FACS staining
5 × 105 cells were stained with biotinylated scFv173 (0.4 μg) in ice-cold RPMI/10% FCS for 30 min at 4°C with rotation. After washing 3 times with ice-cold RPMI/10% FCS, the cells were incubated with FITC-conjugated streptavidin (1:75 in PBS/10% FCS) for 1 h at 4°C. Cells were washed three times as described above and analysed on a FACS (BD LSR II system, BD Biosciences, Franklin Lakes, NJ). Data acquisition and analysis was performed using CELLQuest software (BD Biosciences, Franklin Lakes, NJ).
Immunohistochemistry
Immunohistochemical studies were carried out with the biotinylated scFv173 on human normal and tumour samples, and on murine tissue sections.
Frozen tissue sections were air dried and stored frozen. After thawing, the slides were fixed for 10 min in ice-cold acetone, washed with buffer and blocked for 10 min at room temperature (RT) with biotin blocking system part one. After washing thoroughly, the sections were incubated for 10 min at RT with biotin blocking system part two. After washing thoroughly, sections were incubated with biotinylated scFv173 (16 μg/ml in ChemMate antibody diluent) overnight at 4°C. Next day the sections were washed, incubated with HRP–streptavidin complex for 1 h at RT and subsequently washed before the sections were stained with DAB + chromatin substrate, counterstained with hematoxylin and mounted. Negative control sections were treated as described above except for the incubation with primary antibody.
Cell viability assay (MTS assay)
The CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was used to determine the number of viable cells in proliferation assays as described by the manufacturer. Briefly, the cells were seeded in 96-well plates in a concentration optimised for each cell line. Next day the medium was replaced with serum-reduced growth medium added antibodies in the range from 1 μM to 1 pM. The cells were incubated as indicated in results, medium was changed and MTS added to each well. The plates were incubated for 1–4 h at 37°C before the absorbance was read at 490 nm in a Wallac Victor2 plate reader (Perkin Elmer, Waltham, MA).
Adhesion
Cells were incubated with scFv antibodies at 4°C on a rotating device for 30 min. The cells were then seeded into a 96-well plate pre-coated for 2 h at 37°C with either laminin or fibronectin at 1 μg protein per cm2. After seeding, the cells were allowed to adhere for 1 h before half of the wells are washed twice with PBS. The plates were incubated either at 4- or at 37°C since the scFv173 antibody is rapidly internalized at 37°C (Online resource, Fig. 4). At 4°C the internalization of membrane proteins is highly reduced. The number of cells was quantified using the MTS assay and incubated for 1–4 h at 37°C before the plates were read as described over. Adhesion of antibody-treated cells were represented as the ratio of bound cells (washed wells) and total number of cells seeded (not washed), and compared to the ratio obtained for control cells without antibodies added. The number of cells seeded and the adhesion time was optimised for each cell line.
Invasion
BD BioCoat Matrigel invasion chambers (BD Biosciences, Franklin Lakes, NJ) were used essentially as described by the manufacturer. The cells were labelled with 3H-thymidine (1:200 dilution of 1 mCi stock) for 24 h before they were detached using EDTA. The cells were resuspended in RPMI medium with 4% FCS and mixed with antibodies before 5 × 104 cells were seeded in the upper part of the invasion chamber. The bottom wells contain RPMI medium with 4% FCS. After 48 h incubation cotton tip swabs were used to harvest the cells on each side of the filter and the samples counted in a scintillation counter. The ratio of invading cells over non-invading cells was compared between the various treatments. The anti-ALCAM antibody (RnD systems, Abingdon, UK) was used in a concentration of 10 μg to 1 × 105 cells. The amount of antibody is the same as recommended by the manufacturer for use in an adhesion blockade assay. The scFv antibodies were used in comparable dose (20 μg to 1 × 105 cells) to compensate for lower avidity since these antibodies only have one binding site per molecule.
Animal model
Human HCT 116 colorectal carcinoma cells, 2.5 × 106 cells in 200 μl buffer, were injected subcutaneously (s.c.) into the flank of in-house breed Balb-c nu/nu mice on day 1. Antibody treatment started on day 2 and was repeated on days 3–5 and days 8–12 (in total 9 antibody injections). The scFv173 antibody was injected s.c. on one flank and for control, 200 μl antibody storage buffer (PBS with 50 mM imidazole and 1% BSA) was injected on the other side. In another experiment, tumour cells were injected on one side only to exclude influence of the treatment on the cells on the other side and the mice were divided in two groups treated either with buffer or with antibody. The amount of antibody per injection is described in the following section. Housing and all procedures involving animals were performed according to protocols approved by the animal care and use committee, in compliance with the National Committee for Animal Experiments guidelines on animal welfare.
Results
Antigen detection on cells and tissues
ScFv173 was characterized using flow cytometry with respect to its binding to various normal and tumour cell lines, and freshly isolated human cells. As shown in Table 1, scFv173 bound to all the breast tumour cell lines, as well as to the majority of the other cancer cell lines tested. The antibody did not bind to normal bone marrow cells, but binding was observed to the human fibroblast cell line LL86 in accordance with ALCAM expression on fibroblasts [18].
Table 1.
FACS analysis showing the binding of the biotinylated scFv 173 to breast cancer cell lines, normal bone marrow, and cancer cell lines of different origin
| Tumour tissue | Cell line | scFv 173a |
|---|---|---|
| Breast | MCF-7 | + |
| PM1 | ++ | |
| SK-BR-3 | ++ | |
| MA11 | +++ | |
| BT-474 | +++ | |
| MDA-MB-231 | >+++ | |
| T-47D | >+++ | |
| Colon | HT-29 | ++ |
| HCT 116 | +++ | |
| Skin | FEMX | – |
| SK-MEL-28 | + | |
| Lung | BÜX | – |
| HTB-182 | ++ | |
| EKVX | >+++ | |
| SW900 | >+++ | |
| H146 | >+++ | |
| Bone | OHS | – |
| OSA | >+++ | |
| Cervix | HeLa | – |
| Ovary | SKOV-3 | + |
| OVCAR-3 | ++ | |
| Prostate | PC-MMI | + |
| LNCaP | ++ | |
| DU145 | ++ | |
| Brain | SF-295 | ++ |
| D54MG | ++ | |
| U-138MG | +++ |
| Normal tissue | Cell line | scFv 173 |
|---|---|---|
| Fibroblast | LL 86 | >+++ |
| Bone marrow | NBM I | – |
| NBM II | – | |
| NBM III | – |
aThe notions +, ++, +++ and >+++ represent respectively 1.5–3×, 3–5×, 5–10× and >10× increase in median binding signal over the negative control with streptavidin–FITC. For an example see Online resource, Fig. 2
Staining of frozen tumour tissue was positive in tumours of different histologies. The table in Fig. 1a summarises results from immunohistochemical (IHC) staining of different tumour tissues and in Fig. 1b examples of positive staining of breast (i), prostate (ii) and ovarian (iii) tumours are shown. IHC staining of human (Online resource, Table 1) or murine frozen normal tissue sections did not show any positive staining except for weak staining of normal human breast. Four different breast samples were stained and of these three were negative and one was positive. Fifty-two patient samples from a panel of breast cancer biopsies [19] were compared for positive scFv173 staining. IHC staining was scored on a scale from 0 to 3+, where 0 was no staining and grades 1+ to 3+ represented increasing staining as described previously [19]. Since the number of samples in each group was small, the results were further grouped into a negative group (21 samples) where the staining was recorded as 0 or 1+ and a positive group (31 samples) for staining of 2+ or 3+. The histological grades were only known for 36 samples. In the negative IHC group, 11 of 17 samples were histological grade 3, compared to 4 of 19 samples in the positive group (Table 2), showing that loss of positive ALCAM staining correlated with a more severe histological tumour grade (p = 0.029, Fisher’s exact test).
Fig. 1.
Immunohistochemical ALCAM staining of frozen human tumour tissue biopsies. Positive staining (a) was observed in 46 of 52 representative breast tumour samples, in all prostate tumour samples, and in 11 of 13 ovarian tumour samples. In b examples of positive staining of breast (i), prostate (ii) and ovarian (iii) tumour tissue are shown. IHC staining was scored on a scale from 0 to 3+ where 0 was no staining and grades 1+ to 3+ represented increasing staining
Table 2.
Cross-tabulation of scFv173 IHC staining and histological grading of breast tumour samples
| IHC staining | Histological gradea | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| scFv173 negative | 1 | 5 | 11 | 17 |
| scFv173 positive | 4 | 11 | 4 | 19 |
| Total | 5 | 16 | 15 | 36 |
aOther prognostic factors investigated were age, side, lymph node metastasis, distant metastasis, death, and tumour diameter. None of these factors associated with scFv173 staining
Cell growth
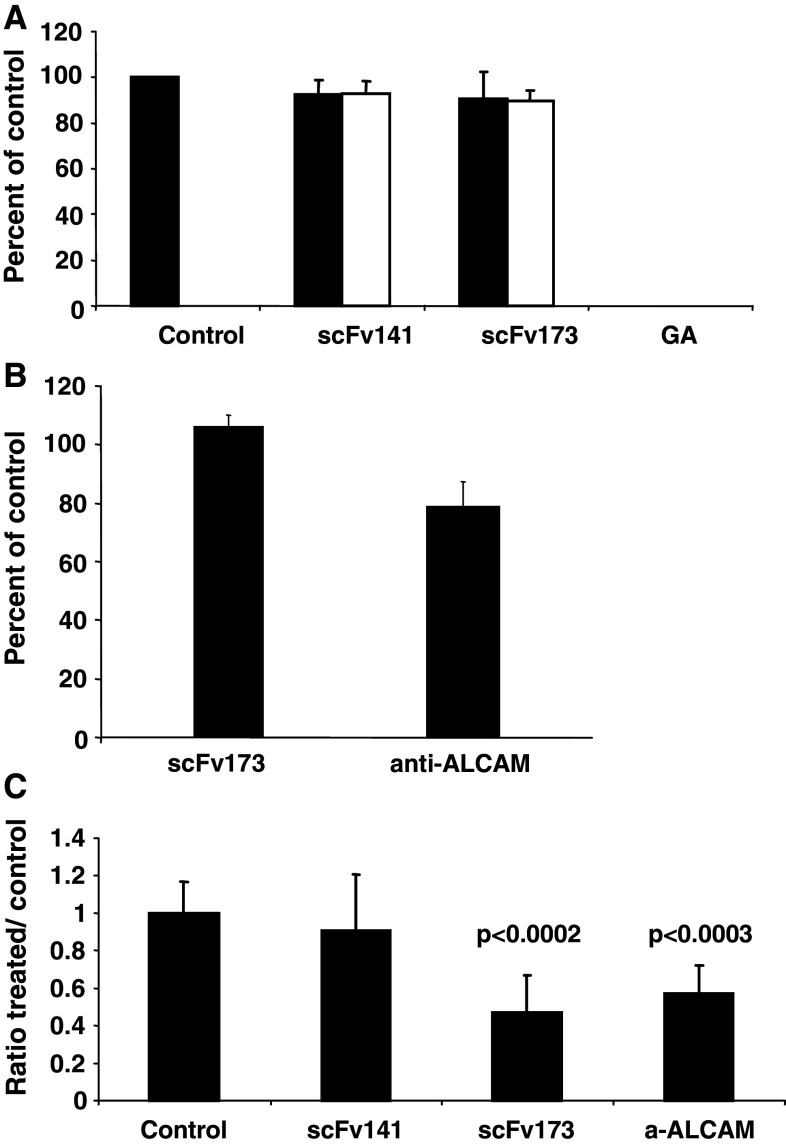
The potential effect of the scFv173 antibody on different aspects of cell growth was tested and no significant effects on DNA synthesis (3H-thymidine labelling), or on cell viability (MTS assay), or on the ability of single cells to form colonies in a clonogenic assay were observed. As an example, results of testing MDA-MB-231 cells are shown in Fig. 3a. We conclude that, under the experimental conditions used, the scFv173 antibody did not influence cell growth or colony formation.
Fig. 3.
Effects of scFv173 on MDA-MB-231 cell viability, adhesion and Matrigel invasion assay. a Breast cancer MDA-MB-231 cells were added antibody, scFv173 or scFv141, to a final concentration of 1 μM (black bar) or 1 nM (white bar) and the cell viability measured after 48 h in a MTS assay. Control cells were grown with ordinary medium or added gambogic acid (GA) known to induce apoptosis in tumour cell lines. b Cell adhesion to laminin at 4°C as previously described using MDA-MB-231 cells. c 3H-thymidine labelled MDA-MB-231 cells were mixed with antibodies prior to seeding and harvested after 48 h. The ratio of invading cells over non-invading cells in treated wells are normalised to the ratio in untreated wells. The bars represents the average with standard deviation of 2 (a, b) or 3 (c) independent experiments with triplicate wells for each treatment. p values were determined using a two tailed t test comparing controls and scFv173-treated cells or controls and anti-ALCAM treated cells
Adhesion
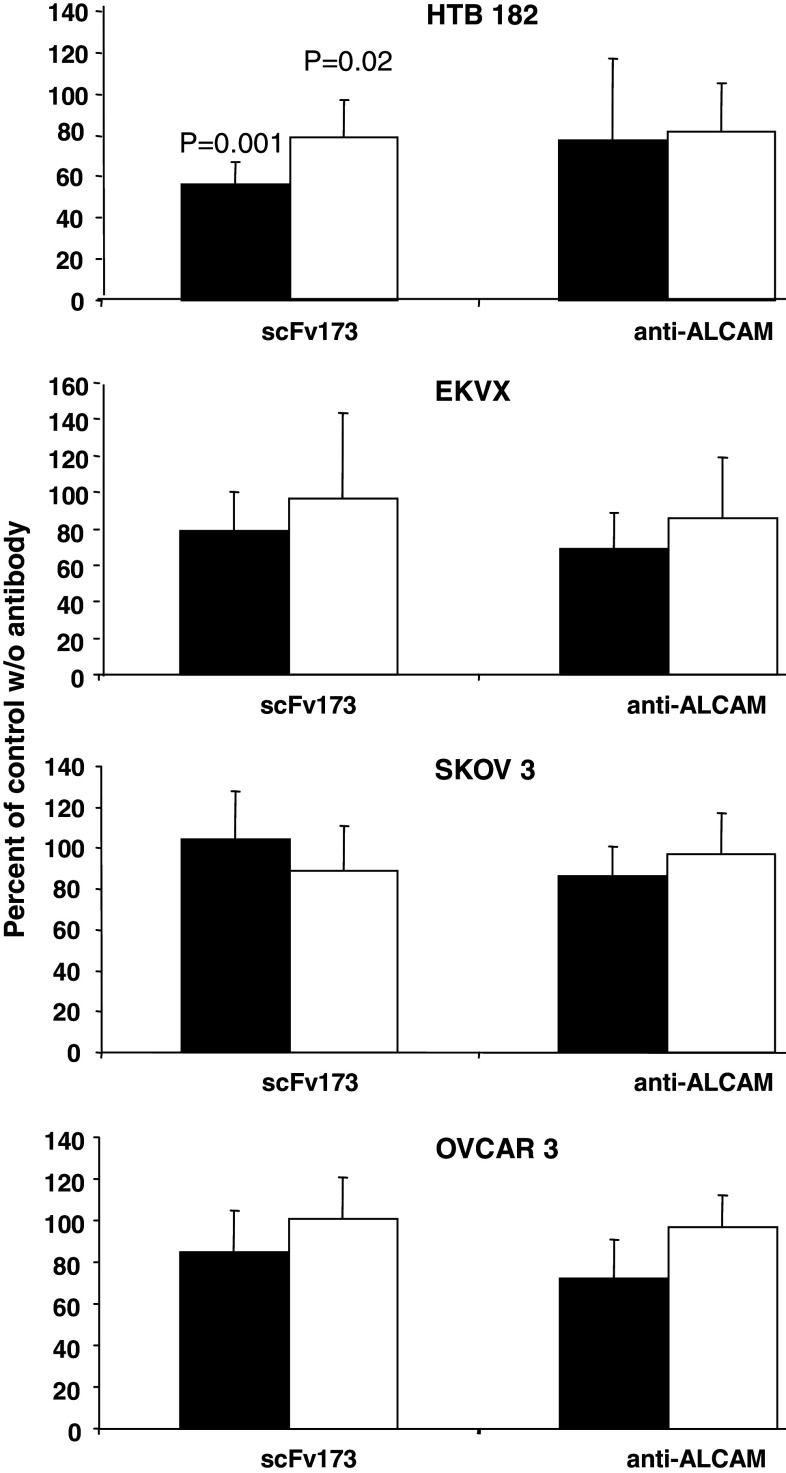
We investigated whether addition of scFv173 to various cell lines influenced the ability of the cells to adhere to the extracellular matrix proteins laminin or fibronectin since ALCAM is classified as an adhesion molecule. A commercially available anti-ALCAM antibody was used as positive control. The cells were preincubated with the antibodies at 4°C before seeded in laminin or fibronectin coated wells. Decreased adhesion to laminin by 44 ± 11% (SD) at 4°C, compared to the control without antibodies added, was observed in the non-small cell lung cancer cell line HTB-182 (Fig. 2, p = 0.001). Adhesion at 37°C was also slightly reduced (p = 0.02). In addition, the non-small cell lung cancer cell line EKVX and two ovarian cancer cell lines, OVCAR3 and SKOV3, were tested. These cell lines express different amounts of ALCAM (Table 1) and no statistically significantly altered adhesion to laminin was observed with any of the three cell lines. We conclude that, although scFv173 reduced the adhesion of HTB182, this antibody most likely does not inhibit adhesion of cells to extracellular matrix proteins. Adhesion of the four cell lines to fibronectin were not statistically significantly affected by scFv173 (Online resource, Fig. 4).
Fig. 2.
Effects of scFv173 on adhesion of cells to extracellular matrix proteins. Adhesion assay of cells (HTB-182, EKVX, OVCAR-3 or SKOV-3) preincubated with antibodies and seeded in 96-well tissue culture plates coated with laminin. The cells were allowed to adhere for 1 h before half of the wells were washed twice with PBS and the number of cells in each well quantified using MTS. Inhibition of cell adhesion to laminin is represented as the ratio of MTS measurement in wells washed with PBS and wells that were not washed. Black bars shows adhesion at 4°C and white bars adhesion at 37°C. For comparison the ratio of control wells without antibodies (scFv173 or anti-ALCAM from RnD systems) added is 100%. Five independent experiments, each with triplicate wells
Invasion
Invasion studies were performed in BD BioCoat Matrigel invasion chambers using the highly invasive breast cancer cell line MDA-MB-231. Prior to this we found that neither cell viability nor adhesion to laminin of these cells were affected by scFv173 (Fig. 3a, b). However, scFv173 inhibited invasion of MDA-MB-231 cells by approximately 50%, in three independent experiments, compared to that observed in the controls without antibody (Fig. 3c). The monoclonal anti-ALCAM antibody did reduce the invasion to the same extent as scFv173. Another single-chain Fv antibody scFv141, targeting the transferrin receptor and isolated in the same screening as scFv173, did not significantly alter the invasion when compared to the buffer control.
To investigate whether scFv173 inhibited invasion of the other cell lines used in the adhesion assay their ability to migrate was tested. After 48 h around 45% of the seeded OVCAR-3 cells migrated to the bottom of the filter, 10% of HCT 116 cells and only 3% of the HTB-182 cells. In the Matrigel assay there was a tendency that scFv173 decreased also the invasion of OVCAR cells, although statistical significance was not obtained (Online resource, Fig. 5). No invasion through Matrigel was observed with HCT 116 colorectal carcinoma cells.
In vivo activity
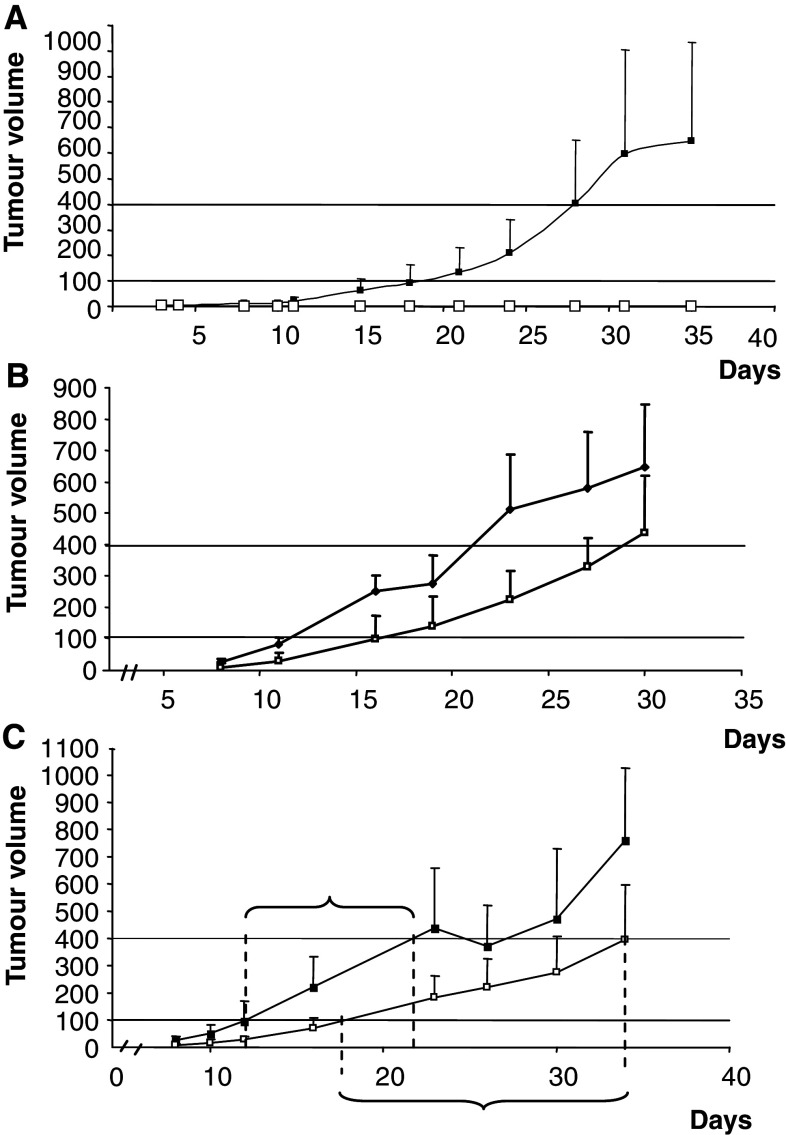
Effects of the fully human scFv173 single-chain antibody on tumour formation were tested in a Balb-c nu/nu model where the antibody was injected subcutaneously in vicinity to the forming tumour. We chose to use the colorectal carcinoma cell line HCT 116 since the tumour take rate and growth are fast [20]. HCT 116 cells express ALCAM (Table 1) and three of five tested colon tumour tissues displayed strong positive ALCAM staining in IHC (Fig. 1). A pilot experiment was performed where three mice were injected with cells on both flanks and next day 200 μg antibody or buffer was injected, and the treatment was repeated every day for 8 days during the first 2 weeks. No tumour take was observed in any of the three flanks treated with antibody, but the flanks treated with buffer only showed tumour take and measurable growth already on day 4 (Fig. 4a). When the mice were killed on day 35 there were still no tumours on the antibody-treated flanks. The experiment was repeated with eight animals treated with 1/10 of the initial antibody dose (20 μg antibody per injection) or buffer control. Even with this low antibody dose reduced growth of the tumours treated with antibody was observed (Fig. 4b). In an independent experiment, experimental groups of six mice were injected with cells on one flank only and treated with buffer or antibody to exclude influence of the treatment on the cells on the opposite flank. On day 34 after cell injection four mice in the control group were killed, since the tumour had reached the maximum tolerated size, compared to one animal treated with scFv173. Reduced tumour volume was observed (Fig. 4c) and, as indicated on the figure, the tumours in the antibody-treated group increased from 100 to 400 mm3 in approximately 16 days compared to 8 days in the control group, suggesting that the scFv173 inhibited both tumour onset and growth in the HCT 116 colorectal cancer model.
Fig. 4.
Inhibitory effect of scFv173 on in vivo tumour growth. The colon cell line HCT 116 and the scFv173 antibody were injected s.c. in Balb-c nu/nu mice. a–c shows the increase in average tumour volume with standard deviation of buffer treated (black box) or scFv173 treated (white box) tumours followed from injection of cells until the mice were killed. The scFv173 antibody was dissolved in PBS pH 7.4 added 50 mM imidazole and 1% BSA and this buffer was used as control. The bars represent the standard deviation
Discussion
Our overall goal in this project was to identify antibodies for possible use in diagnostics and therapy utilizing phage-display technology and cell-specific antibody selection. Two high-affinity antibodies scFv173 and scFv141 were initially characterized; respectively recognising the ALCAM molecule and the transferrin receptor. The ALCAM antibody was most promising and initial characterisation using flow cytometry revealed binding to most of the cancer cell lines tested, and in addition to LL 86, fibroblasts isolated from normal tissue from an osteosarcoma patient. Evaluation using IHC revealed cancer-specific binding and a correlation between ALCAM positivity and histological tumour grade. The scFv173 antibody showed two unique features; it inhibited breast cancer cell invasion through Matrigel and reduced tumour growth in a mouse model.
To identify novel antibodies other groups have used similar approaches to ours and isolated antibodies targeting both antigens. For example did 84 antibodies react with 21 distinct antigens expressed on various carcinomas, among these antibodies 11 recognised ALCAM and 6 the transferrin receptor [21] showing that these proteins are highly antigenic and over-expressed in carcinomas. ALCAM scFv antibodies have also been produced after panning of ovarian [22] and prostate [23] cancer cells. These antibodies were selected for internalization and have been used as immunotoxin [22] or for delivery of liposomal drugs [24]. Our antibody fulfils two important prerequisites for use as targeting molecule to cancer cells; it is internalized (Online resource, Fig. 6), and, importantly, it does not stain normal human tissues except for weak staining of one of four normal breast samples. Another anti-ALCAM scFv was also found negative for normal tissue staining except for bronchial epithelial staining [25]. The antibody libraries were in both cases pre-screened for binding to normal cells to exclude those antibodies reacting with common antigenes. It seems that most antigens are expressed in a low basal level by normal cells and that transformation into tumour cells results in overexpression of these antigens. In this context, scFv antibodies have an advantage compared to monoclonal antibodies in that they have one antigen binding site and thus lower avidity which may result in less binding to normal cells with low expression of the target. To increase tumour specificity it is possible to engineer antibody fragments recognising more than one antigen. Other advantages of using scFv antibodies for therapy instead of conventional antibodies are that the smaller size allows deeper and more rapid penetration into the tumour, and binding to epitopes not accessible to full size antibodies. Due to the small size of the scFv antibodies they are rapidly cleared from the circulation. Under some circumstances this is an advantage, for example to reduce toxicity when the scFv is used as immunotoxin, but this may also be a disadvantage since too few molecules may reach the target site to obtain a therapeutic relevant dose [1].
The scFv173 antibody inhibited Matrigel invasion of MDA-MB-231 cells and in vivo HCT 116 tumour growth. This is in agreement with effects observed when soluble ALCAM [26] or truncated ALCAM [27] were over-expressed in metastatic melanoma cells. Expression of soluble ALCAM made the cells less invasive. This soluble version consist of the N-terminal Ig-domain that together with the neighbour domain is responsible for ligand binding [18] and important for ALCAM mediated cell–cell adhesion [5]. Reduced primary tumour growth, but increased metastasis to the lung was observed when melanoma cells transfected with N-terminally truncated ALCAM were injected s.c. in a nude mouse model. In this truncated ALCAM molecule two domains most distal to the membrane were deleted. Over expression of the two ALCAM variants inhibited the MMP2 activation cascade via inhibition of MMP2 activity caused by reduced MMP14 expression [26]. In contrast, incubation of MDA-MB-231 cells or HCT 116 cells with the scFv173 did not result in reduced MMP2 activation or increased TIMP-2 levels as assayed by zymography and reverse zymography, respectively (Online resource, Fig. 7) suggesting that the observed reduced Matrigel invasion and tumour growth was not due to inhibition of MMP2 activity.
Cell adhesion molecules are involved in cell–cell interactions and cell–extracellular matrix interactions. It has been hypothesised that ALCAM forms a network in the plasma membrane [28] and that the ALCAM variants disturb this network that seems to be important for cell–cell interacting for primary tumour growth. We hypothesise that the scFv173 antibody in a similar manner disturbs the ALCAM–ALCAM network and thereby reduce in vivo primary tumour growth.
Altered ALCAM expression has been suggested as prognostic indicator in breast carcinoma but some discrepancy exists as to whether low ALCAM mRNA expression [29] or high ALCAM protein expression [30] is a bad prognostic indicator. In a recently published paper both mRNA and protein expression was measured and a positive correlation obtained [31] clarifying that discordant ALCAM mRNA and protein expression cannot explain the conflicting reported results. Several studies point at increased ALCAM expression in the cytoplasm as a negative prognostic factor in other cancers for example in oral [32], bladder [33], ovarian [34] and pancreatic cancer [35]. In other tumour types high ALCAM protein expression is associated with poor prognosis [6]. In a study of colorectal cancer patients examining ALCAM expression by immunohistochemistry (IHC) shortened survival time was observed in patients with membranous and cytoplasmic ALCAM staining when compared to patients without membranous ALCAM staining [36]. We observed reduced positive ALCAM staining in frozen sections of human breast cancer of high histological grade compared to those of lower grade. No concomitant increased intracellular staining was observed. This is in agreement with published results [29, 37] but not with data showing that increased cytoplasmic ALCAM expression is linked to higher tumour grading [30]. Since we used live cells to screen for antigen–antibody binding one could speculate that the epitope recognised by scFv173 is unavailable for binding the cytoplasmic form of ALCAM. The majority of reports on cytoplasmic ALCAM staining have been done on formaldehyde fixed paraffin embedded sections. Our antibody does not recognise its epitope under such conditions indicating that the epitope is susceptible to chemical alteration induced by formaldehyde. We suggest that the conflicting results are caused by the use of frozen tissues versus formaldehyde fixed paraffin embedded cancer tissues and not due to the use of different antibodies. This is supported by data reporting that one monoclonal antibody gave opposite results on frozen tissue [29] compared to formaldehyde fixed tissue [30].
In conclusion, the potential of the new fully human scFv anti-ALCAM antibody as a therapeutic candidate is supported by its in vivo anti-tumour activity in colorectal carcinoma and its negative staining of normal human tissue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank MSc Ellen Hellesylt and Prof. Jahn Nesland (Department of Pathology, Oslo University Hospital Radiumhospitalet) for technical assistance and scoring of IHC sections and Dr. Kjetil Boye (Department of Tumour Biology) for performing statistical comparison of data from a cancer mammae panel and ALCAM IHC staining. Marike Stassar and Bruno Mainnemard discovered and produced the scFv antibodies (Affitech A/S, Oslo, Norway).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat Biotechnol. 2009;27:331–337. doi: 10.1038/nbt0409-331. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM. Monoclonal antibodies as innovative therapeutics. Curr Pharm Biotechnol. 2008;9:423–430. doi: 10.2174/138920108786786358. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, Kamal N, Batra SK. Engineering antibodies for clinical applications. Trends Biotechnol. 2007;25:307–316. doi: 10.1016/j.tibtech.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bowen MA, Patel DD, Li X, Modrell B, Malacko AR, Wang WC, Marquardt H, Neubauer M, Pesando JM, Francke U, Haynes BF, Aruffo A. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995;181:2213–2220. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kempen LCLT, Nelissen JMDT, Degen WGJ, Torensma R, Weidle UH, Bloemers HPJ, Figdor CG, Swart GWM. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)–ALCAM interaction. J Biol Chem. 2001;276:25783–25790. doi: 10.1074/jbc.M011272200. [DOI] [PubMed] [Google Scholar]
- 6.Ofori-Acquah SF, King JA. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res. 2008;151:122–128. doi: 10.1016/j.trsl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Myklebust AT, Beiske K, Pharo A, Davies CD, Aamdahl S, Fodstad Ø. Selection of anti-SCLC antibodies for diagnosis of bone marrow metastasis. Br J Cancer. 1991;63:49–53. [PMC free article] [PubMed] [Google Scholar]
- 8.Fodstad Ø, Brøgger A, Bruland Ø, Solheim OP, Nesland J, Pihl A. Characteristics of a cell line established from a patient with multiple osteosarcoma, appearing 13 years after treatment for bilateral retinoblastoma. Int J Cancer. 1986;38:33–40. doi: 10.1002/ijc.2910380107. [DOI] [PubMed] [Google Scholar]
- 9.Fodstad Ø, Aamdahl S, Pihl A, Boyd MR. Activity of mitozolomide (NSC 353451), a new imidazoterazine, against xenografts from human melanomas, sarcoma, and lung and colon carcinomas. Cancer Res. 1985;45:1778–1786. [PubMed] [Google Scholar]
- 10.Aamdahl S, Fodstad Ø, Kaalhus O, Pihl A. Chemosensitivity profiles of human cancers assessed by the 6-day SRC assay on serially xenografted tumors. Int J Cancer. 1986;37:579–587. doi: 10.1002/ijc.2910370417. [DOI] [PubMed] [Google Scholar]
- 11.Wen DY, Hall WA, Fodstad Ø. Rapid detection of transferrin receptor expression in glioma cell lines by using magnetic microspheres. Neurosurgery. 1993;33:878–881. doi: 10.1227/00006123-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Rye PD, Norum L, Olsen D-R, Garman-Vik S, Kaul S, Fodstad Ø. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int J Cancer. 1996;68:682–687. doi: 10.1002/(SICI)1097-0215(19961127)68:5<682::AID-IJC20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Månsson JE, Fredman P, Bigner DD, Molin K, Rosengren B, Friedman HS, Svennerholm L. Characterization of new gangliosides of the lactotetraose series in murine xenografts of a human glioma cell line. FEBS Lett. 1986;201:109–113. doi: 10.1016/0014-5793(86)80580-4. [DOI] [PubMed] [Google Scholar]
- 14.Løset Gå, Løbersli I, Kavlie A, Stacy JE, Borgen T, Kausmally L, Hvattum E, Simonsen B, Hovda MB, Brekke OH. Construction, evaluation and refinement of a large human antibody phage library based on the IgD and IgM variable gene repertoire. J Immunol Methods. 2005;299:47–62. doi: 10.1016/j.jim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Stassar M, Reiersen H (2006) Methods for antibody library screening. International patent application WO 2006/038022
- 16.Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200:69–77. doi: 10.1016/S0022-1759(96)00188-3. [DOI] [PubMed] [Google Scholar]
- 17.Warren DJ, Bjerner J, Paus E, Bormer OP, Nustad K. Use of an in vivo biotinylated single-chain antibody as capture reagent in an immunometric assay to decrease the incidence of interference from heterophilic antibodies. Clin Chem. 2005;51:830–838. doi: 10.1373/clinchem.2004.046979. [DOI] [PubMed] [Google Scholar]
- 18.Swart GWM. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002;81:313–321. doi: 10.1078/0171-9335-00256. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen KB, Nesland JM, Fodstad O, Maelandsmo GM. Expression of S100A4, E-cadherin, [alpha]- and [beta]-catenin in breast cancer biopsies. Br J Cancer. 2002;87:1281–1286. doi: 10.1038/sj.bjc.6600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flatmark K, Mælandsmo GM, Martinsen M, Rasmussen H, Fodstad Ø. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–1598. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Kurosawa G, Akahori Y, Morita M, Sumitomo M, Sato N, Muramatsu C, Eguchi K, Matsuda K, Takasaki A, Tanaka M, Iba Y, Hamada-Tsutsumi S, Ukai Y, Shiraishi M, Suzuki K, Kurosawa M, Fujiyama S, Takahashi N, Kato R, Mizoguchi Y, Shamoto M, Tsuda H, Sugiura M, Hattori Y, Miyakawa S, Shiroki R, Hoshinaga K, Hayashi N, Sugioka A, Kurosawa Y. Comprehensive screening for antigens overexpressed on carcinomas via isolation of human mAbs that may be therapeutic. Proc Natl Acad Sci. 2008;105:7287–7292. doi: 10.1073/pnas.0712202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piazza T, Cha E, Bongarzone I, Canevari S, Bolognesi A, Polito L, Bargellesi A, Sassi F, Ferrini S, Fabbi M. Internalization and recycling of ALCAM/CD166 detected by a fully human single-chain recombinant antibody. J Cell Sci. 2005;118:1515–1525. doi: 10.1242/jcs.02280. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Conrad F, Cooperberg MR, Kirpotin DB, Marks JD. Mapping tumor epitope space by direct selection of single-chain Fv antibody libraries on prostate cancer cells. Cancer Res. 2004;64:704–710. doi: 10.1158/0008-5472.CAN-03-2732. [DOI] [PubMed] [Google Scholar]
- 24.Roth A, Drummond DC, Conrad F, Hayes ME, Kirpotin DB, Benz CC, Marks JD, Liu B. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol Cancer Ther. 2007;6:2737–2746. doi: 10.1158/1535-7163.MCT-07-0140. [DOI] [PubMed] [Google Scholar]
- 25.Ruan W, Sassoon A, An F, Simko JP, Liu B. Identification of clinically significant tumor antigens by selecting phage antibody library on tumor cells in situ using laser capture microdissection. Mol Cell Proteomics. 2006;5:2364–2373. doi: 10.1074/mcp.M600246-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.van Kilsdonk JWJ, Wilting RH, Bergers M, van Muijen GNP, van Kempen LCLT, Swart GWM. Attenuation of melanoma invasion by a secreted variant of activated leukocyte cell adhesion molecule. Cancer Res. 2008;68(10):3671–3679. doi: 10.1158/0008-5472.CAN-07-5767. [DOI] [PubMed] [Google Scholar]
- 27.van Kempen LCLT, Meier F, Egeblad M, Kersten-Niessen MJF, Garbe C, Weidle UH, van Muijen GNP, Herlyn M, Bloemers HPJ, Swart GWM. Truncation of activated leukocyte cell adhesion molecule: a gateway to melanoma metastasis. J Investig Dermatol. 2004;122:1293–1301. doi: 10.1111/j.0022-202X.2004.22531.x. [DOI] [PubMed] [Google Scholar]
- 28.Swart GWM, Lunter PC, Kilsdonk JWJ, Kempen LCLT. Activated leukocyte cell adhesion molecule (ALCAM/CD166): signaling at the divide of melanoma cell clustering and cell migration? Cancer Metastasis Rev. 2005;24:223–236. doi: 10.1007/s10555-005-1573-0. [DOI] [PubMed] [Google Scholar]
- 29.King J, Ofori-Acquah S, Stevens T, Al-Mehdi AB, Fodstad O, Jiang W. Activated leukocyte cell adhesion molecule in breast cancer: prognostic indicator. Breast Cancer Res. 2004;6:R478–R487. doi: 10.1186/bcr815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burkhardt M, Mayordomo E, Winzer KJ, Fritzsche F, Gansukh T, Pahl S, Weichert W, Denkert C, Guski H, Dietel M, Kristiansen G. Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J Clin Pathol. 2006;59:403–409. doi: 10.1136/jcp.2005.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihnen M, Muller V, Wirtz RM, Schroder C, Krenkel S, Witzel I, Lisboa BW, Janicke F, Milde-Langosch K. Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res Treat. 2008;112:419–427. doi: 10.1007/s10549-007-9879-y. [DOI] [PubMed] [Google Scholar]
- 32.Sawhney M, Matta A, Macha M, Kaur J, DattaGupta S, Shukla N, Ramanathan R. Cytoplasmic accumulation of activated leukocyte cell adhesion molecule is a predictor of disease progression and reduced survival in oral cancer patients. Int J Cancer. 2009;124(9):2098–2105. doi: 10.1002/ijc.24192. [DOI] [PubMed] [Google Scholar]
- 33.Tomita K, van Bokhoven A, Jansen CFJ, Kiemeney LA, Karthaus HFM, Vriesema J, Bussemakers MJG, Witjes JA, Schalken JA. Activated leukocyte cell adhesion molecule (ALCAM) expression is associated with a poor prognosis for bladder cancer patients. Urooncology. 2003;3:121–129. doi: 10.1080/15610950310001632322. [DOI] [Google Scholar]
- 34.Mezzanzanica D, Fabbi M, Bagnoli M, Staurengo S, Losa M, Balladore E, Alberti P, Lusa L, Ditto A, Ferrini S, Pierotti MA, Barbareschi M, Pilotti S, Canevari S. Subcellular localization of activated leukocyte cell adhesion molecule is a molecular predictor of survival in ovarian carcinoma patients. Clin Cancer Res. 2008;14:1726–1733. doi: 10.1158/1078-0432.CCR-07-0428. [DOI] [PubMed] [Google Scholar]
- 35.Kahlert C, Weber H, Mogler C, Bergmann F, Schirmacher P, Kenngott HG, Matterne U, Mollberg N, Rahbari NN, Hinz U, Koch M, Aigner M, Weitz J. Increased expression of ALCAM/CD166 in pancreatic cancer is an independent prognostic marker for poor survival and early tumour relapse. Br J Cancer. 2009;101:457–464. doi: 10.1038/sj.bjc.6605136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichert W, Knosel T, Bellach J, Dietel M, Kristiansen G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol. 2004;57:1160–1164. doi: 10.1136/jcp.2004.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jezierska A, Olszewski W, Pietruszkiewicz J, Olszewski W, Matysiak W, Motyl T. Activated leukocyte cell adhesion molecule (ALCAM) is associated with suppression of breast cancer cells invasion. Med Sci Monit. 2006;12:BR245–BR256. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.