Abstract
Background The human immunodeficiency virus (HIV) infectiousness of anal intercourse (AI) has not been systematically reviewed, despite its role driving HIV epidemics among men who have sex with men (MSM) and its potential contribution to heterosexual spread. We assessed the per-act and per-partner HIV transmission risk from AI exposure for heterosexuals and MSM and its implications for HIV prevention.
Methods Systematic review and meta-analysis of the literature on HIV-1 infectiousness through AI was conducted. PubMed was searched to September 2008. A binomial model explored the individual risk of HIV infection with and without highly active antiretroviral therapy (HAART).
Results A total of 62 643 titles were searched; four publications reporting per-act and 12 reporting per-partner transmission estimates were included. Overall, random effects model summary estimates were 1.4% [95% confidence interval (CI) 0.2–2.5)] and 40.4% (95% CI 6.0–74.9) for per-act and per-partner unprotected receptive AI (URAI), respectively. There was no significant difference between per-act risks of URAI for heterosexuals and MSM. Per-partner unprotected insertive AI (UIAI) and combined URAI–UIAI risk were 21.7% (95% CI 0.2–43.3) and 39.9% (95% CI 22.5–57.4), respectively, with no available per-act estimates. Per-partner combined URAI–UIAI summary estimates, which adjusted for additional exposures other than AI with a ‘main’ partner [7.9% (95% CI 1.2–14.5)], were lower than crude (unadjusted) estimates [48.1% (95% CI 35.3–60.8)]. Our modelling demonstrated that it would require unreasonably low numbers of AI HIV exposures per partnership to reconcile the summary per-act and per-partner estimates, suggesting considerable variability in AI infectiousness between and within partnerships over time. AI may substantially increase HIV transmission risk even if the infected partner is receiving HAART; however, predictions are highly sensitive to infectiousness assumptions based on viral load.
Conclusions Unprotected AI is a high-risk practice for HIV transmission, probably with substantial variation in infectiousness. The significant heterogeneity between infectiousness estimates means that pooled AI HIV transmission probabilities should be used with caution. Recent reported rises in AI among heterosexuals suggest a greater understanding of the role AI plays in heterosexual sex lives may be increasingly important for HIV prevention.
Keywords: HIV, anal intercourse, infectivity, transmission probability, review, meta-analysis, HAART
Introduction
Studies systematically reviewing much-needed estimates of human immunodeficiency virus (HIV) infectiousness for various modes of transmission have recently been published,1–4 partly in response to discussions regarding the relative importance of each mode for HIV epidemics world wide.1,2 However, none has specifically focused on anal intercourse (AI), despite its role driving HIV epidemics among men who has sex with men (MSM). AI may also contribute substantially to heterosexual epidemics in sub-Saharan Africa and elsewhere.3
AI within heterosexual relationships is not an uncommon practice but is often underreported.4,5 It is estimated that the absolute number of women in the USA practising unprotected receptive AI (URAI) is ∼7-fold higher than the number of MSM practising URAI,6 while 75% of study participants in the South African site of a multi-centre microbicides trial reported URAI during follow-up.7
Highly active antiretroviral therapy (HAART) is likely to substantially reduce risk from AI, as demonstrated by randomized controlled trials for mother-to-child transmission8 and observational studies for heterosexual partnerships.9,10 Although some ecological evidence suggests a reduction in AI infectiousness due to HAART may have occurred,11 no direct empirical evidence is yet available. However, the high infectiousness associated with AI, as reviewed here, indicates that even with a substantial reduction due to HAART, the residual infectiousness could still present a high risk to partners, especially if coupled with risk compensation.11
Our aims were systematically to review the literature on estimates of unprotected AI (UAI) per-act and per-partner transmission probabilities for heterosexuals and MSM, to investigate the relationship between per-act and per-partner summary estimates and to explore the implications of practising URAI for prevention of HIV transmission in the presence of HAART.
Methods
The systematic review was undertaken following MOOSE guidelines for reviews of observational studies.12
Search strategy
As previously reported13 (details provided in Supplementary data available at IJE online).
Selection criteria and data extraction
Empirical per-act and per-partner (irrespective of partnership duration and frequency of sex acts) estimates were extracted. Abstracts of pre-1990, studies using sample sizes of less than 10 and estimates derived from dynamic modelling studies fitted to empirical HIV prevalence curves were excluded. Estimates where infection of partners was ascertained clinically14 or only through questioning the index,15 rather than by laboratory HIV diagnosis were excluded. Per-partner estimates from studies of heterosexuals were restricted to including only those sexual partners where AI was practised for ≥50% of all sex acts within the partnership. There was no other restriction by study design or language of publication. Each relevant publication was examined by two investigators for data extraction.
Quantitative data synthesis and statistical methods
Meta-analysis
Stata 10.0 produced random effects model summary estimates. For studies not providing a point estimate, the arithmetic midpoint of the confidence bounds or estimate range was used. For studies reporting estimates with an uncertainty range (reflecting uncertainty to model assumptions) rather than a 95% confidence interval (95% CI), a standard error or sufficient information to derive these directly, we approximated the standard error from symmetric and asymmetric intervals as 1/1.96 the largest absolute value (to account for asymmetric intervals) of the widths between the point estimate and the sensitivity bounds.
Relationship between per-act and per-partner transmission probability
We investigated the relationship between per-act and per-partner AI transmission probabilities over n sex acts using the following Bernouilli process that assumes independence of risk for each sex act within a partnership16,17:
| (1) |
where βp,a and βc,a are the per-partner (p) and per-act (c) transmission probability for AI (a), respectively. For heterosexual populations practising both vaginal intercourse (VI) and AI, Equation (1) becomes:
| (2) |
where βp,all is risk per-partner for VI and AI; βc,v and βc,a are per-act transmission probabilities for VI (v) and receptive AI (a), respectively; and d is the proportion of n sex acts which are AI rather than VI.
Intervention impact: HAART
We assessed the potential reduction in HIV infectivity caused by HAART reducing viral load, using two published17–21 functions of infectivity by viral load. In brief, the difference between the two functions is that Function 1 was based on results from the Rakai study of HIV transmission in heterosexual couples (presumed through VI transmission)22 and assumes a linear relationship between infectiousness and log serum viral load; Function 2 was based on data from a Zambian cohort of discordant couples23 and assumed a logistic function between infectivity and plasma viral load, which provides better fits to the low number of transmissions observed for low viral loads of index individuals.21 We assume that successful HAART reduces blood viral load from an average, baseline V0 to V1 copies/ml. Further details are provided in the Supplementary data available at IJE online.
Results
Search results
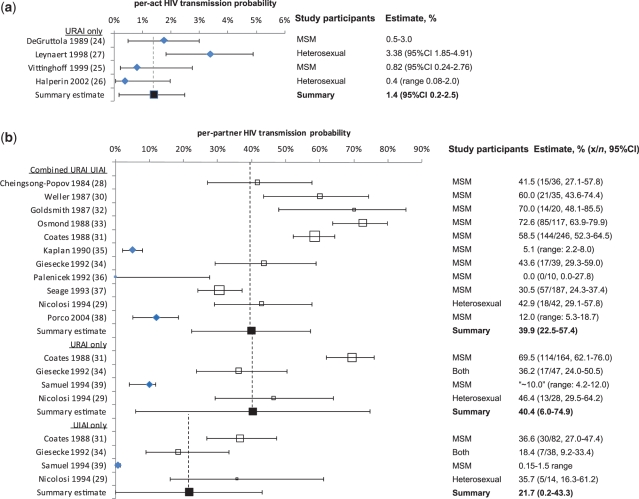
A total of 62 643 titles were searched and 27 potentially appropriate publications were identified, three of which were identified through bibliographies of searched articles. Four publications reporting per-act24–27 and 12 reporting per-partner28–39 estimates were included. These were from MSM (n = 1224,25,28,30–37,39), heterosexual (n = 326,27,29) or mixed (n = 134) study populations. Per-partner estimates from Nicolosi et al.’s study29 of heterosexuals, where AI was practised ‘often or always’ (≥50% of all intercourse) within the partnership were included; those with less frequent practise were excluded. Supplementary Figure S1 (available as Supplementary data at IJE online) summarizes the search strategy. All identified studies were from industrialized countries. Figure 1 summarizes study estimates for per-act and per-partner AI transmission probabilities as forest plots, including summary estimates from the meta-analyses. Details of included and excluded studies are in Supplementary Tables S1 and S2 (available as Supplementary data at IJE online), respectively. Supplementary Table S3 (available as Supplementary data at IJE online) summarizes heterosexual per-partner estimates that have been stratified by frequency of AI practice. Supplementary Table S4 (available as Supplementary data at IJE online) summarizes AI study estimates stratified by different risk factors.
Figure 1.
Forest plot of studies estimating HIV transmission probabilities for AI expressing risk as (a) per-act and (b) per-partner. For crude estimates (unfilled boxes), the size of box represents relative study sample size. Adjusted estimate (filled Rhombus), Crude estimate based on x number of seroconverting partners among n couples with an infected index partner (open square), Summary estimate (filled squre)
The only per-act estimates included were for URAI. Vittinghoff et al.’s25 estimates for protected receptive AI (0.18%, 95% CI 0.10–0.28), unprotected insertive AI (UIAI) (0.06%, 95% CI 0.02–0.19) and protected insertive AI (0.04%, 95% CI 0.01–0.11), based on partners of index cases who were “HIV infected or of unknown serostatus”, were excluded because no attempt was made to estimate the unknown HIV status of index cases by using HIV prevalence as a proxy for exposure to an HIV-infected partner.
Study design and estimate type
The included studies employed three study designs: retrospective-partner (n = 1124,26–34,37), prospective discordant-couple (n = 136) and simple-prospective (longitudinal cohort, n = 425,35,38,39) studies. In retrospective-partner studies, the infection status of each partner becomes known only at the time of the study. The index case and time of infection are determined based on exposure to a salient risk factor. In prospective discordant-couple studies, stable (preferably monogamous) HIV-serodiscordant couples are followed up after diagnosis of the index partner. These studies also provide per-partner HIV transmission rates but, with only one included study (and only 10 couples36), we report and use cross-sectional results at the end of follow-up for the meta-analysis. With simple-prospective studies, individuals (not necessarily monogamous) are recruited following sexual contact with potentially infected, high-risk partners and serostatus monitored. As index cases are not recruited, HIV exposure is estimated using HIV prevalence in the pool of potential partners and the reported coital frequency. Therefore, prospective studies suffer from problems of selection bias (prospective discordant-couple) and uncertainty in estimating the numbers of HIV exposures (simple-prospective) and are not necessarily superior to the retrospective-partner study design.
Per-act estimates were derived from retrospective-partner (n = 324,26,27) and simple-prospective (n = 125) studies. Per-partner estimates were derived from retrospective-partner (n = 828–34,37), prospective-discordant couple (n = 136) and simple-prospective (n = 335,38,39) studies. For both URAI-only and UIAI-only per-partner estimates, three were derived from retrospective-partner29,31,34 and one from simple-prospective39 study data.
We categorized study estimates into ‘crude’ and ‘adjusted’. ‘Crude’ estimates are based on the HIV status of partners of index cases and assume that the index case was the only source of exposure. In high-risk populations, multiple exposures to HIV through sexual contact with other partners and through other types of sexual practice may lead to overestimation of infectivity. Thus, ‘adjusted’ estimates are based on statistical models to control for multiple exposures. One study also adjusted for multiple modes of sexual transmission: URAI, UIAI, protected AI and oro-genital intercourse.25 No study provided both crude and adjusted estimates. Brief details on adjusted estimates are provided in Supplementary Table S1 (available as Supplementary data at IJE online). Numbers of per-act and per-partner estimate by adjustment type and study design are given in Table 1. For per-partner transmission probabilities, retrospective-partner studies reported crude estimates, whereas prospective studies tended to report adjusted estimates, which made it difficult to disentangle the effect of study design from adjustment for multiple exposures. In addition, all estimates where the standard error had to be approximated using an uncertainty range quoted by authors were adjusted estimates.
Table 1.
Summary transmission probability estimates for AI: meta-analyses results
| Estimate type | Median (%) | Min. (%) | Max. (%) | Summary random effects estimate, % (95% CI) | Qa | Pa | I2b (%) | N | References and study design |
|---|---|---|---|---|---|---|---|---|---|
| Per-act | |||||||||
| URAI | 1.0 | 0.4 | 3.4 | 1.4 (0.2–2.5) | 10.8 | 0.013 | 72 | 4 | 3 R-P24,26,27 |
| 1 S-P25 | |||||||||
| Retrospective | 1.3 | 0.4 | 3.4 | 1.6 (0.0–3.2) | 10.5 | 0.005 | 81 | 3 | 3 R-P24,26,27 |
| Prospective | 0.8 | – | – | 0.8 (0.2–2.8) | – | – | – | 1 | 1-S-P25 |
| Per-partner | |||||||||
| Combined URAI–UIAI | 42.9 | 0.0 | 72.6 | 39.9 (22.5–57.4) | 497.1 | <0.001 | 98 | 11 | 8 R-P28–34,37 |
| 1 P-DC36 | |||||||||
| 2 S-P35,38 | |||||||||
| Crude | 43.6 | 0.0 | 72.6 | 48.1 (35.3–60.8) | 91.0 | <0.001 | 91 | 9 | 8 R-P28–34,37 |
| 1 P-DC36 | |||||||||
| Adjusted | 8.6 | 5.1 | 12.0 | 7.9 (1.2–14.5) | 3.5 | 0.063 | 71 | 2 | 2 S-P35,38 |
| Retrospective | 51.1 | 30.5 | 72.6 | 52.3 (39.7–64.9) | 78.1 | <0.001 | 91 | 8 | 8 R-P28–34,37 |
| Prospective | 5.1 | 0.0 | 12.0 | 7.3 (1.8–12.8) | 3.7 | 0.161 | 46 | 3 | 2 S-P35,38 |
| 1 P-DC36 | |||||||||
| Per-partner | |||||||||
| URAI | 41.3 | 10.0 | 69.5 | 40.4 (6.0–74.9) | 164.9 | <0.001 | 98 | 4 | 3 R-P29,31,34 |
| 1 S-P39 | |||||||||
| Crude | 46.4 | 36.2 | 69.5 | 51.4 (28.1–74.7) | 20.5 | <0.001 | 90 | 3 | 3 R-P29,31,34 |
| Adjusted | 10.0 | – | – | 10.0 (4.2–15.8) | – | – | – | 1 | 1-S-P39 |
| Per-partner | |||||||||
| UIAI | 27.1 | 0.7 | 36.6 | 21.7 (0.2–43.3) | 60.6 | <0.001 | 95 | 4 | 3 R-P29,31,34 |
| 1 S-P39 | |||||||||
| Crude | 35.7 | 18.4 | 36.6 | 29.4 (16.0–42.9) | 5.1 | 0.077 | 61 | 3 | 3 R-P29,31,34 |
| Adjusted | 0.7 | – | – | 0.7 (0.0–1.3) | – | – | – | 1 | 1 S-P39 |
aQ-statistic calculated using Cochran’s Q-test for heterogeneity, summing the squared deviations of each study’s estimate from the overall pooled estimate, weighting the contribution of each study by its inverse variance.72 Under the hypothesis of homogeneity among the transmission probability estimates, the Q-statistic follows a chi-square distribution with k − 1 degrees of freedom, with k being the number of studies. From this, the P-value for heterogeneity can be derived.
bI2 is calculated as described in Higgins et al.73 I2 lies between 0 and 100%; 0% indicates no observed heterogeneity and larger values show increasing heterogeneity.
N, number of study estimates; P, P-value; P-DC, prospective discordant-couple study design; Q, heterogeneity statistic; R-P, retrospective-partner study design; S-P, simple-prospective study design; crude estimates, estimates calculated through simple derivation as number of seroconversions out of number of sexual acts involving exposure; adjusted estimates, estimates derived using more sophisticated calculation of transmission probability. Fixed effects summary estimates can be found in Supplementary Table S5 (available as Supplementary data at IJE online).
Infectiousness of AI for heterosexuals and MSM
Table 1 shows the per-act and per-partner summary estimates by exposure (combined URAI–UIAI, URAI-only and UIAI-only). Two per-act URAI estimates were based on studies among MSM24,25 and two among heterosexual couples.26,27 The per-act summary estimate was 1.4% (95% CI 0.2–2.5) (or 1.8% (95% CI 0.3–3.2) if Halperin et al.’s26 abstract estimate is excluded due to lack of further detail on methods). No significant differences in per-act URAI estimates between heterosexual couples and MSM were found (P = 0.674). However, while MSM estimates24,25 were similar to each other (Q = 0.2; P = 0.635; I2 = 0%), heterosexual estimates26,27 were heterogeneous (Q = 10.5; P = 0.001; I2 = 90%; Figure 1; Table 1). Most per-partner estimates were derived from studies on MSM. Exceptions were the studies by Nicolosi et al.29 studying heterosexual couples, and Giesecke et al.34 who enrolled a small proportion of heterosexual participants. Cheingsong-Popov et al.28 did not describe study participants but it appears likely, given the 1984 publication date, that the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex patients reporting AI were MSM. There was no evidence that the heterosexual combined URAI–UIAI crude per-partner estimate29 was significantly different from the eight crude estimates from MSM (P = 0.821).
Reliability of estimates
All per-partner summary estimates displayed considerable heterogeneity. Summary estimates calculated using adjusted estimates were considerably lower than those using crude estimates (Figure 1; Table 1). However, the reliability of summary adjusted estimates is questionable because only the crude combined URAI–UIAI summary estimate was based on more than five estimates. Interestingly, while crude per-partner estimates from MSM populations may overestimate per-partner infectivity because of competing exposures due to frequent lack of monogamy,28,30–34,36,37,39 they were in good agreement with crude per-partner estimates from heterosexual couples reporting high frequency of AI and 100% monogamy29 (Figure 1).
For combined URAI–UIAI, the summary estimate based on crude estimates only was 48.1% (95% CI 35.3–60.8), for URAI-only 51.4% (95% CI 28.1–74.7) and for UIAI-only 29.4% (95% CI 16.0–42.9) (Table 1). The forest plot (Figure 1) and Q- and I2-statistics (Table 1) highlight significant residual heterogeneity across estimates, even after excluding adjusted estimates. Nicolosi et al.’s29 study participants reported 100% monogamy, yet the 42.9% (95% CI 29.1–57.8) combined URAI–UIAI risk is more consistent with the crude MSM study estimates despite potential contamination from competing HIV exposures, than the adjusted MSM estimates. Separating combined URAI–UIAI estimates by study design (prospective35,36,38 versus retrospective28–34,37) gave similar findings because both adjusted estimates were derived from simple-prospective studies and the remaining prospective study, of serodiscordant couples, was small (n=1036).
Heterogeneity of infectiousness
Despite providing a single per-act URAI estimate, DeGruttola et al.24 discussed variability of infectiousness between individuals and suggested that 10–20% of infected MSM may have far greater infectiousness (about ≥10% per-act). Supplementary Table S4 (available as Supplementary data at IJE online) summarizes the few per-act and per-partner estimates stratified by risk factors. The only per-act estimate stratified by infection stage [primary infection and AIDS stages each separately estimated as 18.35% (95% CI 2.08–34.6); asymptomatic incubation stage 1.38% (95% CI 0.0–3.38)]27 reflects the variability in infectiousness within an individual over time. However, it is likely that none of the four average per-act AI infectivity estimates adequately captures the contribution of high infectiousness during acute, pre-seroconversion infection.24–27 The study by Leynaert et al.27 was a retrospective-partner study where the exposure period was only crudely estimated. Although some transmissions in Vittinghoff et al.’s prospective study might have occurred as a result of acute infection exposure, the study estimate included only study participants reporting partners known to be HIV infected. Therefore, the index cases were unlikely to be in the highly infectious acute HIV stage because of the time lag between infection and HIV diagnosis and disclosure of their status to their partners included in the study. Therefore, the true average per-act infectivity across all infection stages may be higher than our 1.4% summary estimate because retrospective-partner studies may miss the acute infection stage, or may be lower because Vittinghoff’s prospective study may have misattributed some transmission events from unidentified HIV exposures.
Relationship between per-act and per-partner infectivity for AI
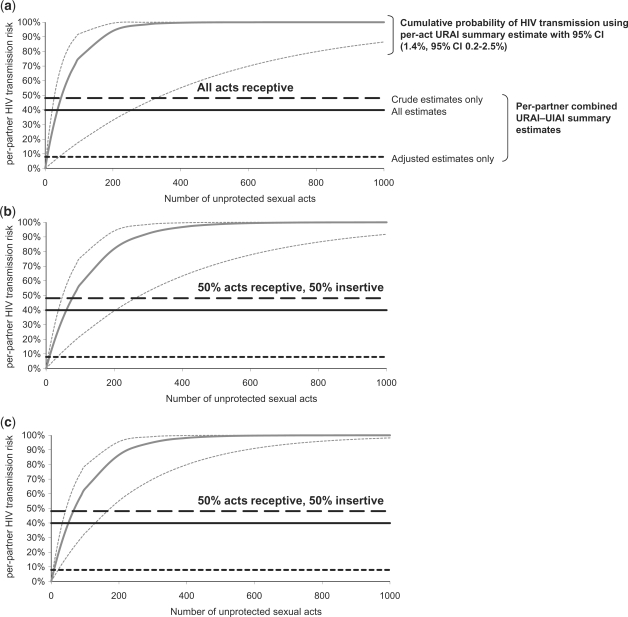
Figure 2 illustrates the relationship between per-act and cumulative HIV risk over a partnership through URAI (by number of sexual acts), compared with per-partner combined URAI–UIAI summary estimates from our meta-analysis (drawn as horizontal lines). The figure indicates, for example, that only 36 URAI acts would be required to produce the per-partner combined URAI–UIAI summary estimate of 39.9% if we assume that per-act URAI HIV transmission probability is 1.4%. If 50% of acts are receptive and 50% insertive, 60 UAI acts are required if we assume that UIAI per-act risk is 0.3% (same as for VI; see Figure 2 legend for details) and 51 acts if the UIAI per-act risk is 0.6%. Competing risk from UIAI increases the total number of unprotected acts necessary for transmission per partnership only by relatively modest amounts, especially when the increase in transmission probability of UIAI compared with VI is large, because UIAI infectiousness becomes closer to that of URAI. As suggested previously,24 under the model assumptions, it is difficult to explain per-partner risk estimates as a function of per-act estimates. The results imply relatively few UAI acts per relationship among the partnerships included in the per-partner studies. As too few MSM per-partner studies reported length of partnership or number of sex acts per partnership (Supplementary Table S1, available as Supplementary data at IJE online), it is difficult to test this hypothesis. Although some of the partnerships in the per-partner studies may have been relatively short with few sexual acts, this is unlikely to be the case for all partnerships and all studies. For example, Nicolosi et al.29 reported a median partnership duration of 2.9 years, implying a relatively large number of acts. Supplementary Figure S2 (available as Supplementary data at IJE online) shows how empirical per-partner study estimates do not show the expected increase in infectivity with increasing number of sexual exposures to the index partner predicted by the Bernoulli process [Equation (1)] in absence of heterogeneity. The discrepancies between per-act and per-partner estimates (Figure 2; Supplementary Figure S2, available as Supplementary data at IJE online) may partly be explained by condom use, competing risks from exposure to HIV outside the main partnership or some degree of heterogeneity in transmission probability per-act between individuals and within individuals over time, implying that our assumption of independence of risk per-act within a partnership is invalid. If there is heterogeneity in infectiousness or susceptibility between individuals, this may explain the saturation of per-partner risk at lower levels than predicted using our per-act summary estimate. The four per-act estimates represent exposure without condoms: DeGruttola et al.24 and Leynaert et al.27 reported <1% condom use and Vittinghoff et al.25 and Halperin et al.26 adjusted for it. In contrast, many per-partner studies reported ‘some condom use’ but generally did not quantify frequency (although it appears that condom use was generally very inconsistent within partnerships).
Figure 2.
Relation between per-partner HIV transmission risk (cumulative probability of HIV transmission) and the number of sexual acts with an HIV infected partner, using our summary per-act URAI estimate of 1.4% (95% CI 0.2–2.5). The intersection between the modelled per-partner HIV transmission risk (y-axis) and our meta-analytic per-partner combined URAI–UIAI summary estimates (plotted as horizontal lines) predicts the required average number of acts per partnership (x-axis), under our model assumptions (see ‘Methods’ section). Adjusted estimates control for exposures due to multiple partners and crude estimates do not. (a) All acts assumed to be URAI; (b) 50% acts URAI, 50% acts UIAI, assuming that per-act UIAI has the same HIV transmission probability as penile–vaginal intercourse (summary estimate of per-act penile–vaginal intercourse, male-to-female transmission for developing countries: 0.3%13; (c) as for (b) but UIAI HIV transmission probability is 0.6%. Competing risk from UIAI increases the total number of unprotected acts necessary for transmission per partnership only by relatively modest amounts, especially when the increase in transmission probability of UIAI compared with VI is large, because UIAI infectiousness becomes closer to that of URAI.
If we assume the 18.35% per-act URAI estimate of Leynaert et al.27 associated with primary infection, only three URAI acts were necessary for per-partner HIV risk to exceed 40.4% (per-partner URAI-only summary estimate, results not shown). Therefore, high infectiousness associated with primary infection may account for the high per-partner estimates observed for some partnerships consisting of few acts. However, relatively few short duration partnerships are likely to occur while infected individuals experience primary infection, because this period is very short [∼3 months40], although this will depend on the sexual network structure. Late-stage infection is also associated with high infectiousness,27 so the same argument could apply, although sexual activity of AIDS patients is likely to be much lower.
Implications for the effectiveness of interventions: HAART
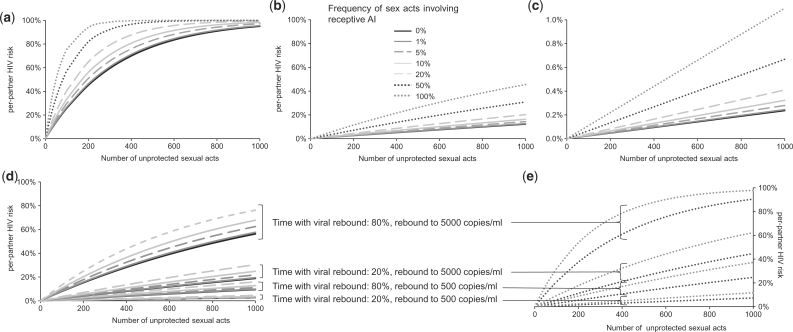
Figure 3a illustrates the relationship between per-partner HIV risk and total sex acts involving exposure to HIV-infected partners not on HAART, considering both VI/UIAI and URAI exposure (see legend for details). Using Function 1 (Figure 3b), the predicted HIV transmission probabilities per-act for VI/UIAI and URAI with successful HAART are 0.013 and 0.061%, respectively, i.e. 96% lower than without therapy. Under these assumptions, 1000 sex acts leads to a male-to-female per-partner HIV risk of 12.2% if no AI is practised (i.e. only VI is practised) and 12.6, 14.3, 16.3 and 20.2% if AI is practised for 1, 5, 10 and 20% of all sex acts, respectively. For MSM, 1000 acts lead to per-partner risk of 30.9% if partners alternate URAI and UIAI, and 45.6% if the initially uninfected partner is always receptive. As expected, our per-partner HIV risk estimates with HAART, even assuming continuous viral suppression, are larger than Wilson et al.’s17 estimates using the same function, because we used recent and higher baseline per-act VI infectivity estimates from the studies of developing country only (due to the lack of AI infectivity studies from developing countries, per-act URAI was informed by the studies of developed country only).13 Using Function 2 (Figure 3c–e), the predicted per-act VI/UIAI and URAI estimates with successful HAART are 0.0002 and 0.0011%, respectively, i.e. 99.9% lower than without therapy. Under these assumptions, 1000 sex acts lead to a male-to-female per-partner HIV risk of <0.5% even where AI constitutes 20% of all sex acts, <1% for MSM practising URAI and UIAI with equal frequency and 1.1% for MSM solely practising URAI (Figure 3c). However, if viral rebound occurs due to treatment failure, per-partner transmission risks become much larger (Figure 3d–e).
Figure 3.
Relation between per-partner HIV risk (cumulative probability of HIV transmission) and the number of all sexual acts (whether penile–vaginal or penile–anal) that uninfected MSM or heterosexual women are exposed to with HIV-infected men, exploring the impact of different frequencies of URAI within the partnership (if in a monogamous HIV discordant relationship) or among all sexual HIV exposures that an uninfected individual encounters. Frequency of sexual acts involving URAI: 0–20% represent ranges for women in heterosexual partnerships, with the remainder of sexual exposures assumed to be penile–vaginal; 50% represents MSM partnerships where each partner practises URAI and UIAI equally often; 100% represents MSM where the seronegative partner is always receptive. Scenario (a) represents the impact of URAI on per-partner HIV risk assuming a constant per-act probability for URAI (1.4%, Table 1) and for penile–vaginal intercourse (summary estimate of per-act penile–vaginal intercourse, male-to-female transmission for developing countries: 0.3%13; we assume, in the absence of per-act HIV estimates for UIAI identified by our review, that HIV transmission probability is the same as for female-to-male penile–vaginal intercourse). Scenario (b) uses Function 1 to investigate impact of HAART, predicting per-partner HIV risk within a discordant couple where the index male has successful viral suppression due to HAART. Scenario (c) investigates the same, by using Function 2 (note change of y-axis scale). Scenarios (d) and (e) use Function 2 (separating graphs for women and MSM for clarity), additionally including viral rebound as a result of treatment failure for a proportion of the duration of exposure. The derived relationship between URAI infectiousness and plasma viral load calculated in Function 2 is illustrated in Supplementary Figure S3 (available as Supplementary data at IJE online).
Discussion
Four per-act URAI estimates produced a summary estimate of 1.4% (95% CI 0.2–2.5) and per-partner summary estimates were 39.9% (95% CI 22.5–57.4), 40.4% (95% CI 6.0–74.9) and 21.7% (95% CI 0.2–43.3) for combined URAI–UIAI, URAI and UIAI transmission, respectively. Competing risk from UIAI only marginally increases HIV transmission per partnership (39.9% for combined, 40.4% for URAI-only), which supports the hypothesis that UIAI is substantially less infectious than URAI. However, the significant heterogeneity between per-partner estimates led to wide CIs, primarily due to differences in analytic methods and study design. Thus, these ‘average’ AI transmission probabilities should be used with caution.
The large discrepancy between crude and adjusted per-partner estimates (Table 1) makes interpretation of the results particularly difficult, especially because similar adjustments used to quantify per-act VI estimates were found to have little impact.13,41 The combined URAI–UIAI summary estimate using adjusted per-partner estimates was approximately six times lower than for the summary of crude estimates. As many MSM study subjects may have multiple partners, adjusted estimates may be more reliable. However, the 42.9% (95% CI 29.1–57.8) crude per-partner estimate reported by Nicolosi et al.29 for heterosexual relationships with high levels of monogamy, together with the high per-act estimates identified, are difficult to reconcile with the low summary estimate for adjusted per-partner infectiousness. Most studies did not collect the necessary sexual activity information required to gain a better understanding of the relationship between per-act and per-partner risk. Variation across study estimates may also partly be explained by differences in distributions of risk factors in sampled populations, study designs and various (time-varying) characteristics of the type of sexual behaviour, characteristics of the infected partner and those of the uninfected partner. For example, duration of exposure to an infected partner, frequency of unprotected acts per-partner and presence of various cofactors for transmission, such as condom use, will differ (Supplementary Table S1, available as Supplementary data at IJE online). While HIV transmission for heterosexual men engaging in AI will predominantly occur by insertive intercourse, MSM experience risk through both insertive and/or receptive AI.
As suggested previously,24 the difficulty in reconciling per-act and per-partner estimates highlights the difficulty in specifying a unique estimate of AI and supports that there is considerable heterogeneity in infectivity between individuals, over the course of infection and/or that our assumption of independence of risk per-act within a partnership is invalid.42 DeGruttola et al.24 suggested in 1989 that such heterogeneity leads to underestimation of the number of partners who would be infected after few acts and overestimation of numbers who would be infected after many acts. Heterogeneity in per-act or per-partner HIV infectiousness has also been reported in other studies for VI13,41 and intercourse for MSM.27,33,34 Additional evidence of heterogeneity in per-partner estimates by potential risk factors such as STI history are summarized in Supplementary Table S4 (available as Supplementary data at IJE online), but the number of studies is limited. Estimates stratified by risk categories (Supplementary Tables S3 and S4, available as Supplementary data at IJE online) may suffer from publication bias, as they are more likely to be reported if differences are significant.
URAI per-act estimates are substantially higher than for male-to-female VI (0.08%, 95% CI 0.06–0.11 in developed countries; 0.30%, 95% CI 0.14–0.63 in developing countries).13 Most studies of heterosexual couples have found an increased male-to-female transmission risk among couples practising AI, even if only occasionally.13,41,43–50 Rectal mucosa lacks the protective humoral immune barrier present in cervicovaginal secretions51 and is more susceptible to traumatic abrasions which may facilitate transmission.52 We found no evidence of a difference in per-act AI infectivity between heterosexual and MSM couples, possibly because they are biologically similar practices, yet per-partner infectivities may differ due to dissimilar frequencies of practising AI within heterosexual and MSM relationships. However, the only heterosexual per-partner study consisted of couples with high frequency of AI (≥50% of sexual acts)29 and therefore, given the much higher risk of transmission during AI than VI, estimates should be comparable with those from MSM studies. Our review extends previous HIV infectivity research because we investigated per-act and per-partner AI infectivity among MSM and heterosexuals. However, we found no AI estimates from developing countries, and given the different distributions in risk factors such as STI prevalences and HIV subtypes between settings, we may underestimate overall AI infectiousness for developing countries, as has been suggested for per-act VI infectiousness.13,41
Our AI transmission probability estimates are considerably higher than oro-genital risks, which were found to be very low, but non-zero.53 For example, our per-act URAI summary estimate is 35-fold larger than the highest per-act oro-genital estimate (0.04% for unprotected receptive oro-genital intercourse, 95% CI 0.01–0.1725). Thus, practising oral sex with an HIV-infected individual considerably reduces the risk of HIV acquisition compared with that for URAI and UIAI, but does not reduce it to zero. Individuals often make sophisticated choices regarding the balance of risk and pleasure;54,55 this difference in risk should be appropriately communicated to relevant populations.
Studies have demonstrated that a substantial percentage of heterosexuals engage in AI with an opposite-gender partner56–60 and that rates of condom use for heterosexual AI are lower than for VI.6,61 More recently, research in the USA and the UK has demonstrated an increase in the proportion of heterosexuals reporting practicing AI,56,62–64 although this rise may be attributable to social changes affecting reporting bias. The proportion of heterosexually acquired HIV infection attributable to AI depends largely on the frequency of UAI, which varies greatly by population and setting, and the HIV risk profile of the partners of heterosexuals. Table 2 presents a summary of studies identified through a non-exhaustive review of PubMed, documenting the proportion of participants reporting any AI over a defined period for various populations and settings published in the last 10 years. Although these findings may not all be representative of the general population due to small samples and selection biases, the high rates of AI are in line with those investigated with our model. The majority of surveys were conducted in industrialized countries; more and carefully collected data on frequency of protected and unprotected AI are required from populations in developing countries to explore the influence of AI on generalized epidemics.65
Table 2.
Summary of selected epidemiological studies investigating practice of AI among heterosexual populations published in the last 10 years
| Study | Population | Age (years) | Sample size | AI reported (%) | Exposure period |
|---|---|---|---|---|---|
| Industrialized countries | |||||
| Pollack, 1999, unpublished data; National AIDS Behavioral Methodology Studya,b | USA population survey, women | 18–49 | 1071 | 6.10 | Past 6 months |
| Laumann et al., 199474,a | USA population survey | 18–59 | 3432 | 23 | Ever |
| 10 men | Past year | ||||
| 9 women | Past year | ||||
| 2.3 men | Last sex | ||||
| 1.2 women | Last sex | ||||
| 50–54 | 3 men | Past year | |||
| 2 women | Past year | ||||
| 25–29 | 2.4 women | Last sex | |||
| Gross et al., 200060 | USA HIV negative women ‘at high risk of HIV infection’ | 11% 18–25 | 1268 | 32 | Past 6 months |
| 38% 26–35 | |||||
| 51% ≥36 | |||||
| Baldwin and Baldwin, 200057 | USA random sample of sexually experienced university students (oversampling ethnic groups) | Mean 21, all <30 | 647 | 23 | Ever |
| Johnson et al., 200164 | UK population-based survey (NATSAL) | 16–44 | 111 613c | 7.0 men | Past year (1990) |
| 6.5 women | |||||
| 12.3 men | Past year (2000) | ||||
| 11.3 women | |||||
| Friedman et al., 200158 | USA women, inner city minority neighbourhood | 18–24 | 202 | 14 | Past year |
| Flannery et al., 200375 | USA sexually experienced female college students 1993–2000 | NR | 761 | 32 | Ever |
| Leichliter et al., 200761 | USA general population survey | 15–44 | 12 571 | 30 women | Ever |
| 34 men | |||||
| Houston et al., 200776 | USA inner city adolescent, sexually experienced females | 12–18 | 350 | 16 main partners | Past 3 months |
| 12 casual partners | |||||
| Tian et al., 200877 | USA STD clinic attendees | 15–39 | 2357 | 18.30 | Past 3 months |
| 39.30 | Past year | ||||
| Developing countries | |||||
| Karim and Ramjee, 199878 | South Africa FSW surveyed at truck stops | Mean 24 | 145 | 43 with clients | Ever |
| Matasha et al., 199879 | Tanzania cohort of sexually experienced male and female school pupils | Median 15, 12–20 | 661 | 6 | First sexual experienced |
| Sallah et al., 199980 | Togo female college students | 20–29 | 817 | 9 | Likely currentlye |
| 37.80 | Likely evere | ||||
| Fonck et al., 200081 | Kenya FSW cohort | Mean 32 | 318 | 14 | Likely evere |
| Ramjee and Gouws, 200282 | South Africa truck drivers | Mean 37, 18–71 | 184 | 42 | Likely evere |
| Ferguson and Morris, 200383 | Kenya FSW cohort | NR | 339 | 20 | Ever |
| Lane et al., 200684 | South Africa national survey of adolescent sexual behaviours, sexually active respondents | 15–24 | ∼7976 | 5.3 women | Ever |
| 5.5 men | |||||
| Schwandt et al., 200685 | Kenya FSW cohort | Mean 35, 15–63 | 147 | 40.80 | Ever |
| Skoler-Karpoff et al., 200870 | South Africa, baseline characteristics from a microbicide RCT, sexually active, HIV-negative women | ≥16 (4% 16–17, 33% 18–24, 63% ≥25) | 6202 | 2f | Past 3 months |
| Subramanian et al., 200886 | India, survey of clients of FSW | Median 30, 18–60 | 4821 | 13.3 with FSW | Ever |
| 6.2 with main regular female partner | Ever | ||||
| 8.3 with male or transgender | |||||
| Past 6 months | |||||
| Munro et al., 200887 | India, community-based survey | Mean 30, 15–49 | 4653 | 2.6g,h men | Ever |
| 0.3h women | |||||
| Kalichman et al., 200988 | South Africa, urban township community-based and urban STI clinic surveys | Mean 31, median 30, minimum 18 (men and women combined) | 2471 | 14.6 men | Past 3 months |
| 1646 | 10.4 women |
FSW, female sex worker; NATSAL, National Survey of Sexual Attitudes and Lifestyles; NR, not recorded; RCT, randomized controlled trial; STD, sexually transmitted disease.
aResults taken from review by Halperin.6
bLance M. Pollack, personal communication, September 1999 to D. Halperin.6
cTotal completing NATSAL 2000 questionnaire, including non-heterosexuals (percentage reporting AI is for heterosexual practices only).
dAuthors report that results must be interpreted with caution as some younger pupils may have had difficulty in understanding some of the more sensitive questions (questionnaire was self-administered), ‘in particular questions on oro-genital and anal sex’.
eNot specified in the publication.
fUAI only.
gA further 0.6% of men reported ever having had AI with another man.
hSeventeen (0.8%) of men and 68 (2.3%) of women reported not knowing if they had ever experienced AI. None of these men was HIV positive, but among women, ‘not knowing’ was significantly associated with HIV infection, compared with the group of women reporting no AI experience.
Our analysis of how the impact of HAART on HIV transmission may be mitigated by AI suffers from several limitations due to its simplicity and the difficulty in quantifying AI infectiousness highlighted in this review (e.g. per-act VI risk was taken from a meta-analysis for male-to-female transmission in developing countries,13 but our per-act URAI summary estimate represents industrialized countries only because no relevant studies from developing countries were identified; we assume that infectiousness varies with viral load similarly for AI and VI and that coital frequency remains constant over time; and again we stress the need for caution in utilizing our quantitative pooled estimates). Nevertheless, it serves to vividly illustrate the large excess in HIV risk that individuals may experience over time if they occasionally engage in UAI with an infected partner. Thus, prevention messages must emphasize the high risk associated with AI and that control measures such as condoms must be used for both VI and AI.
Drawing conclusions on the use of HAART for HIV prevention is beyond the scope of this article. However, the contrasting quantitative results obtained regarding the impact of HAART on per-partner transmission risk using the two functions (Figure 3b–e) highlight that caution is required when relying on viral load data to predict the potential impact of HAART on transmission and the importance of clearly describing the model assumptions. Different viral load functions produce different predictions (Figure 3). Figure 3d–e demonstrates the sensitivity of results to frequency of viral rebound and to the infectiousness associated with these rebounds. Further work in this area is necessary given the increasing interest in HAART use as a prevention tool.66,67 Modelling cannot be used as a substitute for empirical evidence, and data are starting to become available.68
In terms of product development, oral pre-exposure prophylaxis may be more appropriate for use among populations where there is a high frequency of AI if adherence to condom use or rectal microbicide use is poor, or if microbicide efficacy is lower for AI than VI. All microbicide trials potentially suffer from bias from AI.69–71 There must be greater understanding of the role that AI plays in heterosexual as well as MSM sex lives, particularly in regions with high HIV incidence, so that we can design and implement measures to minimize the role that it plays in HIV transmission.
Supplementary data
Supplementary data are available at IJE online.
Funding
Wellcome Trust (GR082623MA to R.F.B., GR078499MA to R.G.W.); UK Medical Research Council (MRC) (to R.G.W.); Bill and Melinda Gates Foundation (to M.C.B. and R.G.W.).
Supplementary Material
Acknowledgements
We thank Dr Steven Shiboski for providing further data. We thank the MRC for Centre funding. The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Bill and Melinda Gates Foundation, Wellcome Trust or MRC.
Conflicts of interest: None declared.
KEY MESSAGES.
UAI is a high-risk practice for HIV transmission (higher risk than VI or oro-genital intercourse), probably with substantial variation in infectiousness and susceptibility to infection between individuals, and in infectiousness over the duration of infection. The significant heterogeneity between infectiousness estimates means that pooled AI HIV transmission probabilities should be used with caution.
Most studies did not collect the necessary sexual activity information required to gain a better understanding of the relationship between per-act and per-partner risk. Variation across study estimates may also partly be explained by differences in distributions of risk factors in sampled populations, study designs and various (time-varying) characteristics of the type of sexual behaviour, characteristics of the infected partner and those of the uninfected partner.
We found no evidence of a difference in per-act AI infectivity between heterosexual and MSM couples.
Model estimates of the impact of HAART in reducing HIV transmission and the mitigating effect of AI can vary substantially, depending on the assumptions made. Empirical evidence is, therefore, urgently needed in order to inform model estimates, which remain highly uncertain. Nevertheless, our analysis illustrates the large excess in HIV risk that individuals may experience over time if they occasionally engage in UAI with an infected partner. Prevention messages must emphasize the high risk associated with AI and that control measures such as condoms must be used for both VI and AI.
References
- 1.Gisselquist D, Potterat JJ, Brody S. Running on empty: sexual co-factors are insufficient to fuel Africa’s turbocharged HIV epidemic. Int J STD AIDS. 2004;15:442–52. doi: 10.1258/0956462041211216. [DOI] [PubMed] [Google Scholar]
- 2.Gisselquist D, Rothenberg R, Potterat J, Drucker E. HIV infections in sub-Saharan Africa not explained by sexual or vertical transmission. Int J STD AIDS. 2002;13:657–66. doi: 10.1258/095646202760326390. [DOI] [PubMed] [Google Scholar]
- 3.Brody S, Potterat JJ. Assessing the role of anal intercourse in the epidemiology of AIDS in Africa. Int J STD AIDS. 2003;14:431–36. doi: 10.1258/095646203322025704. [DOI] [PubMed] [Google Scholar]
- 4.Kloos H, Mariam DH. Some neglected and emerging factors in HIV transmission in Ethiopia. Ethiop Med J. 2007;45:103–7. [PubMed] [Google Scholar]
- 5.Smith LB, Adler NE, Tschann JM. Underreporting sensitive behaviors: the case of young women’s willingness to report abortion. Health Psychol. 1999;18:37–43. doi: 10.1037//0278-6133.18.1.37. [DOI] [PubMed] [Google Scholar]
- 6.Halperin DT. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, Part I. AIDS Patient Care STDS. 1999;13:717–30. doi: 10.1089/apc.1999.13.717. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–77. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 8.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds S, Makumbi F, Kagaayi J, et al. Uganda: 16th Conference on Retroviruses and Opportunistic Infections; ART reduced the rate of sexual transmission of HIV among HIV-discordant couples in rural Rakai. Montreal, Abstract 52a, 2009. [Google Scholar]
- 10.Sullivan P, Kayitenkore K, Chomba E, et al. Reduction of HIV transmission risk and high risk sex while prescribed ART: results from discordant couples in Rwanda and Zambia, 16th Conference on Retroviruses and Opportunistic Infections. Montreal, Abstract 52b, 2009. [Google Scholar]
- 11.Bezemer D, de Wolf F, Boerlijst MC, et al. A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS. 2008;22:1071–77. doi: 10.1097/QAD.0b013e3282fd167c. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzard BG, Shanson DC, Farthing C, et al. Clinical findings and serological evidence of HTLV-III infection in homosexual contacts of patients with AIDS and persistent generalised lymphadenopathy in London. Lancet. 1984;2:480–83. doi: 10.1016/s0140-6736(84)92563-7. [DOI] [PubMed] [Google Scholar]
- 15.Flateby G, Eskild A, Brekke T, Moi H. Steady sexual relationship with an HIV-positive partner and the progression rate to AIDS. AIDS. 1996;10:1749–51. doi: 10.1097/00002030-199612000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Rottingen JA, Garnett GP. The epidemiological and control implications of HIV transmission probabilities within partnerships. Sex Transm Dis. 2002;29:818–27. doi: 10.1097/00007435-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–20. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Raddad LJ, Boily MC, Self S, Longini IM., Jr Analytic insights into the population level impact of imperfect prophylactic HIV vaccines. J Acquir Immune Defic Syndr. 2007;45:454–67. doi: 10.1097/QAI.0b013e3180959a94. [DOI] [PubMed] [Google Scholar]
- 19.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon JA, Hogan DR. Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS. 2008;22(Suppl. 1):S149–59. doi: 10.1097/01.aids.0000327636.82542.87. [DOI] [PubMed] [Google Scholar]
- 21.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007;104:17441–46. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New England J Med. 2000;342:921–29. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 23.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Human Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGruttola V, Seage GR, 3rd, Mayer KH, Horsburgh CR., Jr Infectiousness of HIV between male homosexual partners. J Clin Epidemiol. 1989;42:849–56. doi: 10.1016/0895-4356(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 25.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 26.Halperin DT, Shiboski SC, Palefsky SC, Padian NS. High level of HIV-1 infection from anal intercourse: a neglected risk factor in heterosexual AIDS prevention. Int Conf AIDS. [abstract ThPeC7438], 7–12 July 2002, 14 [Google Scholar]
- 27.Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 28.Cheingsong-Popov R, Weiss RA, Dalgleish A, et al. Prevalence of antibody to human T-lymphotropic virus type III in AIDS and AIDS-risk patients in Britain. Lancet. 1984;2:477–80. doi: 10.1016/s0140-6736(84)92562-5. [DOI] [PubMed] [Google Scholar]
- 29.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994;5:570–75. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Weller IV, Carne CA, Sattentau Q, et al. Human immunodeficiency virus (HIV) infection in the regular sexual partners of homosexual men with AIDS and persistent generalised lymphadenopathy. J Med Virol. 1987;22:91–98. doi: 10.1002/jmv.1890220111. [DOI] [PubMed] [Google Scholar]
- 31.Coates RA, Calzavara LM, Read SE, et al. Risk factors for HIV infection in male sexual contacts of men with AIDS or an AIDS-related condition. Am J Epidemiol. 1988;128:729–39. doi: 10.1093/oxfordjournals.aje.a115026. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith JM, Kalish SB, Ostrow DG, et al. Antibody to human lymphotropic virus type III: immunologic status of homosexual contacts of patients with the acquired immunodeficiency syndrome and the acquired immunodeficiency-related complex. Sex Transm Dis. 1987;14:44–47. [PubMed] [Google Scholar]
- 33.Osmond D, Bacchetti P, Chaisson RE, et al. Time of exposure and risk of HIV infection in homosexual partners of men with AIDS. Am J Public Health. 1988;78:944–48. doi: 10.2105/ajph.78.8.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giesecke J, Ramstedt K, Granath F, Ripa T, Rado G, Westrell M. Partner notification as a tool for research in HIV epidemiology: behaviour change, transmission risk and incidence trends. AIDS. 1992;6:101–7. doi: 10.1097/00002030-199201000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan EH. Modeling HIV infectivity: must sex acts be counted? J Acquir Immune Defic Syndr. 1990;3:55–61. [PubMed] [Google Scholar]
- 36.Palenicek J, Fox R, Margolick J, et al. Longitudinal study of homosexual couples discordant for HIV-1 antibodies in the Baltimore MACS Study. J Acquir Immune Defic Syndr. 1992;5:1204–11. [PubMed] [Google Scholar]
- 37.Seage GR, 3rd, Mayer KH, Horsburgh CR., Jr Risk of human immunodeficiency virus infection from unprotected receptive anal intercourse increases with decline in immunologic status of infected partners. Am J Epidemiol. 1993;137:899–908. doi: 10.1093/oxfordjournals.aje.a116751. [DOI] [PubMed] [Google Scholar]
- 38.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18:81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel MC, Mohr MS, Speed TP, Winkelstein W. Infectivity of HIV by anal and oral intercourse among homosexual men. Estimates from a prospective study in San Francisco. In: Kaplan EH, Brandeau ML, editors. Modeling the AIDS Epidemic: Planning, Policy and Prevention. New York: Raven Press; 1994. pp. 423–38. [Google Scholar]
- 40.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 41.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–63. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV transmission under highly active antiretroviral therapy. Lancet. 2008;372:1806–7; author reply 7. doi: 10.1016/S0140-6736(08)61753-5. [DOI] [PubMed] [Google Scholar]
- 43.Lazzarin A, Saracco A, Musicco M, Nicolosi A. Man-to-woman sexual transmission of the human immunodeficiency virus. Risk factors related to sexual behavior, man’s infectiousness, and woman’s susceptibility. Italian Study Group on HIV Heterosexual Transmission. Arch Intern Med. 1991;151:2411–16. [PubMed] [Google Scholar]
- 44.Mayer KH, Anderson DJ. Heterosexual HIV transmission. Infect Agents Dis. 1995;4:273–84. [PubMed] [Google Scholar]
- 45.Silverman BG, Gross TP. Use and effectiveness of condoms during anal intercourse. A review. Sex Transm Dis. 1997;24:11–17. doi: 10.1097/00007435-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Scott GR. Genital herpes: audit of cases referred by general practitioners to a department of genito-urinary medicine. Br J Clin Pract. 1992;46:256–57. [PubMed] [Google Scholar]
- 47.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994;5:570–75. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Seidlin M, Vogler M, Lee E, Lee YS, Dublin N. Heterosexual transmission of HIV in a cohort of couples in New York City. AIDS. 1993;7:1247–54. doi: 10.1097/00002030-199309000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Chamberland ME, Dondero TJ. Heterosexually acquired infection with HIV: a view from the III International Conference on AIDS. Ann Intern Med. 1987;107:763–66. doi: 10.7326/0003-4819-107-5-763. [DOI] [PubMed] [Google Scholar]
- 50.Padian N, Marquis L, Francis DP, et al. Male-to-female transmission of human immunodeficiency virus. JAMA. 1987;258:788–90. [PubMed] [Google Scholar]
- 51.Belec L, Dupre T, Prazuck T, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–97. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 52.Levy JA. The transmission of HIV and factors influencing progression to AIDS. Am J Med. 1993;95:86–100. doi: 10.1016/0002-9343(93)90237-j. [DOI] [PubMed] [Google Scholar]
- 53.Baggaley RF, White RG, Boily MC. Systematic review of orogenital HIV-1 transmission probabilities. Int J Epidemiol. 2008;37:1255–65. doi: 10.1093/ije/dyn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frost DM, Stirratt MJ, Ouellette SC. Understanding why gay men seek HIV-seroconcordant partners: intimacy and risk reduction motivations. Cult Health Sex. 2008;10:513–27. doi: 10.1080/13691050801905631. [DOI] [PubMed] [Google Scholar]
- 55.Davies PM, Simpson P. On male homosexual prostitution and HIV. In: Aggleton P, Davies PM, Hart G, editors. AIDS: Individual, Cultural and Policy Dimensions. London: Falmer Press; 1990. [Google Scholar]
- 56.Aral SO, Patel DA, Holmes KK, Foxman B. Temporal trends in sexual behaviors and sexually transmitted disease history among 18- to 39-year-old Seattle, Washington, residents: results of random digit-dial surveys. Sexually Trans Dis. 2005;32:710–17. doi: 10.1097/01.olq.0000175370.08709.72. [DOI] [PubMed] [Google Scholar]
- 57.Baldwin JI, Baldwin JD. Heterosexual anal intercourse: an understudied, high-risk sexual behavior. Arch Sexual Behav. 2000;29:357–73. doi: 10.1023/a:1001918504344. [DOI] [PubMed] [Google Scholar]
- 58.Friedman SR, Flom PL, Kottiri BJ, et al. Prevalence and correlates of anal sex with men among young adult women in an inner city minority neighborhood. AIDS. 2001;15:2057–60. doi: 10.1097/00002030-200110190-00025. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DK, Watters JK, Case P. The prevalence of high-risk sexual behavior in male intravenous drug users with steady female partners. Am J Public Health. 1990;80:465–66. doi: 10.2105/ajph.80.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gross M, Holte SE, Marmor M, Mwatha A, Koblin BA, Mayer KH. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J Acquir Immune Defic Syndr. 2000;24:393–98. doi: 10.1097/00126334-200008010-00015. [DOI] [PubMed] [Google Scholar]
- 61.Leichliter JS, Chandra A, Liddon N, Fenton KA, Aral SO. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. J Infect Dis. 2007;196:1852–59. doi: 10.1086/522867. [DOI] [PubMed] [Google Scholar]
- 62.Satterwhite CL, Kamb ML, Metcalf C, et al. Changes in sexual behavior and STD prevalence among heterosexual STD clinic attendees: 1993–1995 versus 1999–2000. Sex Transmit Dis. 2007;34:815–19. doi: 10.1097/OLQ.0b013e31805c751d. [DOI] [PubMed] [Google Scholar]
- 63.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15-44 years of age, United States, 2002. Adv Data. 2005;15:1–55. [PubMed] [Google Scholar]
- 64.Johnson AM, Mercer CH, Erens B, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001;358:1835–42. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- 65.Boily MC, Baggaley RF, Masse B. The role of heterosexual anal intercourse for HIV transmission in developing countries: are we ready to draw conclusions? Sex Transm Infect. 2009;85:408–10. doi: 10.1136/sti.2009.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 67.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 68.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 69.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–97. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 71.Buve A. Microbicide trials. 16th Conference on Retroviruses and Opportunistic Infections. Montreal: Abstract 121; 2009. [Google Scholar]
- 72.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 73.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laumann E, Gagnon JH, Michael RT, Michaels S. The Social Organization of Sexuality. Chicago: University of Chicago Press; 1994. [Google Scholar]
- 75.Flannery D, Ellingson L, Votaw KS, Schaefer EA. Anal intercourse and sexual risk factors among college women, 1993–2000. Am J Health Behav. 2003;27:228–34. doi: 10.5993/ajhb.27.3.4. [DOI] [PubMed] [Google Scholar]
- 76.Houston AM, Fang J, Husman C, Peralta L. More than just vaginal intercourse: anal intercourse and condom use patterns in the context of “main” and “casual” sexual relationships among urban minority adolescent females. J Pediatr Adolesc Gynecol. 2007;20:299–304. doi: 10.1016/j.jpag.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Tian LH, Peterman TA, Tao G, et al. Heterosexual anal sex activity in the year after an STD clinic visit. Sex Trans Dis. 2008;35:905–9. doi: 10.1097/OLQ.0b013e318181294b. [DOI] [PubMed] [Google Scholar]
- 78.Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88:1265–66. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matasha E, Ntembelea T, Mayaud P, et al. Sexual and reproductive health among primary and secondary school pupils in Mwanza, Tanzania: need for intervention. AIDS Care. 1998;10:571–82. doi: 10.1080/09540129848433. [DOI] [PubMed] [Google Scholar]
- 80.Sallah ED, Grunitzky-Bekele M, Bassabi K, et al. [Sexual behavior, knowledge and attitudes to AIDS and sexually transmitted diseases of students at the University of Benin (Togo)] Sante. 1999;9:101–19. [PubMed] [Google Scholar]
- 81.Fonck K, Kaul R, Kimani J, et al. A randomized, placebo-controlled trial of monthly azithromycin prophylaxis to prevent sexually transmitted infections and HIV-1 in Kenyan sex workers: study design and baseline findings. Int J STD AIDS. 2000;11:804–11. doi: 10.1258/0956462001915327. [DOI] [PubMed] [Google Scholar]
- 82.Ramjee G, Gouws E. Prevalence of HIV among truck drivers visiting sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis. 2002;29:44–49. doi: 10.1097/00007435-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Ferguson A, Morris C. Assessing the role of anal intercourse in the epidemiology of AIDS in Africa. Int J STD AIDS. 2003;14:856. doi: 10.1258/095646203322556228. [DOI] [PubMed] [Google Scholar]
- 84.Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H. Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS. 2006;20:123–25. doi: 10.1097/01.aids.0000198083.55078.02. [DOI] [PubMed] [Google Scholar]
- 85.Schwandt M, Morris C, Ferguson A, Ngugi E, Moses S. Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya. Sex Transm Infect. 2006;82:392–96. doi: 10.1136/sti.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramanian T, Gupte MD, Paranjape RS, et al. HIV, sexually transmitted infections and sexual behaviour of male clients of female sex workers in Andhra Pradesh, Tamil Nadu and Maharashtra, India: results of a cross-sectional survey. AIDS. 2008;22(Suppl. 5):S69–79. doi: 10.1097/01.aids.0000343765.00573.ce. [DOI] [PubMed] [Google Scholar]
- 87.Munro HL, Pradeep BS, Jayachandran AA, et al. Prevalence and determinants of HIV and sexually transmitted infections in a general population-based sample in Mysore district, Karnataka state, southern India. AIDS. 2008;22(Suppl. 5):S117–25. doi: 10.1097/01.aids.0000343770.92949.0b. [DOI] [PubMed] [Google Scholar]
- 88.Kalichman SC, Simbayi LC, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009;85:411–15. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Padian NS, Marquis L, Francis DP, et al. Male-to-female transmission of human immunodeficiency virus. JAMA. 1987;258:788–90. [PubMed] [Google Scholar]
- 90.Osmond D, Bacchetti P, Chaisson RE, et al. Time of exposure and risk of HIV infection in homosexual partners of men with AIDS. Am J Public Health. 1988;78:944–48. doi: 10.2105/ajph.78.8.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grant RM, Wiley JA, Winkelstein W. Infectivity of the human immunodeficiency virus: estimates from a prospective study of homosexual men. J Infect Dis. 1987;156:189–93. doi: 10.1093/infdis/156.1.189. [DOI] [PubMed] [Google Scholar]
- 92.Seage GR, Mayer KH, Horsburgh CRJ. Risk of human immunodeficiency virus infection from unprotected receptive anal intercourse increases with decline in immunologic status of infected partners. Am J Epidemiol. 1993;137:899–908. doi: 10.1093/oxfordjournals.aje.a116751. [DOI] [PubMed] [Google Scholar]
- 93.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18:81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.May RM, Anderson RM. Transmission dynamics of HIV infection. Nature. 1987;326:137–42. doi: 10.1038/326137a0. [DOI] [PubMed] [Google Scholar]
- 95.DeGruttola V, Seage GR, 3rd, Mayer K, Horsburgh CR., Jr . In: Program and abstracts of the 4th International Conference on AIDS. Stockholm: Swedish Ministry of Health and Social Affairs; 1988. Infectivity of HIV in homosexual partners, abstract 4112. [Google Scholar]
- 96.Ahlgren DJ, Gorny MK, Stein AC. Model-based optimization of infectivity parameters: a study of the early epidemic in San Francisco. J Acquir Immune Defic Syndr. 1990;3:631–43. [PubMed] [Google Scholar]
- 97.Eisenberg B. The effect of variable infectivity on the risk of HIV infection. Stat Med. 1991;10:131–39. doi: 10.1002/sim.4780100117. [DOI] [PubMed] [Google Scholar]
- 98.Jacquez JA, Koopman JS, Simon CP, Longini IM., Jr Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7:1169–84. [PubMed] [Google Scholar]
- 99.Rapatski BL, Suppe F, Yorke JA. HIV epidemics driven by late disease stage transmission. J Acquired Immune Deficiency Syndromes. 2005;38:241–53. [PubMed] [Google Scholar]
- 100.Weber JN, McCreaner A, Berrie E, et al. Factors affecting seropositivity to human T cell lymphotropic virus type III (HTLV-III) or lymphadenopathy associated virus (LAV) and progression of disease in sexual partners of patients with AIDS. Genitourin Med. 1986;62:177–80. doi: 10.1136/sti.62.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozturk GE, Kohler PF, Horsburgh CR, Jr, Kirkpatrick CH. The significance of antilymphocyte antibodies in patients with acquired immune deficiency syndrome (AIDS) and their sexual partners. J Clin Immunol. 1987;7:130–39. doi: 10.1007/BF00916007. [DOI] [PubMed] [Google Scholar]
- 102.Goedert JJ, Eyster ME, Biggar RJ, Blattner WA. Heterosexual transmission of human immunodeficiency virus: association with severe depletion of T-helper lymphocytes in men with hemophilia. AIDS Res Hum Retroviruses. 1987;3:355–61. doi: 10.1089/aid.1987.3.355. [DOI] [PubMed] [Google Scholar]
- 103.Johnson AM, Petherick A, Davidson SJ, et al. Transmission of HIV to heterosexual partners of infected men and women. AIDS. 1989;3:367–72. doi: 10.1097/00002030-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 104.de Vincenzi I. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. European Study Group on Heterosexual Transmission of HIV. N Engl J Med. 1994;331:341–46. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 105.Guimaraes MD, Munoz A, Boschi Pinto C, Castilho EA. HIV infection among female partners of seropositive men in Brazil. Am J Epidemiol. 1995;142:538–47. doi: 10.1093/oxfordjournals.aje.a117672. [DOI] [PubMed] [Google Scholar]
- 106.Nagachinta T, Duerr A, Suriyanon V, et al. Risk factors for HIV-1 transmission from HIV-seropositive male blood donors to their female partners in northern Thailand. AIDS. 1997;11:1765–72. doi: 10.1097/00002030-199714000-00014. [DOI] [PubMed] [Google Scholar]
- 107.Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–57. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 108.Panda S, Chatterjee A, Bhattacharya SK, et al. Transmission of HIV from injecting drug users to their wives in India. Int J STD AIDS. 2000;11:468–73. doi: 10.1258/0956462001916137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.