Abstract
Background Head and neck cancer (HNC) risk is elevated among lean people and reduced among overweight or obese people in some studies; however, it is unknown whether these associations differ for certain subgroups or are influenced by residual confounding from the effects of alcohol and tobacco use or by other sources of biases.
Methods We pooled data from 17 case–control studies including 12 716 cases and the 17 438 controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for associations between body mass index (BMI) at different ages and HNC risk, adjusted for age, sex, centre, race, education, tobacco smoking and alcohol consumption.
Results Adjusted ORs (95% CIs) were elevated for people with BMI at reference (date of diagnosis for cases and date of selection for controls) ≤18.5 kg/m2 (2.13, 1.75–2.58) and reduced for BMI >25.0–30.0 kg/m2 (0.52, 0.44–0.60) and BMI ≥30 kg/m2 (0.43, 0.33–0.57), compared with BMI >18.5–25.0 kg/m2. These associations did not differ by age, sex, tumour site or control source. Although the increased risk among people with BMI ≤18.5 kg/m2 was not modified by tobacco smoking or alcohol drinking, the inverse association for people with BMI > 25 kg/m2 was present only in smokers and drinkers.
Conclusions In our large pooled analysis, leanness was associated with increased HNC risk regardless of smoking and drinking status, although reverse causality cannot be excluded. The reduced risk among overweight or obese people may indicate body size is a modifier of the risk associated with smoking and drinking. Further clarification may be provided by analyses of prospective cohort and mechanistic studies.
Keywords: BMI, head and neck cancer, smoking
Introduction
Cancers of the oral cavity, pharynx and larynx—referred to collectively as head and neck cancer (HNC)—account for a half a million cancer diagnoses worldwide.1 Although in Europe and North America ∼75% of these cancers are attributed to tobacco smoke and alcohol consumption,2–4 the large proportion of patients who are non-smokers and non-drinkers indicates that other factors may affect risk of these cancers. Most previous studies5–13 have found that people with a body mass index (BMI) <18.5 kg/m2 experience a higher risk of HNC, compared with people with a normal BMI (18.5 to <25.0 kg/m2). Among overweight (25.0 to <30.0 kg/m2) or obese (>30.0 kg/m2) people, HNC risk appears to be lower than among those with a normal BMI.5–13
The independent effect of body size on HNC is difficult to assess because it is strongly affected by alcohol drinking and cigarette smoking,14–16 the two major risk factors for these malignancies. Even following adjustment for alcohol and tobacco consumption, the influence of residual confounding cannot be easily discounted. The limited sample size of previous reports based on individual studies has hindered detailed examination of the association with body size in the small proportion of non-smokers and non-drinkers, among whom confounding by cigarette smoking and alcohol consumption would be especially minimized.
The International Head and Neck Cancer Epidemiology (INHANCE) Consortium has pooled epidemiologic studies of HNC to estimate the effects of risk factors on HNC risk.3 The large number of cases assembled by INHANCE allowed us to estimate more precisely the association with BMI in non-smokers and non-drinkers, as well as in smokers and drinkers. We also examined other potentially important subgroups, such as tumour site, sex and age, as well as the possible methodological issue of whether controls were selected from hospital populations or the general population. In addition to weight at the time of diagnosis, we also examined self-reported weight 2–5 years prior to diagnosis as an estimate of weight prior to weight loss secondary to disease, and also weight at age 20–30 years to evaluate the effects of early body size.
Methods
Data pooling methods for the INHANCE consortium have been previously described in detail elsewhere.3 The INHANCE consortium originally included 15 individual case–control studies (version 1.1), including 10 244 cases and 15 227 controls.17–30 After our earlier publication,3 four additional studies joined the INHANCE consortium including two multicentre studies based in the USA: the New York multicentre study31 (covering New York City, Chicago, Hines, IL, Detroit, MI, New Hyde Park, NY, East Meadow, NW and Philadelphia) and the US multicentre study (covering metropolitan Atlanta, Los Angeles, Santa Clara and San Mateo counties south of San Francisco-Oakland, and the state of New Jersey),32 and studies in Rome33 and Boston.34 In the current analysis, we excluded the Iowa study (556 cases and 760 controls)30 that did not collect information about weight and height, and the Rome study (278 cases and 295 controls)33 because only weight was collected. A total of 17 studies, including 9 in North America, 7 in Europe and 1 in South America (Table 1), contributed data to the current analyses that included 12 716 cases and 17 438 controls, which excluded 49 cases and 101 controls with missing data for age, sex or race/ethnicity and the site of origin of their tumour.
Table 1.
Characteristics of the 12 716 cases and the 17 438 controls with data on body sizea
| Characteristic | Cases |
Controls |
||
|---|---|---|---|---|
| n | % | n | % | |
| Age (years) | ||||
| 12–39 | 500 | 3.9 | 1135 | 6.5 |
| 40–45 | 706 | 5.6 | 1240 | 7.1 |
| 45–49 | 1364 | 10.7 | 1920 | 11.0 |
| 50–54 | 1934 | 15.2 | 2701 | 15.5 |
| 55–59 | 2346 | 18.4 | 1994 | 11.4 |
| 60–64 | 2199 | 17.3 | 2724 | 15.6 |
| 65–69 | 1786 | 14.0 | 2305 | 13.2 |
| 70–74 | 1205 | 9.5 | 1627 | 9.3 |
| 75–94 | 676 | 5.3 | 791 | 4.5 |
| Sex | ||||
| Women | 2759 | 21.7 | 5124 | 29.4 |
| Men | 9957 | 78.3 | 12314 | 70.6 |
| Race | ||||
| Non-Hispanic White | 8870 | 69.8 | 13691 | 78.5 |
| Black | 682 | 5.4 | 770 | 4.4 |
| Hispanic | 208 | 1.6 | 428 | 2.5 |
| Asian | 629 | 4.9 | 665 | 3.8 |
| Other | 136 | 1.1 | 178 | 1.0 |
| Brazilian | 2191 | 17.2 | 1706 | 9.8 |
| Study location | ||||
| Italy multicentre | 1261 | 9.9 | 2716 | 15.6 |
| Latin America multicentre | 2191 | 17.2 | 1706 | 9.8 |
| IARC multicentre | 1559 | 12.3 | 1676 | 9.6 |
| Milan | 416 | 3.8 | 1531 | 8.8 |
| US multicentre | 1114 | 8.8 | 1268 | 7.3 |
| Los Angeles | 417 | 3.3 | 1005 | 5.8 |
| Central Europe | 762 | 6.0 | 907 | 5.2 |
| New York multicentre | 1118 | 8.8 | 906 | 5.2 |
| Tampa | 207 | 1.6 | 897 | 5.1 |
| Switzerland | 516 | 4.1 | 883 | 5.1 |
| Houston | 829 | 6.5 | 865 | 5.0 |
| Aviano | 482 | 3.8 | 855 | 4.9 |
| Boston | 584 | 4.6 | 659 | 3.8 |
| Seattle | 407 | 3.2 | 607 | 3.5 |
| Puerto Rico | 350 | 2.8 | 521 | 3.0 |
| France | 323 | 2.5 | 234 | 1.3 |
| North Carolina | 180 | 1.4 | 202 | 1.2 |
| Study design | ||||
| Hospital-based studies | 9844 | 77.4 | 13 378 | 76.7 |
| Population-based studies | 2872 | 22.6 | 4060 | 23.3 |
| Cigarette smoking status | ||||
| Never | 1341 | 10.5 | 6465 | 37.1 |
| Ever | 11 372 | 89.5 | 10 959 | 62.9 |
| Missing | 3 | 14 | ||
| Pack-years of cigarette smoking | ||||
| Never | 1901 | 15.1 | 6827 | 39.6 |
| 1–10 | 858 | 6.8 | 2539 | 14.7 |
| 11–20 | 1146 | 9.1 | 1986 | 11.5 |
| 21–30 | 1620 | 12.9 | 1747 | 10.1 |
| 31–40 | 1838 | 14.6 | 1391 | 8.1 |
| 41–50 | 1546 | 12.3 | 977 | 5.7 |
| >50–441 | 3681 | 29.2 | 1790 | 10.4 |
| Missing | 126 | 181 | ||
| Alcohol drinking status | ||||
| Never | 1786 | 14.1 | 4462 | 25.6 |
| Ever | 10915 | 85.9 | 12964 | 74.4 |
| Missing | 15 | 12 | ||
| Number of alcohol drinks per day | ||||
| Never | 1786 | 14.7 | 4462 | 26.4 |
| 0 to <1 | 2192 | 18.1 | 4761 | 28.2 |
| 1 to <3 | 2338 | 19.3 | 3618 | 21.4 |
| 3 to <5 | 1522 | 12.5 | 1725 | 10.2 |
| 5 to 8512 | 4290 | 35.4 | 2305 | 13.7 |
| Missing | 588 | 567 | ||
| Height (in metres) | ||||
| 0.65–1.64 | 3168 | 27.2 | 4434 | 26.9 |
| 1.65–1.69 | 2145 | 18.4 | 3138 | 19.0 |
| 1.70–1.74 | 2584 | 22.2 | 3508 | 21.3 |
| 1.75–2.13 | 3764 | 32.3 | 5414 | 32.8 |
| Subsite of tumour | ||||
| Oral cavity | 3740 | 29.4 | ||
| Oral cavity/pharynx NOS | 3690 | 29.0 | ||
| Oropharynx | 917 | 7.2 | ||
| Hypopharynx | 1226 | 9.6 | ||
| Larynx | 2837 | 22.3 | ||
| Head and neck overlap | 306 | 2.4 | ||
| Histology of tumour | ||||
| Squamous cell | 800 | 71.4 | ||
| Other | 320 | 28.6 | ||
| Unknown | 3596 | |||
aINHANCE pooled case–control study of HNC.
IARC = International Agency on Research for Cancer.
Cases were defined as newly diagnosed cancers of the oral cavity, pharynx, oral/pharynx not otherwise specified (NOS) or larynx. For each study, controls were frequency-matched on age and sex, and for some studies: centre (Italy multicentre, Central Europe, IARC multicentre and Latin America studies), hospital (France study), ethnicity (Tampa and Los Angeles studies) and neighbourhood (Los Angeles study). The date of reference was defined as the date of diagnosis for cases and the date of selection for controls with the exception of the Seattle study. In the Seattle study, the date of reference for controls was selected at random from among the possible case diagnosis dates.29 The wording of interview questions was compared across studies to determine data similarities. Variables for ethnicity, education, tumour site and histology, cigarette smoking, other tobacco habits, alcohol consumption, height and weight were pooled across studies, as described in detail elsewhere3 and in brief below.
All height and weight variables were self-reported at the time of interview. Adult height was missing for 1055 cases and 944 controls. Because each study queried weight for different time periods, the total number of cases and controls differ by analysis. Weight at the date of reference was self-reported for 11 547 cases and 15 924 controls with the exception of Central Europe and Seattle. Weight 2–5 years prior to reference was collected for 7654 cases and 9799 controls (from studies: Central Europe, New York Multicentre, Seattle, Boston, Latin America and IARC multicentre studies), and weight between 20 and 30 years of age for 7654 cases and 9799 controls (from studies: Central Europe, Puerto Rico, Los Angeles, Latin America, IARC multicentre, Italy multicentre and US multicentre studies). BMI values were calculated as weight (in kilograms) divided by height squared (in square metres). We were not able to systematically verify self-reported height and weight; therefore, we included all BMI values. Few subjects had values outside the physiological range (BMI < 11 or >60.0 kg/m2); specifically, three cases and five controls for BMI at reference, four cases and four controls for BMI 2–5 years prior to reference and six cases and two controls for BMI at age 20–30 years had BMI values >60.0 kg/m2. BMI values were categorized according to the World Health Organization definitions (≤18.5, >18.5–25.0, >25.0–30.0, >30.0 kg/m2).35 Height was categorized using quartiles of the control distribution.
We used the definitions of smoking of cigarettes, pipes and cigars, and alcohol drinking categories adopted in a previous INHANCE publication.3 Although questions about tobacco use varied across studies, never users of cigarettes, pipes and cigars did not exceed either 1 year of cigarette smoking, 100 cigarettes in a lifetime or ever smoked ‘regularly’ (France and Central Europe studies). Pack-years of cigarette smoking was calculated by multiplying packs (defined as 20 cigarettes) of cigarettes per day and number of years smoking. In the alcohol section of the study questionnaires, subjects were asked if they had been alcohol drinkers, then asked subsequent questions on frequency of drinking, duration of drinking and different types of alcoholic beverages consumed (beer, wine, hard liquors and aperitif). Definition of never drinkers also varied by study, from 0 drinks in a lifetime (France, Central Europe, Aviano, Milan, Italy multicentre and Switzerland studies) to <4 drinks per month (North Carolina study).3 To handle the different volume specification for each type of alcoholic beverage by study, the number of drinks per day was calculated by the frequency of consumption of each alcoholic beverage type weighted by the corresponding duration with the exception of France, Iowa and Tampa studies, in which the average of the frequency of all alcoholic beverage type was used in lieu of missing data for duration.3
For missing data on education level (703 cases and 487 controls for the analysis on BMI at diagnosis; 367 cases and 227 controls for the analysis on BMI 2–5 years prior to diagnosis; 318 cases and 229 controls for the analysis on BMI at age 20–30 years), we applied multiple imputation with the PROC MI procedure in SAS Institute Inc software 9.0 (Cary, NC), for the studies within each of the four geographic regions. We used a logistic regression model36 to predict education level with age, sex, race/ethnicity, study and case–control status.
Unconditional logistic models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Minimally adjusted models controlled for age, study, ethnicity and education level (categorical, see Table 1). Multivariate-adjusted models were further adjusted for pack-years of cigarette smoking (continuous) and drinks per day (continuous). Because cigarette smoking status was associated with BMI in our populations (Supplementary Table 1 available as Supplementary data at IJE online), we were concerned about residual confounding by cigarette smoking. Therefore, we explored separate models with alternate definitions of cigarette smoking, including log-transformed cigarette-years, square root of pack-years with ever smoking status and duration of cigarette smoking with ever smoking status.37,38 The P-values for linear trend were calculated using the ordinal values for the categorical variable in logistic regression models.
Multivariate-adjusted models among all subjects were further stratified by cigarette smoking and alcohol drinking status (non-drinking and non-smoking, and non-smoking and non-drinking subjects), age (<50, ≥50 years), sex, study centre and geographical region (Europe, North America, Latin America and Asia) and whether the study collected population-based or hospital-based controls. Cigarette smokers were further stratified by smoking status (never, current, former), duration of smoking (≤20, >20 to <40, ≥40 years), number of cigarettes smoked per day (<15, 15–20, 21–30, ≥31) among current smokers, and for former smokers time since quitting smoking (≤10, >10 to <20, ≥20 years). HNC cases were stratified by tumour histology and site. Effect measure modification was evaluated by testing for deviation from a multiplicative interaction model, using the log-likelihood ratio test to compare the fit of logistic models with and without an interaction term. Between-study heterogeneity was also examined using the likelihood ratio test.
Results
Study population characteristics
A total of 12 716 cases and 17 438 controls had anthropometry data. Among these participants, cases were older and more likely to be male, shorter in stature (<1.65 m), cigarette smoker and alcohol drinker compared with controls (Table 1). Furthermore, cases were heavier cigarette smokers and alcohol drinkers than controls. Most (71.4%) cases were squamous cell carcinomas. Approximately 70% of cases were diagnosed with cancers of the oro- and hypo-pharynx or larynx, and 30% of the cases were diagnosed with cancers of the oral cavity. A majority of the studies selected controls from hospital patients.
BMI
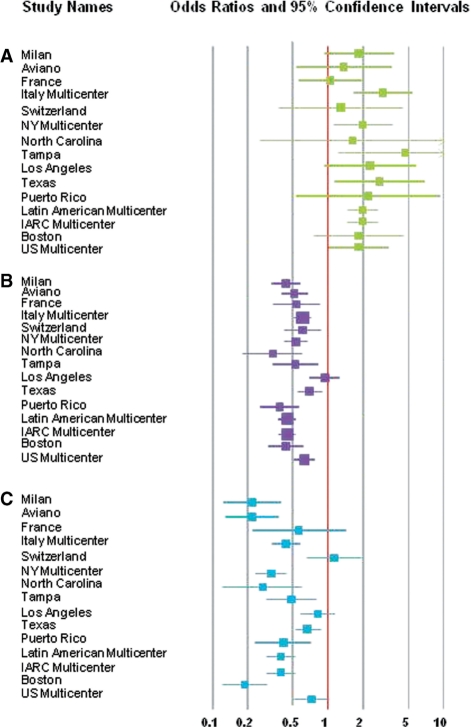
Lean subjects at reference (BMI ≤ 18.5 kg/m2) had twice the risk of HNC (multivariate-adjusted OR = 2.13, 95% CI 1.75–2.58; Table 2), compared with subjects with a BMI between >18.5 and 25.0 kg/m2. Conversely, HNC risk was inversely associated with being overweight (BMI >25.0–30.0 kg/m2) or obese (BMI > 30.0 kg/m2) at reference (multivariate-adjusted OR = 0.52, 95% CI 0.44–0.60; multivariate-adjusted OR = 0.43, 95% CI 0.33–0.57, respectively; Table 2), adjusting for pack-years of cigarette smoking, duration of cigar and pipe smoking and duration of alcohol consumption (Figure 1).
Table 2.
Adjusteda ORs and 95% CIs for the association between HNC and BMI
| BMI measures | Cases | Controls | Minimally-adjustedb |
Multivariate-adjustedc |
||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR | (95% CI) | OR | (95% CI) | |
| BMI (kg/m2) at referenced,e | ||||||
| <18.5 | 918 (8.7) | 382 (2.5) | 2.57 | (2.20–2.99) | 2.13 | (1.75–2.58) |
| 18.5–24.9 | 5749 (54.6) | 6255 (41.5) | 1.00 | 1.00 | ||
| 25.0–29.9 | 2855 (27.1) | 6069 (40.3) | 0.47 | (0.41–0.53) | 0.52 | (0.44–0.60) |
| ≥30.0 | 1011 (9.6) | 2359 (15.7) | 0.43 | (0.35–0.53) | 0.43 | (0.33–0.57) |
| P-value for study heterogeneity | <0.01 | <0.01 | ||||
| P for trendf | <10−6 | <10−6 | ||||
| BMI (kg/m2) at 2–5 years before referenceg | ||||||
| <18.5 | 481 (8.4) | 203 (3.6) | 1.88 | (1.06–3.33) | 1.56 | (0.80–3.02) |
| 18.5–24.9 | 3159 (55.4) | 2456 (43.0) | 1.00 | 1.00 | ||
| 25.0–29.9 | 1523 (26.7) | 2072 (36.3) | 0.55 | (0.44–0.68) | 0.57 | (0.48–0.68) |
| ≥30.0 | 538 (9.4) | 980 (17.2) | 0.43 | (0.31–0.61) | 0.46 | (0.30–0.72) |
| P-value for study heterogeneity | <0.01 | <0.01 | ||||
| P-value for trendf | 0.000295 | 0.0011 | ||||
| BMI (kg/m2) between 20 and 30 years of ageh | ||||||
| <18.5 | 415 (6.3) | 579 (6.7) | 0.99 | (0.80–1.22) | 0.91 | (0.72–1.15) |
| 18.5–24.9 | 4708 (71.9) | 5911 (68.6) | 1.00 | 1.00 | ||
| 25.0–29.9 | 1232 (18.8) | 1748 (20.3) | 0.88 | (0.74–1.05) | 0.88 | (0.73–1.04) |
| ≥30.0 | 191 (2.9) | 375 (4.4) | 0.66 | (0.48–0.91) | 0.62 | (0.44–0.86) |
| P-value for study heterogeneity | <0.01 | 0.03 | ||||
| P-value for trendf | 0.13 | 0.11 | ||||
aINHANCE pooled case–control study of HNC.
bAdjusted for age, sex, study centres, race and education level.
cAdjusted for age, sex, race, education level, study centres, pack-year of cigarette smoking, lifetime duration of pipe use, lifetime duration of cigar use and alcohol drinks per day.
dDate of reference is defined as the date of diagnosis for cases and date of selection for controls.
eAggregate estimates are based on data from the following studies: France, Milan, Tampa, Puerto Rico, Switzerland, North Carolina, Aviano, Italy multicentre, Houston, Boston, New York, Los Angeles and South America.
fP-values for linear trend was calculated using the ordinal values for the categorical variable.
gAggregate estimates are based on data from the following studies: Central Europe, New York, Seattle, Boston, South America and IARC Multicentre.
hAggregate estimates are based on data from the following studies: Italy Multicentre, Centrale Europe, Los Angeles, Puerto Rico, South America and IARC Multicentre.
Figure 1.
Forest plot of study-specific ORs and 95% CIs for the association between HNC risk and BMI reported at reference with categories of <18.5 kg/m2 (leanness, A), 25.0 to <30.0 kg/m2 (overweight, B) and 30.0–60.0 kg/m2 (obese, C), compared with 18.5 to <25.0 kg/m2 (normal). Studies are weighted according to the inverse of the variation of the log OR. The size of the boxes indicates the variance of the log OR.
Comparing the minimally adjusted and the multivariate-adjusted ORs, it is evident that pack-years of cigarette smoking, lifetime duration of pipe use, lifetime duration of cigar use and alcohol drinks per day did not meaningfully confound the association between BMI and HNC risk. However, we further evaluated the influence of possible residual confounding from cigarette smoking among all subjects by controlling for alternative definitions of cigarette smoking (including log-transformed cigarette-years, square root of pack-years with ever smoking status and duration of cigarette smoking with ever smoking status);37,38 however, these alternate definitions of cigarette smoking did not alter the results for BMI (data not shown).
Results for BMI 2–5 years prior to the reference date were similar to the results for BMI at reference (Table 2). Furthermore, BMI > 30.0 kg/m2 at age 20–30 years was associated with a lower risk of HNC (multivariate-adjusted OR = 0.62, 95% CI 0.44–0.86) compared with subjects with BMI >18.5–25.0 kg/m2. No association was found between leanness (BMI < 18.5 kg/m2) at age 20–30 years and risk of HNC (multivariate-adjusted OR = 0.91, 95% CI 0.72–1.15). Additional analyses restricted to studies that reported all three BMI time points (including BMI at reference, 2–5 years prior to reference and at age 20–30 years) were not undertaken due to the small numbers from two studies (Latin American and IARC multicentre studies).
The direction of the risk estimates found in Table 2 was similar to those for individual studies, although the magnitude of the association differed significantly across studies (P-value for between-study heterogeneity was <0.01), and there were no patterns by geographical region (Supplementary Table 2 available as Supplementary data at IJE online). The ORs and 95% CIs from random effects models (data not shown) were similar to those shown in Table 2 from fixed effect models.
Effect measure modification by cigarette smoking and alcohol consumption
Among never tobacco smokers and never alcohol drinkers (Table 3), the associations for overweight (multivariate-adjusted OR = 0.94, 95% CI 0.49–1.80) and obese (multivariate-adjusted OR = 0.95, 95% CI 0.47–1.91) subjects were attenuated towards the null, whereas lean subjects at reference experienced an elevated risk of HNC (multivariate-adjusted OR = 3.13, 95% CI 0.73–13.40). The elevated risk associated with leanness was sustained for BMI 2–5 years prior to reference and for BMI at age 20–30 years among never tobacco smokers and never alcohol drinkers. Overweight and obesity were not associated with a lower risk of HNC at BMI 2–5 years before reference or at age 20–30 years (Table 3). Supplementary Tables S3 and S4 (available as Supplementary data at IJE online) show BMI associations stratified by tobacco use only and adjusted for alcohol intake, and stratified by alcohol use only adjusted for tobacco use, respectively. Overall, leanness at reference was inversely associated with HNC risk in all strata. BMI > 25 kg/m2 was associated with HNC risk in all strata with the exception of never tobacco users (>25.0–30.0 kg/m2: multivariate-adjusted OR = 0.84, 95% CI 0.70–1.00; >30 kg/m2: multivariate-adjusted OR = 0.82, 95% CI 0.65–1.02; Supplementary Table 5 available as Supplementary data at IJE online).
Table 3.
Multivariate-adjusted ORs and 95% CIs for the association between HNC and BMI by alcohol drinking and cigarette smoking statusa
| BMI measures | Among never tobacco smokers and never alcohol drinkers |
Among ever tobacco smokers and ever alcohol drinkers |
||||||
|---|---|---|---|---|---|---|---|---|
| Case n (%) | Control n (%) | ORb | (95% CI) | Case n (%) | Control n (%) | ORc | (95% CI) | |
| BMI (kg/m2) at referenced,e | ||||||||
| <18.5 | 40 (8.3) | 118 (4.8) | 3.13 | (0.73–13.40) | 650 (7.7) | 167 (2.1) | 2.01 | (1.60–2.52) |
| 18.5–24.9 | 183 (38.0) | 1017 (41.4) | 1.00 | 4794 (55.0) | 3287 (42.0) | 1.00 | ||
| 25.0–29.9 | 145 (30.0) | 859 (35.0) | 0.94 | (0.49–1.80) | 2271 (26.1) | 3222 (41.2) | 0.50 | (0.45–0.56) |
| ≥30.0 | 113 (23.5) | 461 (18.8) | 0.95 | (0.47–1.91) | 695 (8.0) | 1142 (14.6) | 0.38 | (0.30–0.49) |
| P-value for study heterogeneity | 0.02 | 0.01 | ||||||
| P for trendf | 0.49 | <10−6 | ||||||
| BMI (kg/m2) 2–5 years before referenceg | ||||||||
| <18.5 | 28 (12.0) | 81 (8.8) | 2.64 | (1.50–4.67) | 245 (5.4) | 73 (2.3) | 1.39 | (0.80–2.44) |
| 18.5–24.9 | 93 (39.9) | 419 (45.5) | 1.00 | 2598 (57.7) | 1335 (42.5) | 1.00 | ||
| 25.0–29.9 | 70 (30.0) | 271 (29.5) | 0.97 | (0.67–1.41) | 1259 (28.0) | 1196 (38.1) | 0.58 | (0.49–0.68) |
| ≥30.0 | 42 (18.0) | 149 (16.2) | 1.09 | (0.70–1.71) | 398 (8.8) | 539 (17.1) | 0.42 | (0.29–0.60) |
| P-value for study heterogeneity | 0.02 | 0.03 | ||||||
| P-value for trendf | 0.17 | 0.00042 | ||||||
| BMI (kg/m2) between 20 and 30 years of ageh | ||||||||
| <18.5 | 24 (10.8) | 99 (8.9) | 1.76 | (0.98–3.19) | 326 (5.9) | 271 (5.7) | 0.88 | (0.73–1.07) |
| 18.5–24.9 | 135 (60.5) | 743 (66.6) | 1.00 | 4038 (73.2) | 3333 (69.6) | 1.00 | ||
| 25.0–29.9 | 50 (22.4) | 218 (19.5) | 1.26 | (0.84–1.90) | 1018 (18.4) | 1001 (20.9) | 0.82 | (0.73–0.91) |
| ≥30.0 | 14 (6.3) | 56 (5.0) | 1.15 | (0.57–2.35) | 137 (2.5) | 185 (3.9) | 0.50 | (0.39–0.65) |
| P-value for study heterogeneity | 0.08 | 0.36 | ||||||
| P for trendf | 0.90 | <10−5 | ||||||
aINHANCE pooled case–control study of HNC.
bAdjusted for age, sex, study centres, race and education level.
cAdjusted for age, sex, race, education level, study centres, pack-year of cigarette smoking, lifetime duration of pipe use, lifetime duration of cigar use and alcohol drinks per day.
dDate of reference is defined as the date of diagnosis for cases and date of selection for controls.
eAggregate estimates are based on data from the following studies: France, Milan, Tampa, Puerto Rico, Switzerland, Aviano, Italy multicentre, Houston, Boston, New York, Los Angeles and South America.
fP-values for linear trend was calculated using the ordinal values for the categorical variable.
gAggregate estimates are based on data from the following studies: Central Europe, New York, Seattle, Boston, South America and IARC Multicentre.
hAggregate estimates are based on data from the following studies: Italy Multicentre, Central Europe, Los Angeles, Puerto Rico, South America and IARC Multicentre.
Among tobacco users, we further examined the relationship between BMI and risk of HNC by characteristics of smoking habits (Supplementary Tables 5–7 available as Supplementary data at IJE online). Similar to results presented overall (Table 2), obesity (at reference, 2–5 years prior to reference and at age 20–30 years) was associated with lower risk of HNC among both current and former tobacco users (Supplementary Table 5 available as Supplementary data at IJE online). Leanness was associated with higher risk of HNC among current smokers (multivariate-adjusted OR = 2.11, 95% CI 1.48–3.01; Supplementary Table 5 available as Supplementary data at IJE online), persisted for current smoking of <15, 15–20 and 21–30 cigarettes/day, but was weaker, albeit imprecise, for smokers of >30 cigarettes/day (multivariate-adjusted OR = 1.27, 95% CI 0.79–2.03; Supplementary Table 6 available as Supplementary data at IJE online). Current smokers had a 51 and 62% lower risk, respectively, associated with being overweight or obese, compared with current smokers with BMI >18.5–25.0 kg/m2 (multivariate-adjusted OR = 0.49, 95% CI 0.43–0.56 and OR = 0.38, 95% CI 0.27–0.54, respectively, Supplementary Table 5 available as Supplementary data at IJE online), regardless of number of cigarettes per day (Supplementary Table 6 available as Supplementary data at IJE online). The multivariate-adjusted ORs for BMI at reference and 2–5 years from reference were slightly closer to the null for former tobacco users than those observed for current tobacco users (P-value for interaction was ≤0.0001; Supplementary Table 5 available as Supplementary data at IJE online). As the number of years since quitting increased, the decreased risk associated with obesity and the increased risk associated with leanness diminished (Supplementary Table 7 available as Supplementary data at IJE online). Results were similar whether any tobacco product (data not shown) or cigarette tobacco (as shown in tables presented here) were considered.
The inverse association for obesity appeared to be slightly stronger for heavier drinkers than for lighter drinkers or non-drinkers (Supplementary Table 8 available as Supplementary data at IJE online).
Effect measure modification by other factors
Although the estimates for leanness, compared with BMI 18.5–25.0 kg/m2, were consistently elevated for risk of site-specific HNC, the ORs were stronger, ranging from 2.20 to 2.85, for squamous cell carcinomas risk of the oral cavity, hypopharynx and oropharynx compared with the ∼1.6 fold higher risk of laryngeal/oral cancer (Supplementary Table 9 available as Supplementary data at IJE online). The inverse associations with larger body size also varied by site-specific HNC risk but all estimates exhibited a decreased association (Supplementary Table 9 available as Supplementary data at IJE online). The association between body size and HNC risk was in similar directions for both men and women (Supplementary Table 10 available as Supplementary data at IJE online), although the magnitude of the association for BMI at reference was significantly different than that for men (P-value for interaction was 0.004). Age was also an effect measure modifier for leanness at reference and 2–5 years prior to reference, although estimates for overweight and obesity were similar for subjects <50 and ≥50 years (Supplementary Table 11 available as Supplementary data at IJE online). Supplementary Table 12 (available as Supplementary data at IJE online) displays the ORs for BMI by the type of control selection used in the study designs. ORs were similar despite the source of controls for BMI at reference and BMI at age 20–30 years, although the association for BMI 2–5 years prior to reference was stronger for studies with hospital-based controls compared with those with population-based controls.
Discussion
In a pooled analysis of 17 international studies, we found that lean subjects were at higher risk of HNC, whereas heavy subjects were at a lower risk, compared with subjects with a normal body size, after adjustment for major HNC risk factors, cigarette smoking and alcohol drinking. These results confirm the inverse association between BMI and HNC risk observed in previous studies.5–13 Although these individual studies had fewer subjects who were non-smokers and non-drinkers to obtain estimates that were independent of smoking and alcohol use. We found that the elevated risk of HNC observed among lean subjects was evident among cigarette smokers and alcohol drinkers, as well as non-smokers and non-drinkers. However, the reduced risk related to higher BMI was limited only to cigarette smokers and alcohol drinkers in our analysis. Furthermore, examination of stratification by only tobacco use or only alcohol use suggested that the association between BMI and HNC risk was more confounded by tobacco use.
One explanation for our findings is that, in the time shortly before diagnosis, undiagnosed cancer lesions in the head and neck may cause dysphagia or odynophagia or may alter taste and appetite, leading to a reduction of overall caloric intake and weight loss. For example, in a study of 407 patients with newly diagnosed second primary or recurrent tumours, on average, a 5–10% weight loss before diagnosis in ∼30–53% of cases was observed.39 Reported weight, therefore, may misrepresent usual adult weight, which may be the more biologically relevant time frame. A 5–10% weight loss prior to diagnosis of cases may shift some cases into a lower BMI category at the time of study interview. If it is assumed that 25–50% of the reportedly lean subjects in our study had a ‘true’ normal adult body size then the corrected unadjusted OR for leanness would be 2.01 (95% CI 1.76–2.29) to 1.21 (95% CI 1.05–1.40). These estimates suggest that misclassification bias would need to be quite prevalent before risk estimates for leanness are significantly attenuated towards the null and thus modify our conclusions. Lastly, assuming 25% of our reportedly lean cases had a normal adult BMI and 25% of our reportedly normal weight cases had a true overweight BMI during adulthood, the OR for leanness would move farther from the null (OR = 2.65, 95% CI 2.32–3.02), whereas the OR for overweight would move towards the null (OR = 0.97, 95% CI 0.92–1.03), indicating the robustness of leanness estimates to the influence of differential misclassification, but casting some doubt on our estimates for overweight subjects. Furthermore, we found that leanness self reported 2–5 years prior to reference was also associated with elevated risk of HNC. In aggregate, these observations suggest that recent weight loss in cases is unlikely to cause a substantial bias to our observed results for leanness at reference (at diagnosis for cases).
Obesity, but not leanness, between 20 and 30 years of age was associated with lower risk for HNC. However, among people who never smoked or drank, data suggested an increase in risk with leanness and little to no association with being overweight or obese. Taken together with results for BMI over the life course, a predisposition for leanness at a young age may increase risk of HNC in subjects who do not drink or smoke. In contrast, drinkers and smokers who were also overweight or obese at any age appear to have a lower risk of HNC.
Elevated risks among lean subjects and reduced risks among overweight and obese subjects have also been found for other smoking-related malignancies, including cancers of the lung 40–45 and squamous cell carcinoma of the esophagus.46 These consistent findings from prospective cohort and retrospective case–control studies suggest that weight loss due to pre-existing conditions is unlikely to account for the observed associations. Moreover, exclusions of people who died in the first 5 years of follow-up and those with pre-existing disease in one prospective study did not significantly alter results,42 which further suggests weight loss measured close to disease onset, such as in case–control studies represented here, does not appreciably contribute to exposure misclassification. These results, however, do not provide additional insight to whether cigarette smoking confounds or modifies the relationship between body size and risk of these smoking-related cancers. In the prospective studies of lung cancer,41,42,44 the associations between leanness and mortality or incidence was minimally affected when adjusted for smoking, and weaker, although still evident, in non-smokers.41,42,44
Another possible explanation for our BMI findings stratified by alcohol/smoking status is that the underlying etiology and molecular mechanisms of smoking-related malignancies do not apply to those that develop in non-smokers.47 For example, in lung cancer,47 it has been shown that the somatic alterations in key tumour suppressor and oncogenes, risk factors and clinical features differ among smokers and non-smokers. If this is also true for tumours of the head and neck, it is possible that differences we observed in effect estimates between smokers and non-smokers may indicate true modification of risk.
Inconsistent definitions of a cigarette smoker and an alcohol drinker across studies may have led to the inclusion of some individuals who minimally used tobacco or alcohol into the ‘never user of tobacco’ and ‘never drinker’ categories as defined in this study3,44 and in a study of lung cancer,44 which may be a potential source of study heterogeneity detected in our effect estimates. However, estimated ORs for individual studies were in similar directions and differed only in the magnitude of the association. The exact sources of differences between studies are unknown, but our stratified analyses suggest that they are unlikely to be due to geographical region or sources of controls. The accuracy of self-reported height and weight is high for men and women but slightly lower for obese individuals.48 Yet, even small underestimations of weight, accompanied by overestimations of height, can shift an individual’s BMI into a lower category. A downward shift in BMI, calculated from self-reported height and weight in our study, is likely to be non-differential with respect to case–control status. Our results for BMI at age 20–30 years self-reported at reference may be particularly prone to random misclassification and may explain why these estimates are closer to the null. We were further limited to evaluating the association with body size since we only had information on BMI, which is a measure of overall adiposity.49
There has been limited research on connections between leanness and likely mechanisms of head and neck carcinogenesis, although altered caloric absorption and utilization as well as levels of oxidative stress markers and DNA adducts have been proposed to mediate the relationship between body size and HNC risk.14–16,50–55 Some,50,51 but not all, studies have shown that lean, compared with heavy but otherwise healthy, adults have higher urinary levels of 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative DNA damage that is also elevated in smokers.52–54 This relationship was stronger51 or only evident53 among cigarette smokers but not non-smokers. Similarly, DNA adducts, which represent an integrated indicator of exposure, metabolism and DNA repair, have also been observed to be higher in lean, compared with overweight, smokers.55 If greater propensity for elevated levels of oxidative stress markers and DNA adducts explains the association between leanness and higher HNC risk among smokers, we would not expect to have observed a higher risk of HNC for lean people regardless of smoking status, and an inverse association with overweight and obese people only among smokers and drinkers. Moderate smokers, who on average weigh less than non-smokers,14–16 despite similar caloric intakes, have been hypothesized to have higher smoking-induced metabolism and caloric utilization or loss due to faster bowel motility.14 However, the distribution of BMI in our control populations was unrelated to number of cigarettes smoked per day, and the lower risk for higher BMI and elevated risk for leanness persisted across categories of number of cigarettes smoked per day. Additional studies are needed to explore biological pathways that mediate the relationship between body size and head and neck and other smoking-related cancers.
Based on a large pool of case–control studies, our findings provide the strongest evidence to date that leanness is a risk factor for HNC independent of the confounding effects of cigarette smoking and alcohol drinking. Stronger tests of the relationship between BMI and HNC could come from large prospective cohorts to eliminate the possibility of bias due to pre-existing conditions, as well as functional studies, to explain the relationship between body size and HNC risk. Although only a small portion of adults are lean,56 our findings, if confirmed, may shed light on biological mechanisms independent of tobacco smoke and ethanol consumption.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Grant from the US National Institutes of Health (NIH), National Cancer Institute (NCI) [R03CA113157]. The individual studies were funded by the following grants: Milan study: Italian Association for Research on Cancer (AIRC); Aviano and Italy multicentre studies: Italian Association for Research on Cancer (AIRC), Italian League Against Cancer and Italian Ministry of Research; France study: Swiss League against Cancer [KFS1069-09-2000], Fribourg League against Cancer [FOR381.88], Swiss Cancer Research [AKT 617] and Gustave-Roussy Institute [88D28]; Swiss study: Swiss League against Cancer and the Swiss Research against Cancer/Oncosuisse [KFS-700, OCS-1633]; Central Europe study: World Cancer Research Fund and the European Commission’s INCO-COPERNICUS Programme [Contract No. IC15-CT98-0332]; New York study: National Institutes of Health (NIH) US [P01CA068384 K07CA104231]; Seattle study: National Institutes of Health (NIH) US [R01CA048896, R01DE012609]; Boston study: National Institutes of Health (NIH) US [R01CA078609, R01CA100679]; Iowa study: National Institutes of Health (NIH) US [NIDCR R01DE11979, NIDCR R01DE13110, NIH FIRCA TW01500] and Veterans Affairs Merit Review Funds; North Carolina study: National Institutes of Health (NIH) US [R01CA61188], and in part by a grant from the National Institute of Environmental Health Sciences [P30ES010126]; Tampa study: National Institutes of Health (NIH) US [P01CA068384, K07CA104231, R01DE13158]; Los Angeles study: National Institute of Health (NIH) US [P50CA90388, R01DA11386, R03CA77954, T32CA09142, U01CA96134, R21ES011667] and the Alper Research Programme for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Centre; Houston study: National Institutes of Health (NIH) US [R01ES11740, R01CA100264]; Puerto Rico study: jointly funded by National Institutes of Health (NCI) US and NIDCR intramural programmes; Latin America study: Fondo para la Investigacion Cientifica y Tecnologica (FONCYT) Argentina, IMIM (Barcelona), Fundação de Amparo à Pesquisa no Estado de São Paulo (FAPESP) [No 01/01768-2], and European Commission [IC18-CT97-0222]; IARC Multicentre study: Fondo de Investigaciones Sanitarias (FIS) of the Spanish Government [FIS 97/0024, FIS 97/0662, BAE 01/5013], International Union Against Cancer (UICC), and Yamagiwa-Yoshida Memorial International Cancer Study Grant.
KEY MESSAGES.
Leanness near the time of diagnosis was associated with higher risk of HNC among all participants, including those who did not drink alcohol or smoke cigarettes.
Excess adiposity may be associated with lower risk of HNC, although the relationship may be limited to drinkers and smokers.
Supplementary Material
References
- 1.Ferlay J, Bray F, Pisani P, Parkin D. Lyon: IARC Press; 2004. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No.5. [Google Scholar]
- 2.Negri E, La Vecchia C, Franceschi S, Tavani A. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol Biomarkers Prev. 1993;2:189–93. [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 4.Blot WJ, McLaughlin JK, Devasa SS, Fraumeni JF., Jr . Cancers of the oral cavity and pharynx. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 2nd. New York: Oxford University Press; 1996. [Google Scholar]
- 5.Franceschi S, Dal Maso L, Levi F, Conti E, Talamini R, La Vecchia C. Leanness as early marker of cancer of the oral cavity and pharynx. Ann Oncol. 2001;12:331–36. doi: 10.1023/a:1011191809335. [DOI] [PubMed] [Google Scholar]
- 6.Hashibe M, Sankaranarayanan R, Thomas G, et al. Body mass index, tobacco chewing, alcohol drinking and the risk of oral submucous fibrosis in Kerala, India. Cancer Causes Control. 2002;13:55–64. doi: 10.1023/a:1013991025848. [DOI] [PubMed] [Google Scholar]
- 7.Nieto A, Sanchez MJ, Martinez C, et al. Lifetime body mass index and risk of oral cavity and oropharyngeal cancer by smoking and drinking habits. Br J Cancer. 2003;89:1667–71. doi: 10.1038/sj.bjc.6601347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieto A, Sanchez MJ, Quintana MJ, et al. BMI throughout life, intake of vitamin supplements and oral cancer in Spain. IARC Sci Publ. 2002;156:259–61. [PubMed] [Google Scholar]
- 9.D'A;vanzo B, La Vecchia C, Talamini R, Franceschi S. Anthropometric measures and risk of cancers of the upper digestive and respiratory tract. Nutr Cancer. 1996;26:219–27. doi: 10.1080/01635589609514478. [DOI] [PubMed] [Google Scholar]
- 10.Garavello W, Randi G, Bosetti C, et al. Body size and laryngeal cancer risk. Ann Oncol. 2006;17:1459–63. doi: 10.1093/annonc/mdl166. [DOI] [PubMed] [Google Scholar]
- 11.Kabat GC, Chang CJ, Wynder EL. The role of tobacco, alcohol use, and body mass index in oral and pharyngeal cancer. Int J Epidemiol. 1994;23:1137–44. doi: 10.1093/ije/23.6.1137. [DOI] [PubMed] [Google Scholar]
- 12.Kreimer AR, Randi G, Herrero R, Castellsague X, La Vecchia C, Franceschi S. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: analysis from the IARC multinational case-control study. Int J Cancer. 2006;118:2293–97. doi: 10.1002/ijc.21577. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40:207–13. doi: 10.1016/j.oraloncology.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DR, Jr, Gottenborg S. Smoking and weight: the Minnesota Lipid Research Clinic. Am J Public Health. 1981;71:391–96. doi: 10.2105/ajph.71.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John U, Hanke M, Rumpf HJ, Thyrian JR. Smoking status, cigarettes per day, and their relationship to overweight and obesity among former and current smokers in a national adult general population sample. Int J Obes. 2005;29:1289–94. doi: 10.1038/sj.ijo.0803028. [DOI] [PubMed] [Google Scholar]
- 16.Istvan JA, Cunningham TW, Garfinkel L. Cigarette smoking and body weight in the Cancer Prevention Study I. Int J Epidemiol. 1992;21:849–53. doi: 10.1093/ije/21.5.849. [DOI] [PubMed] [Google Scholar]
- 17.Baron AE, Franceschi S, Barra S, Talamini R, La Vecchia C. A comparison of the joint effects of alcohol and smoking on the risk of cancer across sites in the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2:519–23. [PubMed] [Google Scholar]
- 18.Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50:6502–507. [PubMed] [Google Scholar]
- 19.Hashibe M, Boffetta P, Zaridze D, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006;15:696–703. doi: 10.1158/1055-9965.EPI-05-0710. [DOI] [PubMed] [Google Scholar]
- 20.Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23:1229–34. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Morgenstern H, Greenland S, et al. Polymorphism of Xeroderma Pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006;118:714–20. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Shi Q, Liu Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1188–93. doi: 10.1158/1055-9965.EPI-04-0501. [DOI] [PubMed] [Google Scholar]
- 23.Benhamou S, Tuimala J, Bouchardy C, Dayer P, Sarasin A, Hirvonen A. DNA repair gene XRCC2 and XRCC3 polymorphisms and susceptibility to cancers of the upper aerodigestive tract. Int J Cancer. 2004;112:901–904. doi: 10.1002/ijc.20474. [DOI] [PubMed] [Google Scholar]
- 24.Bosetti C, Gallus S, Trichopoulou A, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12:1091–94. [PubMed] [Google Scholar]
- 25.Hayes RB, Bravo-Otero E, Kleinman DV, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 26.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 27.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77:705–709. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:185–91. [PubMed] [Google Scholar]
- 29.Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64:4049–54. doi: 10.1158/0008-5472.CAN-03-3425. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Ritchie JM, Smith EM, Zhang Z, Turek LP, Haugen TH. Alcohol dehydrogenase 3 and risk of squamous cell carcinomas of the head and neck. Cancer Epidemiol Biomarkers Prev. 2005;14:626–32. doi: 10.1158/1055-9965.EPI-04-0343. [DOI] [PubMed] [Google Scholar]
- 31.Muscat JE, Richie JP, Jr, Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996;56:5192–97. [PubMed] [Google Scholar]
- 32.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–87. [PubMed] [Google Scholar]
- 33.Boccia S, Cadoni G, Sayed-Tabatabaei FA, et al. CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2 polymorphisms, smoking, consumption of alcohol and fruit and vegetables and risk of head and neck cancer. J Cancer Res Clin Oncol. 2008;134:93–100. doi: 10.1007/s00432-007-0254-5. [DOI] [PubMed] [Google Scholar]
- 34.Peters ES, McClean MD, Liu M, Eisen EA, Mueller N, Kelsey KT. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol Biomarkers Prev. 2005;14:476–82. doi: 10.1158/1055-9965.EPI-04-0431. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Geneva: WHO; 1998. Report of a WHO consultation on obesity. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 36.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 37.Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–23. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 38.Siemiatycki J. Synthesizing the lifetime history of smoking. Cancer Epidemiol Biomarkers Prev. 2005;14:2294–95. doi: 10.1158/1055-9965.EPI-05-0775. [DOI] [PubMed] [Google Scholar]
- 39.Jager-Wittenaar H, Dijkstra PU, Vissink A, van der Laan BF, van Oort RP, Roodenburg JL. Critical weight loss in head and neck cancer—prevalence and risk factors at diagnosis: an explorative study. Support Care Cancer. 2007;15:1045–50. doi: 10.1007/s00520-006-0212-9. [DOI] [PubMed] [Google Scholar]
- 40.Knekt P, Heliovaara M, Rissanen A, et al. Leanness and lung-cancer risk. Int J Cancer. 1991;49:208–13. doi: 10.1002/ijc.2910490211. [DOI] [PubMed] [Google Scholar]
- 41.Olson JE, Yang P, Schmitz K, Vierkant RA, Cerhan JR, Sellers TA. Differential association of body mass index and fat distribution with three major histologic types of lung cancer: evidence from a cohort of older women. Am J Epidemiol. 2002;156:606–15. doi: 10.1093/aje/kwf084. [DOI] [PubMed] [Google Scholar]
- 42.Henley SJ, Flanders WD, Manatunga A, Thun MJ. Leanness and lung cancer risk: fact or artifact? Epidemiology. 2002;13:268–76. doi: 10.1097/00001648-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Kark JD, Yaari S, Rasooly I, Goldbourt U. Are lean smokers at increased risk of lung cancer? The Israel Civil Servant Cancer Study. Arch Intern Med. 1995;155:2409–16. [PubMed] [Google Scholar]
- 44.Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18:607–12. doi: 10.1097/ede.0b013e31812713d1. [DOI] [PubMed] [Google Scholar]
- 45.Kabat GC, Wynder EL. Body mass index and lung cancer risk. Am J Epidemiol. 1992;135:769–74. doi: 10.1093/oxfordjournals.aje.a116363. [DOI] [PubMed] [Google Scholar]
- 46.Gallus S, La Vecchia C, Levi F, Simonato L, Dal Maso L, Franceschi S. Leanness and squamous cell oesophageal cancer. Ann Oncol. 2001;12:975–79. doi: 10.1023/a:1011104809985. [DOI] [PubMed] [Google Scholar]
- 47.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 48.Visscher TL, Viet AL, Kroesbergen IH, Seidell JC. Underreporting of BMI in adults and its effect on obesity prevalence estimations in the period 1998 to 2001. Obesity. 2006;14:2054–63. doi: 10.1038/oby.2006.240. [DOI] [PubMed] [Google Scholar]
- 49.Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002;132:3451S–45S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]
- 50.Kasai H, Iwamoto-Tanaka N, Miyamoto T, et al. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative dna damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res. 2001;92:9–15. doi: 10.1111/j.1349-7006.2001.tb01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–47. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 52.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res. 1996;56:2546–49. [PubMed] [Google Scholar]
- 53.Mizoue T, Kasai H, Kubo T, Tokunaga S. Leanness, smoking, and enhanced oxidative DNA damage. Cancer Epidemiol Biomarkers Prev. 2006;15:582–85. doi: 10.1158/1055-9965.EPI-05-0658. [DOI] [PubMed] [Google Scholar]
- 54.Mizoue T, Tokunaga S, Kasai H, Kawai K, Sato M, Kubo T. Body mass index and oxidative DNA damage: A longitudinal study. Cancer Sci. 2007;98:1254–58. doi: 10.1111/j.1349-7006.2007.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godschalk RW, Feldker DE, Borm PJ, Wouters EF, van Schooten FJ. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:790–93. [PubMed] [Google Scholar]
- 56.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.