How did the study come about?
The human immunodeficiency virus (HIV) epidemic in China, with its unique but tragic cohort of former plasma donors (FPD) in central China, has been well reviewed.1–3 Overall, China does not have a big HIV problem, with only ∼0.05% of the total population infected. With 1.3 billion people, however, this equates to an estimated 700 000 infected individuals, with high rates reported among the high-risk cohorts of FPD, injection drug users, female sex workers and men who have sex with men. Among the estimated 85 000 with acquired immunodeficiency syndrome (AIDS), 62 838 have been reported as of October 2007.4
In October 2002, the China Ministry of Health (MOH) directed the National Center for AIDS/STD Control and Prevention (NCAIDS) to initiate a pilot free HIV treatment programme in one county in Henan Province, the epicentre of the FPD epidemic. This pilot, called China Comprehensive AIDS Response (China CARES), was subsequently expanded nationwide in 2003 into the current National Free Antiretroviral Treatment Program (NFATP), providing HIV treatment and care primarily to rural and low-income urban patients who meet the national treatment guidelines.5 By the end of 2003, more than 7000 patients had initiated antiretroviral therapy (ART) and the programme had grown to include nine provinces.6 To monitor and evaluate the rapidly expanding programme more effectively, NCAIDS established a nationwide observational cohort in September 2004. Managed through an electronic ART database system, standardized case report forms (CRFs) were completed at each patient visit and faxed to NCAIDS via DataFax (Clinical DataFax Systems Inc., Hamilton, ON, Canada) to be maintained in the national ART database.7 Patient data from 2003 to 2004 were completed retrospectively once the database was constructed. Information since 2004 has been collected prospectively.
In 2005, the first edition of the China Free ART Manual, which serves as the guideline of NFATP, was published, and requested every county providing HIV treatment to participate in providing data to the national database.8 China's national paediatric NFATP began in six provinces in 2005 with the donation of paediatric HIV medication formulations from the Clinton Foundation and scaled up to cover the entire country in 2006.7 Paediatric data began being added to the national database in 2006, with all paediatric treatment sites participating.
Annual funding from the central government covers the cost of software licensing, data collection, training and data verification. Nearly 1 million Chinese renminbi (RMB) were allocated to purchase facsimile machines for clinical sites based on need; provincial and city-level sources funded the rest.
What does the study cover?
The NFATP utilizes a community care model to provide ART at the county level with follow-up, monitoring and care at the lower township or village levels, due to the critical shortage of experienced health care personnel.7 The ART cohort implements standard, essential procedures for collection of core treatment indicators in accordance with the Free ART Manual. Relatively simple yet vital indicators are emphasized to promote understanding and compliance from inexperienced lower-level healthcare providers, facilitating a mechanism for consistent programme monitoring and evaluation. Additionally, cohort monitoring allows for the planning and management of free antiretroviral (ARV) drugs, as well as enabling research and analysis of treatment outcomes.
The course of treatment for the adult cohort is assessed based on five standardized CRFs that are uploaded to the central database—initial patient assessment, treatment follow-up, treatment regimen change, treatment/follow-up termination and transfer of care. The local healthcare provider completes one or more forms at each patient visit, depending on the purpose of the visit. The county Center for Disease Control (CDC) then transmits the completed forms to the national treatment database in Beijing via DataFax. Each form received is reviewed twice by database managers for missing data and logic errors, with queries sent to the local CDC for data correction.
At the baseline treatment visit, the initial patient assessment CRF, completed only once for each patient, is given. Subsequent routine follow-up visits are captured on the treatment follow-up CRF. If a patient misses a scheduled follow-up visit, the follow-up CRF is still returned with the missed visit noted. The treatment regimen change CRF is completed any time there is a change in medication (dosage changes not included) and captures the old regimen, the new regimen and the date and reason for change. If treatment is withheld or discontinued for any reason, such as death, medication stoppage, lost to follow-up or transfer of care, the treatment/follow-up termination form is completed, capturing the date and reason for discontinuation. If treatment is restarted, the treatment follow-up forms will continue to be completed. For questionnaire content, see Table 1.
Table 1.
Summary of the components of each CRF used for adult patients in the China NFATP
| Initial assessment | Follow-upa | Treatment termination | Regimen change | Transfer of clinical care | |
|---|---|---|---|---|---|
| Patient name and ID | X | X | X | X | X |
| Current treatment ID | X | X | X | X | X |
| Treatment site location code | X | X | X | X | X |
| Patient demographics | |||||
| Age | X | ||||
| Gender | X | ||||
| Marital status | X | ||||
| Examination | |||||
| Pre-treatment symptomsb | X | ||||
| Body weight | X | X | |||
| Laboratory test resultsc | X | X | |||
| HIV status | |||||
| Date of HIV diagnosis | X | ||||
| Route of HIV infection | X | ||||
| Treatment status | |||||
| Duration of follow-up | X | X | |||
| ART payment source | X | ||||
| ART regimens at initiation | X | ||||
| Previous use of Chinese medicine to treat HIV | X | ||||
| Current ART regimen | X | X | X | ||
| Current regimen initiation date | X | X | |||
| Self-reported adherence | X | ||||
| ART adverse side effects | X | ||||
| Spouse ART status | X | ||||
| Date of regimen change | X | ||||
| Reason for regimen change | X | ||||
| Previous medication stoppage | X | ||||
| Time elapsed since stopping previous regimen | X | X | |||
| Termination | |||||
| Type of terminationd | X | ||||
| Cause of termination | X | ||||
| Date of termination event | X | ||||
| Cause of death | X | ||||
| Adverse side effects caused by termination | X |
aFollow-up is performed at 0.5, 1, 2 and 3 months after ART initiation, once every 3 months thereafter.
bThese include herpes zoster, fever, chronic diarrhoea, odynophagia and weight loss.
cLaboratory tests include CD4 count, viral load, haematology, immunology, liver function.
dMortality, medication discontinuation, lost to follow-up, transfer of clinical care.
Similar to the adult cohort, the paediatric portion of the national treatment database is built on five standardized CRFs, with a medication form in place of the treatment regimen change form being the only significant divergence from the adult forms (Table 2). The paediatric medication form includes much more detail about the treatment regimen than the adult form, including dosage amount, formulation, number of pills or millilitres and frequency. Trimethoprim–sulfamethoxazole usage is also captured. All other forms are similar to the adult forms but include additional information on physical exam measurements (weight, height and head circumference). The treatment follow-up form also includes information on signs and symptoms of opportunistic infections.
Table 2.
Summary of the components of each CRF used for paediatric patients in the China NFATP
| Initial assessment | Follow-upa | Treatment termination | Medicationb | Transfer of clinical care | |
|---|---|---|---|---|---|
| Patient name and ID | X | X | X | X | X |
| Current treatment ID | X | X | X | X | X |
| Treatment site location code | X | X | X | X | X |
| Patient demographics | |||||
| Date of birth | X | ||||
| Gender | X | ||||
| Examination | |||||
| Pre-treatment symptoms and diseasesc | X | ||||
| Current symptoms and diseases | X | X | |||
| Current height, weight and head circumference | X | ||||
| Height, weight and head circumference at birth | X | ||||
| Laboratory test resultsd | X | X | |||
| HIV status | |||||
| Date of HIV diagnosis | X | ||||
| Route of HIV infection | X | ||||
| PMTCT therapy | X | ||||
| WHO clinical stage | X | ||||
| Treatment status | |||||
| Duration of follow-up | X | X | |||
| ART payment source | X | ||||
| ART regimens at initiation | X | ||||
| Use of TMP-SMZ | X | X | X | ||
| Current ART regimen | X | ||||
| Current regimen initiation date | X | X | |||
| ART drug and SMZ specificse | X | ||||
| Self-reported adherence | X | ||||
| Reason for regimen changef | X | ||||
| Concurrent use of other drugsg | X | X | |||
| ART adverse side effects | X | ||||
| Termination | |||||
| Type of terminationh | X | ||||
| Cause of termination | X | ||||
| Date of termination event | X | ||||
| Cause of death | X | ||||
| Adverse side effects caused by termination | X |
aFollow-up is performed at 0.5, 1, 2 and 3 months after ART initiation, once every 3 months thereafter.
bCompleted at treatment initiation, change of regimen dosage/medication type, and change of regimen.
cClassified into WHO HIV disease stages.
dLaboratory tests include: CD4 count, viral load, hematology, immunology, liver function, PPD, HBsAg, anti-HCV.
eDrug code, dosage form, amount per dose and frequency.
fContinuing initial regimen with same dosage, continuing initial regimen with change in dosage/medication type, or regimen change.
gAnti-TB drugs, Traditional Chinese Medicine, TMP-SMZ, others.
hMortality, medication discontinuation, lost to follow-up and transfer of clinical care.
PMTCT = Prevention of mother-to-child transmission; TMP-SMZ = trimethoprim-sulfamethoxazole.
Cohort core indicator data are automatically analysed accessible via an encrypted website to enable healthcare staff to view data from their respective coverage areas. Data quality issues can be reported and real-time and time-elapsed data surveillance and treatment management reports can be generated. The cross-sectional report on numbers of patients on ART within various scales of coverage gives the cumulative number of patients that have ever started ART, that are currently on ART and new to ART within the last month, as well as cumulative and recent mortalities, treatment terminations, lost to follow-ups and treatment regimens. The simplified ART cohort report recommended by WHO Patient Monitoring Guidelines for HIV Care and Antiretroviral therapy monitors programme quality by tracking the proportion of patients who remain on treatment and their follow-up rates.
All laboratory tests were carried out by the local hospital or CDC. For patients starting treatment, haematology tests were the most commonly performed baseline laboratory tests, accounting for 85.5% of all patients, followed by CD4 count (76.4%), liver function (61.9%) and viral load (3.0%). Haematology and liver function tests were the most commonly performed tests during follow-up. Because the costs of laboratory tests for adult patients have predominantly been paid for by patients, there are large numbers of missing laboratory data. In recent years, the government has provided additional resources, such as funds for laboratory instruments and test reagents and patient transportation subsidies, with the amount covered determined by each province. The accessibility of laboratory testing is thus improving and more patients will be able to have routine laboratory surveillance according to the recommendations of the Free ART Manual. The paediatric treatment programme covers all laboratory charges and thus paediatric laboratory tests are much more complete. The costs of treating opportunistic infections are not included through the national treatment programme. However, an increasing number of provinces are reimbursing a proportion of these costs, with the exact amount determined by each province. Information on opportunistic infection treatment is maintained by each local treatment site and is not reported to the national database for adults but is captured for children.
Who is in the sample?
The broad coverage of the NFATP generates a continually expanding cohort representing HIV/AIDS treatment across all of China, including an especially noteworthy FPD population in the central regions. In the first edition (2005) of the China Free ART Manual, the criteria for treating adult patients were CD4 cell count <200/μl or World Health Organization (WHO) stage III or IV disease. In the revised (2008) version of the China Free ART Manual, the CD4 cell count criterion was increased to <350/μl without changing the WHO criterion. The ART manual recommends giving treatment to children <12 months of age without considering CD4 cell count. For patients >12 months of age, the manual recommends starting treatment on the CD4 cell count/percentage or WHO stage III or IV disease by age.
The adult cohort comprises AIDS patients of ≥15 years of age who have received treatment through the NFATP. Through constant expansion, by the end of 2007, the NFATP had cumulatively treated 42 126 patients in more than 1200 counties across all 31 provinces and municipalities. Among them, 50% were infected through plasma donation, 23% through heterosexual transmission, 11% through injecting drugs, 10% through blood transfusions, 1% through homosexual transmission and 5% through other or unknown routes. Men comprise 57% of the patients and the mean age of male patients is 39.5 years. Among the 799 paediatric patients, 60% are male, the mean age is 9.4 years, and 15% are <5 years old.
Each year, provincial authorities report the cumulative total number of HIV patients on treatment in their provinces, separately from the database reporting. As of 31 August 2007, the provinces have reported 38 998 patients nationwide as having received free ART. The National ART Database contained patient data on 36 884 individuals, with a reporting rate of 94.6%.
How often are they being followed-up?
After treatment initiation, follow-up visits are scheduled at 0.5, 1, 2 and 3 months, and once every 3 months thereafter. Additional follow-up visits are scheduled as needed, but these are not captured by the national treatment database. In the event of a regimen change, the 0.5-, 1-, 2- and 3-month schedule is restarted. Moreover, if a patient misses four consecutive follow-up appointments, the patient is considered lost to follow-up and the treatment termination form is completed with the termination date as the date of the last follow-up. Finally, if a patient needs to transfer his/her clinical care to a different location, the new sites completes the transfer of care CRF to ensure that subsequent records are linked to the patient's previous records in the database and that follow-up remains consistent.
What is attrition like?
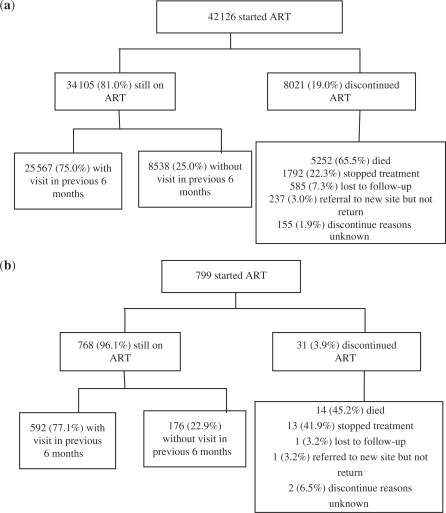
The breakdown of patient attrition rates as of 2007 of the 42 126 adult patients enrolled are shown in Figure 1a, and of the 799 paediatric patients is shown in Figure 1b. Among the adult patients who stopped treatment, the top three reasons were side effects (47.0%), patient self-request (33.6%) and adherence issues (8.7%). Among the paediatric patients who stopped treatment, the primary reasons were adherence issues (six patients), side effects (five patients) and patient self-request (two patients). An additional 8538 (20.3%) adult patients and 176 (22.0%) paediatric patients have not reported a follow-up visit within the most recent 6 months and would be considered lost if missed appointments persist.
Figure 1.
Schematic of patient attrition among adult (a) and paediatric patients (b) enrolled in the China AFATP, as of 31 December 2007
What has been found?
Cohort data serve as an invaluable tool for monitoring the progress and quality of the NFATP. Through 5 years of patient data, a vast quantity of core data have been collected, available to address management and evaluation issues as well as research questions involving ART. One study on the challenges and responses of the Chinese free ART programme on data available through the end of 2006 showed that 64% of the individuals known to have AIDS has received ART. Whereas only domestically produced ARV drugs were initially available in China, after the relatively recent addition of imported 3TC, the recommended D4T + 3TC + NVP combination has become the most common regimen, received by 56% of the cohort. Treatment termination occurred for 5497 of the 30 640 patients (17.9%), consisting of 3381 deaths, 1489 medication stoppages due to intolerance or preference, 461 lost to follow-ups and 166 transfers.7 In addition, an analysis of the effect of highly active antiretroviral therapy (HAART) on mortality among adult FPD AIDS patients showed that their mortality rate declined from 27.3/100 person-years in 2001 to 4.6/100 person-years in 2006. Concomitantly, HAART coverage among this population increased from 0% in 2001 to 70.5% in 2006.9 Further analyses are ongoing involving longer term treatment outcomes on survival and treatment failure.
Preliminary analyses of the paediatric cohort have shown a significant lag time between infection and treatment for children infected through maternal–child transmission (mean 6.7 years in FPD provinces, 4.7 years in injection drug use provinces). Furthermore, ∼30% of children who met the treatment criteria were still untreated.10 Treatment has been successful in another study of 83 children who, after 1 year of HAART, showed weight and CD4 cell count improvements and more than half of the previously ART-naïve children having undetectable viral loads.11 Application of the paediatric cohort database demonstrably enhances tracking of HIV diagnoses and the sensitive paediatric treatment process.
What are the main strengths and weaknesses?
The main strength of the NFATP is its broad, nationwide coverage with over 40 000 Chinese HIV/AIDS patients of all kinds, including a unique FPD population in central China, providing an extremely rich dataset for analysis.
A cornerstone of the NFATP, the cohort receives full endorsement and emphasis from the central government, with all the advantages and potential conferred on perpetually ongoing national programmes. Further measures were adopted in 2007 to emphasize cohort quality control, such as the addition of data quality reports to the routine reports generated and nationwide on-site data validation.
The cohort database has become an essential monitoring and evaluation tool for the NFATP. As treatment outcomes are analysed, modifications to the NFATP can be made to improve the programme with the results analysed again in subsequent years to determine the impact. Through the implementation of online accessibility, all levels of the NFATP are able to analyse and be notified of developments in the patient treatment at each level and perform real-time surveillance and evaluation. Therefore, officials at the provincial level or below may take initiative to track local progress and make adjustments accordingly, rather than wait for monitoring reports and directives from above.
The rapid scale-up of the NFATP, however, has also revealed weaknesses that must be addressed. Among the patients still supposed to be on treatment, 20.3% of the adults and 22.0% of the children did not have a visit recorded in the previous 6 months (Figure 1). This is concerning because the lack of recent records on these patients constitutes a bias in the cohort data, as well as the possibility of ART provider non-compliance with accurate and timely completion of CRFs. Providers may not understand the importance of filling out the CRF completely and accurately because they do not recognize the immediate benefits in increasing efficiency or improving patient follow-up, adherence or treatment results. Thus, although cohort data can be utilized as a significant monitoring tool for NFATP management officials in improving treatment quality, incentives need to be imparted to providers, such as client-side software to demonstrate the direct benefits of patient progress monitoring and even assistance with clinic scheduling.
Data from Henan Province has been problematic. Henan was the first site for the NFATP and has significant numbers of FPD-infected patients. The cumulative number of treated Henan patients account for 52% of the national total, as of the end of 2007. During the pilot study, Henan established its own provincial cohort, and hence did not initially participate in the national treatment cohort. When the cohorts were joined in 2006, directly merging the databases proved impossible. Instead, re-completion of NFATP CRFs by hand, based on previously collected data from Henan, was required. Due to the enormous volume of data, only the initial patient assessment CRF and most recent regimen change and treatment termination forms were completed. All other follow-up data were not transferred. Consequently, there is a substantial amount of missing data and concerns about data accuracy for Henan before 2006.
Where can I find out more and what is the potential for collaboration?
Initial enquiries may be made to the principal investigator (F.J.Z.) at NCAIDS in Beijing. The NCAIDS website, www.chinaaids.cn, provides up-to-date news and official documents on AIDS policy in China, with extensive information on the National Free ART cohort. International collaborations on innovative efforts to improve HIV/AIDS treatment and prevention in China are strongly welcomed. Interested parties should contact the principal investigator to find out more.
Funding
The China Free ART Program, funded by the China Ministry of Health and the U.S. National Institutes of Health, ICOHRTA grant (U2R TW006918).
Conflict of interest: None declared.
References
- 1.Zhang FJ, Chen RY, Lo SN, Ma Y. Country Review: China. In: Zuniga JM, Whiteside A, Ghaziani A, Bartlett JG, editors. A Decade of HAART. Oxford: Oxford University Press; 2008. [Google Scholar]
- 2.Qian HZ, Vermund SH, Wang N. Risk of HIV/AIDS in China: subpopulations of special importance. Sex Transm Infect. 2005;81:442–47. doi: 10.1136/sti.2004.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He N, Detels R. The HIV epidemic in China: history, response, and challenge. Cell Res. 2005;15:825–32. doi: 10.1038/sj.cr.7290354. [DOI] [PubMed] [Google Scholar]
- 4.A Joint Assessment of HIV/AIDS Prevention. Beijing: State Council AIDS Working Committee Office, UN Theme Group on AIDS in China; 2007. Treatment and Care in China. 1 December 1 2007. [Google Scholar]
- 5.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res. 2005;15:877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 6.Zhang FJ, Wen Y, Yu L, Ma Y, Pan J, Zhao Y. Antiretroviral therapy for HIV/AIDS and current situation of China free ARV program. Sci Technol Rev. 2005;23:24–28. [Google Scholar]
- 7.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl 8):S143–48. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 8.China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; January 2005. [Google Scholar]
- 9.Zhang F, Dou Z, Yu L, et al. The effect of highly active antiretroviral therapy on mortality in HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47:825–33. doi: 10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Au MC, Bouey PD, et al. The diagnosis and treatment of HIV-infected children in China: challenges and opportunities. J Acquir Immune Defic Syndr. 2007;44:429–34. doi: 10.1097/QAI.0b013e31803133ac. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Haberer JE, Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007;46:594–98. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]