Abstract
Background Body mass index (BMI) (kg/m2) has a U- or J-shaped relationship with all-cause mortality in Western and East Asian populations. However, this relationship is not well characterized in Bangladesh, where the BMI distribution is shifted towards lower values.
Methods Using data on 11 445 individuals (aged 18–75 years) participating in the Health Effects of Arsenic Longitudinal Study (HEALS) in Araihazar, Bangladesh, we prospectively examined associations of BMI (measured at baseline) with all-cause mortality during ∼6 years of follow-up. We also examined this relationship within strata of key covariates (sex, age, smoking, education and arsenic exposure). Cox proportional hazards models adjusted for these covariates and BMI-related illnesses were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for BMI categories defined by the World Health Organization.
Results Low BMI was strongly associated with increased mortality in this cohort (P-trend < 0.0001). Severe underweight (BMI < 16 kg/m2; HR 2.06, CI 1.53–2.77) and moderate underweight (16.0–16.9 kg/m2; HR 1.39, CI 1.01–2.90) were associated with increased all-cause mortality compared with normal BMI (18.6–22.9 kg/m2). The highest BMI category (≥23.0 kg/m2) did not show a clear association with mortality (HR 1.10, CI 0.77–1.53). The BMI–mortality association was stronger among individuals with <5 years of formal education (interaction P = 0.02).
Conclusions Underweight (presumably due to malnutrition) is a major determinant of mortality in the rural Bangladeshi population.
Keywords: Arsenic, Bangladesh, body mass index, mortality, survival analysis
Introduction
A large body of epidemiological research suggests that body mass index (BMI) (kg/m2) has a U- or J-shaped relationship with all-cause mortality in Western1–3 and East Asian4–6 populations. In other words, underweight (<18.5 kg/m2; also referred to as ‘chronic energy deficiency’7) and obese (>30 kg/m2) individuals tend to die earlier than individuals with intermediate BMI values. However, the relationship between BMI and mortality is not well characterized in many developing nations, which may have unique BMI distributions, environmental exposures and genetic backgrounds.
Compared with Western populations, the BMI distribution in East Asian countries is shifted towards lower values, although the prevalence of obesity and overweight has increased in recent years.8 In South Asian countries, such as India, the BMI distribution is shifted even further towards low values, especially in rural areas,8–11 likely reflecting poor nutritional status.12 In Bangladesh, recent estimates7,13,14 of the population mean BMI range between 19 and 20 kg/m2, lower than estimates in the USA (∼27 kg/m2),15 Japan (∼23.5 kg/m2)6 and India (21.7 kg/m2).10
In this article, we examine the relationship between BMI (measured at baseline) and all-cause mortality using ∼6 years of follow-up data from a large prospective cohort study of individuals chronically exposed to arsenic though drinking water in Araihazar, Bangladesh. We examine the BMI–mortality association within strata of key covariates (sex, age, smoking, education and arsenic exposure).
Methods
Study area and study population
The Health Effects of Arsenic Longitudinal Study (HEALS, described by Ahsan et al.16) is a prospective investigation of health outcomes associated with arsenic exposure through drinking water in a cohort of adults in Araihazar, Bangladesh, a rural area east of Dhaka with relatively homogenous socio-cultural characteristics. Between October 2000 and May 2002, we recruited individuals (aged 18–75 years) who were (i) married, (ii) residents of the study area for at least 5 years and (iii) primarily drinking water from a local well. Using a pre-cohort survey, we enumerated a total of 65 876 individuals residing in Araihazar, from which we identified a sampling frame of 14 828 eligible residents. Of these 14 828 individuals, 2778 were not at home during any of the three attempted recruiting visits. Of the 12 050 remaining eligible residents, 11 746 (97.5% response rate) men and women (4801 married couples and 2144 married individuals whose spouses did not participate) enrolled into the HEALS cohort. All 5966 tubewells in the study area were tested for arsenic. Trained study physicians, blinded to the arsenic measurements, conducted in-person interviews and clinical evaluations and collected urine and blood samples from participants in their homes using structured protocols. The study protocol was approved by the Institutional Review Boards of The University of Chicago, Columbia University, and the Bangladesh Medical Research Council. Informed consent was obtained from all participants prior to the initial interview.
Follow-up in-person interviews were conducted for the entire cohort during the following periods: follow-up 1 during September 2002 to May 2004, follow-up 2 during June 2004 to August 2006 and follow-up 3 during January 2007 to February 2009. Follow-up interviews were conducted using the same data collection procedures developed for the baseline interview. At each follow-up an attempt was made to find each participant (or an informant who could confirm the vital status of that participant) not determined to be deceased in a prior follow-up. At follow-up 1 we identified 103 deaths, at follow-up 2 we identified 116 deaths and at follow-up 3 we identified 181 deaths. At each follow-up we were unable to ascertain death status for some individuals; however, at the end of follow-up 3 we were able to determine vital status for all but one participant.
Assessment of mortality
From 2000 to 2009, vital status was determined at each biennial follow-up interview. Date of death was ascertained by relatives (n = 396) or neighbours (n = 3) of deceased participants. The relationship status of one informant was unknown. An extensive verbal autopsy procedure was used to investigate and assign the causes of death (previously validated in a Bangladeshi population17–20). For deceased individuals, survival time was calculated as the number of days between the date of the baseline interview and date of death. Follow-up time for participants who completed (or were reported to be alive at) all three follow-up interviews was calculated as the number of days between the baseline interview and last interview with the participant or the informant. Participants lost to follow-up with no informant or vital status data were censored at the last point of contact (n = 1).
Assessment of BMI and covariates
At the baseline interview, trained study physicians measured height and weight using a locally manufactured tape measure and a Misaki (Japan) scale (calibrated weekly), respectively. Both height and weight were measured three times at baseline and averaged. BMI was calculated as average weight in kilograms divided by average height in metres, squared. Socio-demographic factors including sex, age (years) and education (years) were obtained. Smoking status was categorized as current, former and never. Participants self-reported experiencing the following health-related symptoms in the 6 months prior to baseline (yes or no): nausea, vomiting, weight loss, hyperhydrosis, asthenia, weakness, diarrhoea and dyspnoea.
Exclusions
Of the 11 746 enrolled study participants, we excluded subjects lacking data on age (n = 1) and BMI (n = 279) from all analyses. An additional 21 individuals were excluded who lacked data on smoking, education or BMI-related symptoms reported at baseline. These exclusions resulted in an effective analysis sample size of 11 445, including 393 deaths.
Arsenic exposure assessment
Nearly half of the Bangladeshi population (approximately 150 million) has been chronically exposed to arsenic through drinking water for >20 years, an exposure known to increase mortality risk in this cohort at levels >150 µg/l (unpublished data). At baseline, participants identified the well they primarily used for drinking water. Arsenic concentrations of all 5966 tubewells in the study area were measured by graphite furnace atomic absorption spectrometry (detection limit of 5 μg/l). Samples below the detection limit (669 wells) were re-analysed by inductively coupled plasma-mass spectrometry (detection limit of 0.1 μg/l).21 We were unable to ascertain well-water arsenic concentration for one tubewell; participants drinking from this well (n = 3) were excluded from analyses of water arsenic.
Statistical analysis
Baseline characteristics were examined stratified by the World Health Organization’s (WHO) International BMI categories22 (kg/m2): <16 (severe underweight), 16.0–16.9 (moderate underweight), 17.0–18.4 (mild underweight), 18.5–22.9 (normal–low), 23.0–24.9 (normal–high), 25–29.9 (pre-obese) and ≥30 (obese). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality by BMI categories, using the ‘normal–low’ category (18.5–22.9 kg/m2) as the reference group. For all analyses, the three highest WHO BMI categories (normal–high, pre-obese and obese) were combined into a single category to avoid having categories with sparse data. Combining the data in this way is further justified by the WHO’s designation of 23.0 kg/m2 as a ‘public health action point’, above which health risks have been observed in Asian countries.22 Trend tests were conducted by including BMI in regression models as an ordinal variable, i.e. 1 (severe underweight) to 5 (normal–high to obese).
Analyses were first conducted adjusted for age and sex only and then with additional adjustment for smoking status, education and self-reported history of BMI-related symptoms experienced <6 months prior to baseline. Confounding by other markers of socio-economic status (SES) (land and television ownership) and arsenic exposure was also explored. BMI-related symptoms were included as potential confounders if they were associated with both BMI and mortality at a modest P = 0.15 level (weight loss and weakness met these criteria). These symptoms serve as surrogates for prevalent medical conditions that may confound the association between BMI and mortality. To further protect against such confounding, we separately analysed deaths occurring >2 years after baseline, under the assumption that individuals who are seriously ill at baseline are more likely to die in years 1 and 2 of follow-up.
Associations were evaluated in subgroups defined by sex, education (<5 and ≥5 years of formal education), age (≤50 and >50 years), smoking status (ever and never smokers) and water arsenic exposure (≤150 and >150 µg/l). We tested for multiplicative interaction between ordinal BMI and each of these subgroup variables by including interaction terms between BMI and stratifying variables in the Cox models. Additive interaction was assessed using the ‘relative excess risk due to interaction’ (RERI) measure (CIs determined using 5000 bootstrap samples).23
Cox regressions were performed using the Statistical Analysis System’s (release 9.2) PHREG procedure (SAS Institute, Inc., Cary, NC). The proportional hazards assumption was tested by modelling interaction terms of time and covariates in the model. Since most HEALS participants are married couples drinking from the same tubewell, we accounted for clustering (on tubewell) using robust standard errors for the proportional hazards model24 analogous to performing a generalized estimating equations analysis in other regression models.25
Results
Distributions of key covariates within WHO BMI categories are shown in Table 1. The mean BMI was 19.8 kg/m2 (19.4 for males, 20.0 for females) with a 3.2 standard deviation (SD). A total of 81 (0.7% of cohort) and 722 individuals (6.3%) were obese and pre-obese, respectively. The proportion of underweight individuals was higher among older individuals, males, less-educated individuals and current smokers, compared with the total cohort. Compared with females, males had a higher mean age and proportion of current and former smokers. Individuals with and without (n = 279) a BMI measure were similar with respect to age, sex, smoking and mortality rate (data not shown).
Table 1.
Baseline characteristics of the HEALS Cohort (Araihazar, Bangladesh), stratified by the WHO’s BMI categories
|
BMI (kg/m2) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Underweight |
Normal |
Overweight |
||||||
| Characteristic | Severe <16.0 | Moderate 16.0–16.9 | Mild 17.0–18.4 | Low 18.5–22.9 | High 23.0–24.9 | Pre-obese 25–29.9 | Obese ≥30.0 | Full Cohort |
| Individuals | 846 | 1128 | 2571 | 5214 | 883 | 722 | 81 | 11 445 |
| Percent of total | 7.4 | 9.9 | 22.5 | 45.6 | 7.7 | 6.3 | 0.7 | 100.0 |
| Sex (%) | ||||||||
| Female | 56.4 | 50.5 | 49.7 | 60.1 | 63.5 | 63.0 | 72.8 | 57.1 |
| Age in years (%) | ||||||||
| 18–30 | 20.6 | 26.1 | 31.2 | 35.3 | 29.9 | 29.2 | 23.5 | 30.1 |
| 31–40 | 29.8 | 33.5 | 34.4 | 35.9 | 40.1 | 42.7 | 40.7 | 35.7 |
| 41–50 | 30.7 | 27.3 | 24.2 | 20.5 | 22.2 | 26.0 | 28.4 | 23.3 |
| 51–75 | 18.9 | 13.1 | 10.2 | 8.4 | 7.8 | 8.0 | 7.4 | 10.0 |
| Mean | 41.1 | 38.7 | 37.1 | 35.9 | 36.8 | 37.7 | 37.8 | 37.1 |
| SD | 10.9 | 10.5 | 10.2 | 9.9 | 9.3 | 9.0 | 8.5 | 10.1 |
| Age for males, years | ||||||||
| Mean | 46.1 | 43.1 | 41.1 | 40.7 | 41.9 | 41.7 | 41.2 | 41.6 |
| SD | 10.3 | 10.0 | 10.0 | 9.8 | 9.3 | 8.8 | 8.1 | 9.9 |
| Age for females, years | ||||||||
| Mean | 37.2 | 34.5 | 33.1 | 32.8 | 33.8 | 35.3 | 36.5 | 33.6 |
| SD | 9.7 | 9.1 | 8.9 | 8.7 | 8.0 | 8.2 | 8.3 | 8.8 |
| Years of education (%) | ||||||||
| 0 | 58.4 | 54.6 | 51.0 | 43.3 | 26.2 | 21.7 | 19.8 | 44.4 |
| 1–4 | 17.6 | 14.8 | 16.5 | 14.2 | 14.0 | 8.7 | 4.9 | 14.6 |
| 5–7 | 15.0 | 18.5 | 20.9 | 22.7 | 26.8 | 25.1 | 27.2 | 21.8 |
| 8–16 | 9.0 | 12.1 | 11.7 | 19.8 | 33.0 | 44.5 | 48.2 | 19.2 |
| Smokinga (%) | ||||||||
| Never | 48.6 | 53.6 | 55.7 | 69.2 | 77.5 | 78.1 | 88.9 | 64.5 |
| Current | 39.3 | 40.9 | 37.6 | 24.8 | 15.4 | 15.8 | 7.4 | 28.9 |
| Former | 12.0 | 5.5 | 6.7 | 5.9 | 7.0 | 6.1 | 3.7 | 6.6 |
| Smokinga in males (%) | ||||||||
| Never | 9.7 | 15.3 | 20.1 | 29.2 | 42.9 | 43.5 | 68.2 | 25.6 |
| Current | 74.9 | 75.9 | 69.9 | 58.5 | 40.4 | 40.5 | 27.3 | 62.4 |
| Former | 15.4 | 8.8 | 10.0 | 12.4 | 16.8 | 16.1 | 4.6 | 12.0 |
| Smokinga in females (%) | ||||||||
| Never | 78.8 | 90.6 | 91.8 | 96.0 | 97.5 | 98.5 | 96.6 | 93.7 |
| Current | 11.7 | 7.4 | 4.9 | 2.4 | 1.1 | 1.3 | 0.0 | 3.8 |
| Former | 9.4 | 2.3 | 3.3 | 1.7 | 1.4 | 0.2 | 3.4 | 2.5 |
| Weight lossb | ||||||||
| Yes | 41.6 | 39.4 | 39.1 | 31.5 | 19.7 | 11.6 | 8.6 | 32.4 |
| Weaknessb | ||||||||
| Yes | 59.9 | 58.1 | 55.1 | 48.8 | 41.0 | 36.8 | 28.4 | 50.5 |
| Water arsenic concentration in μg/l (%) | ||||||||
| 0.1–10 | 22.5 | 23.8 | 21.0 | 23.3 | 24.9 | 28.2 | 45.7 | 23.4 |
| 10.1–50 | 21.6 | 20.0 | 22.2 | 21.2 | 23.8 | 21.0 | 19.8 | 21.5 |
| 50.1–150 | 28.6 | 30.1 | 30.7 | 30.9 | 31.6 | 29.5 | 18.5 | 30.5 |
| 150.1–854 | 27.3 | 26.2 | 26.1 | 24.5 | 19.7 | 21.3 | 16.0 | 24.6 |
aCigarettes or bidi.
bSelf-reported symptoms experienced within the 6 months prior to the baseline interview.
The mean follow-up time was 6.4 years (75 225 total person-years); 393 deaths were observed. Common causes of deaths were related to the circulatory system (International Classification of Diseases (ICD)-10 codes I00–I99, n = 170), neoplasms (C00–D48, n = 65), the respiratory system (J00–J99, n = 33) and infection (A00–B99, n = 29). Other deaths were related to the nervous system (G00–G99, n = 4), digestive system (K00–K99, n = 27), genitourinary system (N00–N99, n = 8), pregnancy complications (O00–O99, n = 10), diabetes (E00–E99, n = 4) and musculoskeletal disorders (M00–M99, n = 1), whereas 39 deaths were not related to any specific health problem (R00–R99, n = 27; S00–T98, n = 2; V01–Y98, n = 10). For three cases, an ICD-10 code was not available.
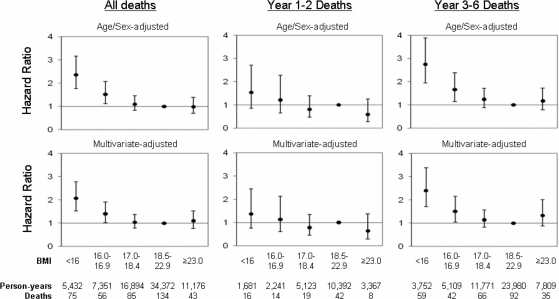
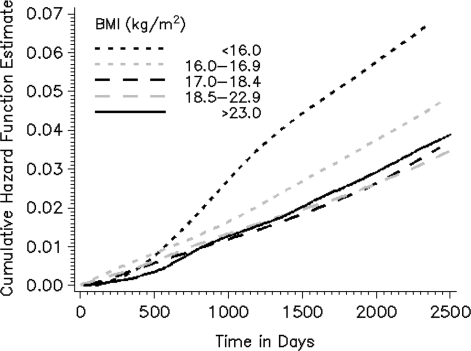
HRs presented in the text are from the multivariate model; P-values are two-sided. Both severe underweight (BMI <16 kg/m2; HR 2.06; CI 1.53–2.78) and moderate underweight (16.0–16.9 kg/m2; HR 1.40; CI 1.02–1.91) were associated with increased mortality when compared with normal–low BMI (18.5–22.9 kg/m2) (P-trend <0.0001) (Figure 1). These associations were stronger for deaths occurring during years 3–6 of follow-up than for deaths in years 1–2. Further adjustment for water arsenic exposure and other SES-related factors (land ownership and television ownership) did not change the magnitude of these associations (data not shown). The multivariate-adjusted cumulative hazard function for each BMI group is plotted in Figure 2. The hazard rate for each group is fairly constant (supporting the proportional hazards assumption), and clear separation of cumulative hazard functions for severe and moderate underweight groups occurs near the second year of follow-up.
Figure 1.
HRs and 95% CIs for mortality, by BMI categories in the HEALS (Araihazar, Bangladesh). Multivariate estimates were adjusted for age, sex, smoking status, education and BMI-related symptoms experienced in the 6 months prior to baseline (weight loss and weakness). Robust standard errors were used to account for clustering on tube well.
Figure 2.
The multivariate-adjusted cumulative hazard function (of mortality) plotted against time (in days) by BMI (kg/m2) category for the HEALS (Araihazar, Bangladesh; n = 11 445). Curves are adjusted to represent non-smoking males (age 18–30 years) with <5 years of formal education (experiencing no weight loss or weakness in the 6 months prior to baseline). Robust standard errors were used to account for clustering on tube well.
Table 2 shows BMI–mortality associations by gender and education. Males accounted for 43% of total person-time and 74% of deaths (n = 289). In males, both severe (HR 2.31; CI 1.34–3.25) and moderate underweight (HR 1.62; CI 1.13–2.33) were associated with increased mortality. Increasing BMI showed a trend towards decreased mortality (P-trend <0.0001), although we observed a borderline association with increased mortality for individuals with BMI ≥23 kg/m2. In females, increasing BMI was modestly associated with reduced mortality (P-trend = 0.03).
Table 2.
HRs and 95% CIs for associations between BMI categories and mortality in the HEALS cohort (Araihazar, Bangladesh), stratified by sex, age, smoking and education levels
|
All-cause mortality |
||||||
|---|---|---|---|---|---|---|
| Person-years | Age/sex-adjusteda |
Multivariate-adjusteda,b |
||||
| of follow-up | Deaths | HR | 95% CI | HR | 95% CI | |
| Men | 32 151 | 289 | ||||
| BMI (kg/m2) | ||||||
| <16.0 | 2317 | 55 | 2.61 | 1.86–3.66 | 2.31 | 1.34–3.25 |
| 16.0–16.9 | 3567 | 46 | 1.78 | 1.24–2.54 | 1.62 | 1.13–2.33 |
| 17.0–18.4 | 8480 | 67 | 1.21 | 0.88–1.67 | 1.14 | 0.82–1.57 |
| 18.5–22.9 | 13 762 | 87 | 1.00 | Ref. | 1.00 | Ref. |
| ≥23.0 | 4025 | 34 | 1.31 | 0.88–1.94 | 1.49 | 1.00–2.23 |
| P for trend | <0.0001 | 0.0004 | ||||
| Women | 43 075 | 104 | ||||
| BMI (kg/m2) | ||||||
| <16.0 | 3090 | 19 | 1.89 | 1.12–3.21 | 1.58 | 0.92–2.69 |
| 16.0–16.9 | 3708 | 10 | 0.97 | 0.49–1.92 | 0.89 | 0.45–1.77 |
| 17.0–18.4 | 8262 | 18 | 0.90 | 0.52–1.56 | 0.84 | 0.49–1.4 |
| 18.5–22.9 | 20 136 | 48 | 1.00 | Ref. | 1.00 | Ref. |
| ≥23.0 | 6979 | 9 | 0.49 | 0.24–0.99 | 0.54 | 0.27–1.12 |
| P for trend | 0.003 | 0.03 | ||||
| P for BMI–sex interactionc | 0.91 | 0.78 | ||||
| Education <5 years | 44 140 | 271 | ||||
| BMI (kg/m2) | ||||||
| <16.0 | 4116 | 63 | 2.65 | 1.88–3.74 | 2.43 | 1.72–3.44 |
| 16.0–16.9 | 5048 | 49 | 1.87 | 1.30–2.69 | 1.80 | 1.25–2.59 |
| 17.0–18.4 | 11 360 | 65 | 1.28 | 0.91–1.80 | 1.23 | 0.87–1.73 |
| 18.5–22.9 | 19 708 | 76 | 1.00 | Ref. | 1.00 | Ref. |
| ≥23.0 | 3910 | 18 | 1.27 | 0.76–2.12 | 1.32 | 0.78–2.22 |
| P for trend | <0.0001 | <0.0001 | ||||
| Education≥5 years | 31 084 | 122 | ||||
| BMI (kg/m2) | ||||||
| <16.0 | 1317 | 12 | 1.56 | 0.83–2.92 | 1.42 | 0.75–2.69 |
| 16.0–16.9 | 2303 | 7 | 0.66 | 0.31–1.44 | 0.62 | 0.29–1.35 |
| 17.0–18.4 | 5534 | 20 | 0.77 | 0.46–1.29 | 0.74 | 0.44–1.25 |
| 18.5–22.9 | 14 665 | 58 | 1.00 | Ref. | 1.00 | Ref. |
| ≥23.0 | 7266 | 25 | 0.84 | 0.53–1.35 | 0.88 | 0.55–1.41 |
| P for trend | 0.54 | 0.85 | ||||
| P for BMI–education interactionc | 0.02 | 0.02 | ||||
aRobust standard errors were used to accounted for clustering on tubewell.
bMultivariate estimates were adjusted for age, sex, smoking status, education and BMI-related symptoms experienced in the 6 months prior to baseline (weight loss and weakness).
cThe multiplicative interaction term tested was ordinal BMI multiplied by the dichotomous strata variable (a one degree of freedom test).
Individuals with <5 years of education (59% female) accounted for 59% of total person-time and 69% of total deaths (n = 271); individuals with ≥5 years of education were 54% females. Increasing BMI was strongly associated with reduced mortality in the low-education group (P-trend < 0.0001), but there was no clear trend-based in the high-education group (P-trend = 0.85), suggesting that education modifies the association between BMI and mortality (multiplicative interaction P = 0.02). The RERI was 0.22 (CI 0.10–0.33), indicating that the relative risk of mortality per 1 category decrease in BMI in the low-education group is 0.22 more than that in the high-education group.
No clear evidence of multiplicative or additive interaction with BMI (ordinal) was observed for age, smoking or arsenic exposure (data not shown).
Discussion
In this prospective cohort study of 11 445 residents of Araihazar, Bangladesh, severe underweight (BMI <16 kg/m2) and moderate underweight (BMI 16.0–16.9 kg/m2) were associated with increased mortality. The prevalence of overweight (25–29.9 kg/m2) and obesity (≥30 kg/m2) in this cohort was 6.3 and 0.7%, respectively, lower than prevalences reported from a rural Indian cohort (15.8 and 2.8%, respectively).10 In this study, overweight and obese BMI categories were combined with the normal–high BMI category (23.0–24.9 kg/m2) for analysis purposes, and this combined category (≥23.0 kg/m2) was not strongly associated with increased mortality. Females had a higher average BMI than males, but prior research does not provide clear support for sex-based differences in BMI in Bangladesh.26–29 The association of mortality to BMI was stronger for individuals with <5 years of education compared with individuals with ≥5 years of education (multiplicative interaction P = 0.02, additive interaction P = 0.0002), suggesting that individuals of lower SES are more susceptible to adverse health effects associated with low BMI.
Previous research in Bangladesh30 supports an increased mortality risk for severely and moderately underweight (<17.3 kg/m2; lowest quartile) individuals compared with individuals in the low end of the normal–low BMI category (18.4–19.6 kg/m2; third quartile), consistent with the results of this work. However, this previous result was based on a smaller cohort (n = 1888) of women only, followed for a longer time period (19 years). In addition, this previous research did not show statistically significant increased risk for the highest average BMI quartile (>19.6 kg/m2; compared with third quartile) or decile (>21.7 kg/m2; compared with 10th through 89th percentile), consistent with our results. Similarly, data from a large rural Indian cohort10 suggest that severe (<16.0 kg/m2) and moderate–mild (16.0–18.4 kg/m2) underweight, but not overweight (25.0–27.4 and ≥27.5 kg/m2), are associated with increased mortality compared with normal–low BMI (18.5–22.9 kg/m2). Data from China,4 Korea5 and Japan6 support a similar relationship between underweight (<18.5 kg/m2) and mortality, but show a clear increase in mortality for the obese (>30 kg/m2), when compared with BMI categories of 24.0–24.9 kg/m2 (China) or 23.0–24.9 kg/m2 (Korea and Japan).
BMI is a marker of nutritional status,12 and low BMI (i.e. chronic energy deficiency) may increase susceptibility to a wide range of diseases, both chronic and infectious, thereby increasing mortality risk. However, associations between low BMI and increased mortality are often controversial, due to the potential confounding effects of smoking, age and SES. In this cohort, female sex, decreasing age, never smoking and increasing education were independently associated with increased BMI and decreased mortality. We attempted to account for these factors by using adjustments and stratified analyses. SES was controlled using education as a proxy; additional adjustment for land ownership and television ownership did not change the results of our education-adjusted analyses, suggesting that education captures the confounding effects of other SES-related factors.
It is also possible that associations between low BMI and increased mortality are confounded by prevalent medical conditions that decrease BMI and increase mortality risk. Although we did not have data on diagnoses of specific medical conditions at baseline, the HEALS cohort consisted of individuals who were apparently healthy at the time of enrolment. Nevertheless, we accounted for potential confounding effects using two strategies: (i) adjusting for baseline symptoms that were associated with both BMI and mortality at a modest P = 0.15 level; and (ii) excluding deaths occurring in the first 2 years of follow-up. Adjustment for symptoms resulted in an attenuation of most significant HRs reported in this analysis, suggesting some partial confounding was present. However, associations between underweight and mortality were stronger in years 3–6 than in years 1–2, suggesting confounding by prevalent medical conditions is not a serious limitation of this analysis.
The low prevalence of overweight/obesity (7%) in this cohort and the lack of a clear association between mortality and BMI ≥23 kg/m2 suggest that overweight/obesity is not a paramount public health issue in this rural Bangladeshi population, a finding consistent with previous reports from rural India10 and Bangladesh.30 However, our ability to observe increased mortality risks for individuals with high BMI may be limited by residual confounding,31 as individuals with high BMI tended to have low mortality-risk characteristics (i.e. female, high education, non-smoking), although we attempt to adjust for these characteristics. The observation that low-education subgroups have the lowest prevalence of overweight/obesity is also consistent with data from other Indian32 and Bangladeshi7 cohorts. However, it is critical to acknowledge that the prevalence of overweight/obesity in South Asian countries may be increasing,7,32 even among low SES subgroups.
Further complicating our conclusions regarding high BMI and mortality is the fact that the relationship between BMI and body composition (i.e. percentage of body fat) is known to vary across populations. For example, for a given BMI value, Asians tend to have a higher body fat percentage and more abdominal fat, compared with Caucasians.33 Consequently, harmful levels of adiposity may be captured at lower BMI values in Asians than in Caucasians.34 Data on waist circumference and waist–hip ratio (measures of abdominal adiposity) were not collected in this study. Due to the limited number of deaths observed in the 6-year follow-up period (n = 393), we were unable to examine the effects of BMI on specific causes of death. Our power was also limited in subgroups experiencing few events, such as women and never smokers. As long-term follow-up data accumulate, we will be able to better assess the relationship between BMI and mortality for specific causes of death and within strata.
In conclusion, severe underweight and, to a lesser degree, moderate underweight are associated with increased mortality in this Bangladeshi cohort, and these associations are stronger among individuals with <5 years of education. We did not find strong evidence of a U- or J-shaped relationship between BMI and mortality in this cohort, emphasizing that underweight (presumably due to malnutrition), is a major determinant of mortality in this rural community.
Funding
National Institutes of Health (grant numbers P42ES010349, R01CA102484, R01CA107431, CA014599).
Conflict of interest statement: None declared.
KEY MESSAGES.
The relationship between BMI and mortality is not well characterized in Bangladesh, where the population’s BMI distribution is shifted towards lower values.
In a large rural Bangladeshi cohort, severe underweight (BMI <16.0 kg/m2) and moderate underweight (16.0–16.9 kg/m2) were associated with increased mortality when compared with normal–low BMI (18.6–22.9 kg/m2).
There was not strong evidence for an association between the highest BMI category (≥23.0 kg/m2) and increased mortality.
The association between low BMI and increased mortality was stronger among individuals with <5 years of education than in those with ≥5 years of education.
References
- 1.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA . 2005;293:1861–67. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 3.Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord . 1996;20:63–75. [PubMed] [Google Scholar]
- 4.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA . 2006;295:776–83. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 5.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med . 2006;355:779–87. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 6.Tsugane S, Sasaki S, Tsubono Y. Under- and overweight impact on mortality among middle-aged Japanese men and women: a 10-y follow-up of JPHC study cohort I. Int J Obes Relat Metab Disord . 2002;26:529–37. doi: 10.1038/sj.ijo.0801961. [DOI] [PubMed] [Google Scholar]
- 7.Shafique S, Akhter N, Stallkamp G, de Pee S, Panagides D, Bloem MW. Trends of under- and overweight among rural and urban poor women indicate the double burden of malnutrition in Bangladesh. Int J Epidemiol . 2007;36:449–57. doi: 10.1093/ije/dyl306. [DOI] [PubMed] [Google Scholar]
- 8.Asia Pacific Cohort Studies Collaboration. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev. 2007;8:191–96. doi: 10.1111/j.1467-789X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 9.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 10.Sauvaget C, Ramadas K, Thomas G, Vinoda J, Thara S, Sankaranarayanan R. Body mass index, weight change and mortality risk in a prospective study in India. Int J Epidemiol . 2008;37:990–1004. doi: 10.1093/ije/dyn059. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian SV, Smith GD. Patterns, distribution, and determinants of under- and overnutrition: a population-based study of women in India. Am J Clin Nutr . 2006;84:633–40. doi: 10.1093/ajcn/84.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ . 1995;73:673–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol . 2006;163:1138–48. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 14.Zaman MM, Yoshiike N. Prevalence of overweight defined by body mass index in a rural adult population of Bangladesh. J Health Popul Nutr . 2003;21:162–63. [PubMed] [Google Scholar]
- 15.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. Int J Obes Relat Metab Disord . 2000;24:807–18. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- 16.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol . 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury ME, Botlero R, Koblinsky M, Saha SK, Dieltiens G, Ronsmans C. Determinants of reduction in maternal mortality in Matlab, Bangladesh: a 30-year cohort study. Lancet . 2007;370:1320–28. doi: 10.1016/S0140-6736(07)61573-6. [DOI] [PubMed] [Google Scholar]
- 18.Hurt LS, Ronsmans C, Saha S. Effects of education and other socioeconomic factors on middle age mortality in rural Bangladesh. J Epidemiol Comm Health . 2004;58:315–20. doi: 10.1136/jech.2003.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronsmans C, Vanneste AM, Chakraborty J, van Ginneken J. Decline in maternal mortality in Matlab, Bangladesh: a cautionary tale. Lancet . 1997;350:1810–14. doi: 10.1016/S0140-6736(97)08012-4. [DOI] [PubMed] [Google Scholar]
- 20.Ronsmans C, Vanneste AM, Chakraborty J, Van Ginneken J. A comparison of three verbal autopsy methods to ascertain levels and causes of maternal deaths in Matlab, Bangladesh. Int J Epidemiol . 1998;27:660–66. doi: 10.1093/ije/27.4.660. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem . 2004;379:512–18. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Knol MJ, van der Tweel, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol . 2007;36:1111–18. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Asso . 1989;84:1074–78. [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics . 1986;42:121–30. [PubMed] [Google Scholar]
- 26.Hussain A, Vaaler S, Sayeed MA, Mahtab H, Ali SM, Khan AK. Type 2 diabetes and impaired fasting blood glucose in rural Bangladesh: a population-based study. Eur J Public Health . 2007;17:291–96. doi: 10.1093/eurpub/ckl235. [DOI] [PubMed] [Google Scholar]
- 27.Pryer JA, Rogers S. Epidemiology of undernutrition in adults in Dhaka slum households, Bangladesh. Eur J Clin Nutr . 2006;60:815–22. doi: 10.1038/sj.ejcn.1602385. [DOI] [PubMed] [Google Scholar]
- 28.Zaman MM, Choudhury SR, Ahmed J, Numan SM, Islam MS, Yoshiike N. Non-biochemical risk factors for cardiovascular disease in general clinic-based rural population of Bangladesh. J Epidemiol . 2004;14:63–68. doi: 10.2188/jea.14.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaman MM, Yoshiike N, Rouf MA, et al. Cardiovascular risk factors: distribution and prevalence in a rural population of Bangladesh. J Cardiovasc Risk . 2001;8:103–8. doi: 10.1177/174182670100800207. [DOI] [PubMed] [Google Scholar]
- 30.Hosegood, Campbell OM. Body mass index, height, weight, arm circumference, and mortality in rural Bangladeshi women: a 19-year longitudinal study. Am J Clin Nutr . 2003;77:341–47. doi: 10.1093/ajcn/77.2.341. [DOI] [PubMed] [Google Scholar]
- 31.Adams KF, Subramanian SV. Commentary: Is the concern regarding overweight/obesity in India overstated? Int J Epidemiol . 2008;37:1005–7. doi: 10.1093/ije/dyn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian SV, Perkins JM, Khan KT. Do burdens of underweight and overweight coexist among lower socioeconomic groups in India? Am J Clin Nutr . 2009;90:369–76. doi: 10.3945/ajcn.2009.27487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rush E, Plank L, Chandu, et al. Body size, body composition, and fat distribution: a comparison of young New Zealand men of European, Pacific Island, and Asian Indian ethnicities. N Z Med J . 2004;117:U1203. [PubMed] [Google Scholar]
- 34.Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev . 2002;3:209–15. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]