Summary
Background
To describe incidence rates and risk factors for loss to follow-up (LTFU) among HIV-infected and HIV-exposed children in a large HIV treatment program in Western Kenya.
Methods
The USAID-AMPATH Partnership has enrolled > 100,000 patients (20% children) at 23 clinic sites throughout western Kenya. LTFU is defined as being absent from the clinic for >3 months if on combination antiretroviral treatment (cART) and >6 months if not. Included in this analysis were children aged <14 years, HIV-exposed or infected at enrolment, and enrolled between April 2002-March 2009. The incidence rates (IR) for LTFU are presented per 100 child-years (CY) of follow-up. Proportional hazards models with time independent and dependent covariates were used to model factors associated with LTFU. Weight for height Z-scores were calculated using EpiInfo, with severe malnutrition being defined as a Z-score ≤−3.0. Immune suppression was defined as per WHO age-specific categories.
Results
There were 13,510 children eligible for analysis, comprising 3106 children who at enrolment were HIV-infected, and 10,404 children who were HIV-exposed. The overall IR of LTFU was 18.4 (17.8-18.9) per 100 CY. Among HIV-infected children, 15.2 (13.8-16.7) and 14.1 (13.1-15.8) per 100 CY became LTFU, pre- and post-cART initiation respectively. The only independent risk factor for becoming LTFU among the HIV-infected children was severe immune-suppression (AHR: 2.17, 95%CI: 1.51-3.12). Among the HIV-exposed children, 20.1 per 100 (19.4-20.7) became LTFU. Independent risk factors for LTFU among them were being severely low weight for height (AHR: 1.69, 95%CI: 1.25-2.28), being orphaned at enrolment (AHR: 1.57, 95% CI: 1.23-1.64), being CDC Class B or C (AHR: 1.41, 95% CI: 1.14-1.74), and having received cART (AHR: 1.56, 95% CI: 1.23-1.99). Protective against becoming LTFU among the HIV-exposed were testing HIV-positive (AHR: 0.26, 95%CI: 0.21-0.32), older age (AHR: 0.90, 95% CI: 0.85-0.96), enrolling in later time periods, and receiving food supplementation (AHR: 0.58, 95% CI: 0.32-1.04).
Conclusions
There is a high rate of LTFU among these highly vulnerable children, particularly among the HIV-exposed. These data suggest that HIV-infected and HIV-exposed children are at especially high risk for LTFU if they are sick or malnourished.
Keywords: pediatrics, HIV, Africa, retention, losses-to-follow-up
Introduction
The advent of combination antiretroviral therapy (cART) has shifted the paradigm of HIV/AIDS by extending the life expectancy of persons living with the disease who have access to treatment towards that of uninfected individuals [1, 2]. As treatment becomes more available and more effective, attention is moving towards prevention, including secondary and tertiary prevention, and to the populations most vulnerable to the ravages of the HIV pandemic. Among the highest risk groups, and one of the most severely under-studied and under-treated [3], are the children of sub-Saharan Africa.
The majority of the 2 million children living with HIV/AIDS globally are in 10 countries, 9 of which are in sub-Saharan Africa [4]. There were 350,000 children who became newly HIV-infected in 2007; another 200,000 died of AIDS [4]. Effective at improving the survival, physical growth and other important outcomes of HIV-infected children [5-13], cART is becoming more available to this highly vulnerable population. Significant strides have been made in improving global pediatric antiretroviral coverage with an increase from 80,000 in 2005 to 198,000 in 2007 [14]. Despite this, only a fraction of the children in urgent need of cART globally are receiving treatment [14].
As in adults, losses to follow-up (LTFU) and patient retention pose critical challenges to the successful care and treatment of HIV-exposed and infected children [5-8, 15, 16]. Given that HIV-infected children will need care and treatment for longer, LTFU may be an even greater threat for them. However, the issues surrounding pediatric LTFU are not yet well characterized. Risk and protective factors that could be influenced or modified for the purposes of increasing pediatric retention have not been described, and rates of LTFU among different sub-sets of HIV-affected children are nearly non-existent.
We sought to elucidate issues related to LTFU among HIV-infected and exposed children attending a large network of HIV treatment and care clinics in western Kenya. Our specific objectives were to (1) calculate the incidence of LTFU among HIV-exposed and HIV-infected children, the latter both pre- and post-cART initiation; and (2) to identify baseline and time-varying risk factors for LTFU for both HIV-exposed and HIV-infected children. It is hoped that these data will inform program planners and policy makers about possible opportunities for intervention to reduce LTFU and improve patient retention in healthcare facilities.
Methods
Study Design
This was a retrospective analysis of prospectively collected routine clinical data. The study was approved by the Moi University School of Medicine Institutional Review and Ethics Committee and the Indiana University School of Medicine Institutional Review Board.
The Program
The Academic Model Providing Access to Healthcare (AMPATH) was initiated in 2001 as a joint partnership between Moi University School of Medicine (Eldoret, Kenya), the Indiana University School of Medicine (Indianapolis, USA), and the Moi Teaching and Referral Hospital (Eldoret, Kenya). The USAID-AMPATH Partnership was initiated in 2004 when AMPATH received ongoing funding through USAID and the United States Presidential Emergency Plan for AIDS Relief (PEPFAR). The initial goal of AMPATH was to establish an HIV care system to serve the needs of both urban and rural patients in western Kenya and to assess the barriers to and outcomes of antiretroviral therapy. Details of the development of this program have been described in detail elsewhere [17]. The first urban and rural HIV clinics were opened in November 2001. Since then, the program has enrolled more than 100,000 HIV-infected patients, including approximately 18,000 children, in 23 Ministry of Health facilities and numerous satellite clinics in western Kenya (data for satellite clinics are incorporated into their ‘parent’ clinic). All HIV and tuberculosis-related care and treatment are provided free at the point of care. Since late 2005, AMPATH has had an outreach program in which trained HIV-positive adults obtain detailed locator information on each new and returning patient in the clinic. When a pediatric patient does not return for a scheduled visit, an outreach attempt is made within 24 hours by telephone or home visit. If and when a patient is found to have died either through active outreach or by information passed to the clinic by relatives or friends, the patient is documented as deceased in the database and is no longer considered LTFU.
Children under 18 months of age are diagnosed with HIV using a DNA PCR test at 6 weeks of age, followed by a confirmatory DNA PCR (Amplicor, Roche, Basel, Switzerland) at 9 months of age or 6 weeks after weaning. For children older than 18 months of age, 2 parallel HIV rapid ELISA tests using Determine and Unigold are used. In the event of a tie between the 2 tests, a DNA PCR and a long-Elisa test are used to make the final determination. Children who are confirmed or suspected to be HIV-infected receive CD4 testing at enrolment and every 6 months thereafter if the CD4% of the total lymphocyte count is ≤30%; otherwise they receive a CD4 test annually. HIV-exposed children only receive CD4 testing after HIV-infection is confirmed, or if there is high clinical suspicion of HIV infection. Care is offered to all children who have enrolled in AMPATH, including those who are found to be HIV-negative, the latter until they are 5 years old. HIV-exposed children return to clinic every month until weaning and every 3 months thereafter, unless their health warrants more frequent clinic visits. During these visits, they are weighed and measured, and are screened for symptoms as per the normal pediatric HIV encounter. Further laboratory investigations are conducted when warranted. HIV-infected children receiving cART are seen monthly; those not on cART return every 2 or 3 months.
Data Collection
Clinicians complete standardized forms capturing demographic, clinical, and pharmacologic information at each patient visit. These data are then hand-entered into the AMPATH Medical Record System (AMRS), a secure computerized database designed for clinical management, with data entry validated by random review of 5% of the forms entered [18]. At the time of registration, patients are provided with a unique identifying number. For this study, all data were stripped of identifying information prior to analysis.
Study Population
The analysis included all patients aged 13 years and under, HIV-exposed or HIV-infected at enrolment (defined as documented HIV status within the first 3 clinic visits), who were attending one of the USAID-AMPATH clinics from April 2002 to March 2009.
Outcomes and Independent Variables
Children enrolled in this program are considered to be HIV exposed if their mother is HIV infected, they have not yet been tested for HIV or are less than 18 months old and have a negative DNA PCR. Children are considered HIV infected if they have a confirmed positive DNA-PCR prior to 18 months of age or a positive HIV ELISA at 15 months or older.
The primary outcome for this analysis was being lost-to-follow-up (LTFU). LTFU is defined as being absent from the clinic for at least 3 months with no information regarding vital status or whereabouts if receiving cART, and at least 6 months if not receiving cART. The outcome was treated as a repeated time-to-event measure, to account for children with multiple episodes of LTFU.
We hypothesized that several baseline (enrolment) and time-varying factors could be associated with a higher or lower risk of becoming LTFU. Cross-sectional variables considered were the sex of the child, their year of enrolment into the program, whether the child was attending an urban or a rural clinic, having ever received food supplementation (vs. never), and the child’s orphan status (having lost one or both parents vs. none). Longitudinal variables examined were age (in years), weight-for-height (Z score calculated with EpiInfo, with severely low weight-for-height defined as a Z score of ≤3 [19]), CD4 cell percentage of total lymphocyte count (CD4%), the use of combination antiretroviral treatment (cART), and CDC clinical stage. For the children defined as HIV-exposed at enrolment, we additionally considered whether they were subsequently found to be HIV-infected. As per country-adopted World Health Organization recommendations [20], severe immune suppression was analyzed as a binary variable as the threshold for severe immune suppression depends on the age of the child. Specifically, severe immune suppression is defined for children aged ≤18 months as a CD4% <25%; CD4% <20% if the child is aged >18 months but <5 years; and CD4% ≤15% if the child aged ≥5 years. HIV-exposed children who received cART did so as a result of their own health issues; cART use for the purposes of prevention of mother-to-child transmission (PMTCT) was not included in this analysis.
Analysis
Continuous variables are summarized by medians and interquartile ranges (IQR). Categorical variables are summarized by frequency and percentage. Time-dependent proportional hazard regression models were used to evaluate the association between loss-to-follow-up and hypothesized predictor variables. Variables were included in the multivariable models if they were statistically significant in univariable analyses (p<0.05). Some univariately significant variables are not included in the multi-variable models because the missing data led to non-estimable regression co-efficients. Gender, orphan status, urban clinic, enrollment period, and receiving food supplementation are included as fixed-covariates; age, immune suppression status, low weight adjusted for height, CDC clinical stage, HIV infection (for exposed cohort only) and receiving antiretroviral therapy are included as time-dependent covariates (in other words: using all available observations for these covariates). For missing observations in these time-dependent covariates, we searched within a 3-month window and imputed the closest observed value. We chose 3 months as the width of the window so that the imputed values would be reasonably accurate and a meaningful number of missing values could be filled. Completeness-of-enrolment data are presented in Table 1; completeness of data for time-updated variables are presented in Table 3 as a proportion of the expected number of measures given clinical protocols and algorithms.
Table 1.
Descriptive summary of HIV-infected and HIV-exposed pediatric populationsin USAID-AMPATH clinics*
| HIV-exposed at enrolment N=10,404 |
N Events | LTFU Rate per 100 CY |
HIV-infected at enrolment N=3106 |
N Events | LTFU Rate per 100 CY |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Sex | n=10,404 | n=3106 | ||||
| Male | 5125 (49.3%) | 247 | 38.8 | 1,513 (48.7%) | 263 | 13.7 |
| Female | 5279 (50.7%) | 297 | 47.1 | 1,593 (51.3%) | 289 | 14.4 |
|
| ||||||
|
Age in years*(me-
dian, IQR**) |
n=10,404 0.25 (0.10,1.14) |
- | - | n=3106 5.51 (3.44,8.54) |
- | - |
| ≤ Median Age | 5426 (52%) | 186 | 311.9 | 1554 (50%) | 267 | 17.8 |
| > Median Age | 5158 (48%0 | 358 | 28.7 | 1552 (50%) | 285 | 9.3 |
|
| ||||||
|
Ever received food
supplementation |
n=10,404 | n=3106 | ||||
| Yes | 331 (3.2%) | 8 | 10.4 | 143 (4.6%) | 32 | 11.1 |
| No | 10,073 (96.8%) | 536 | 45.0 | 2963 (95.4%) | 520 | 14.3 |
|
| ||||||
| Ever received cART | n=10,404 | n=3106 | ||||
| Yes | 2150 (20.7%) | 52 | 12.4 | 1,776 (57.2%) | 291 | 11.3 |
| No | 8254 (79.3%) | 492 | 58.0 | 1330 (42.8%) | 261 | 19.3 |
|
| ||||||
|
CD4% *
(median, IQR) |
n=1750 19 (12,26) |
- | - | n=2321 17 (10,26) |
- | - |
|
| ||||||
|
Severely immune-
suppressed * |
||||||
| Yes | 880 (50.3%) | 11 | 4.7 | 1175 (50.6%) | 92 | 8.9 |
| No | 870 (49.7%) | 26 | 6.1 | 1146 (49.4%) | 135 | 5.0 |
|
| ||||||
|
Weight for height
Z-score * (median, IQR**) |
n=533 −0.58 (−1.71,0.71) |
n=2391 −0.76 (−1.66,0.09) |
||||
|
Severely low
(Z≤−3.0) |
36 (7%) | 6 | 5.8 | 160 (7%) | 29 | 10.2 |
|
Moderately se-
vere to normal (Z>−3.0) |
497 (93%) | 109 | 13.4 | 2231 (93%) | 374 | 11.2 |
|
| ||||||
| CDC Clinical Stage* | n=9753 | n= 2,918 | ||||
| A/N | 8318 (86.9%) | 1715 | 25.7 | 1,683(57.7%) | 285 | 12.6 |
| B/C | 1255 (13.1%) | 337 | 12.2 | 1,235 42.3%) | 360 | 13.5 |
|
| ||||||
| Year of enrolment | n=10,404 | n=3106 | ||||
| <2005 | 766 (7.4%) | 581 | 28.6 | 316 (10.2%) | 172 | 16.4 |
| 2005-2006 | 3695 (35.5%) | 1286 | 19.7 | 1084 (34.9%) | 359 | 13.8 |
| ≥2007 | 5943 (57.1%) | 827 | 16.9 | 1706 (54.9%) | 235 | 13.3 |
|
| ||||||
|
Orphan status at
enrolment |
n=8354 | n=2,559 | ||||
| Orphan | 916 (11.0%) | 260 | 17.1 | 1085 (42.4%) | 250 | 12.8 |
| Non-orphan | 7438 (89.0%) | 1547 | 19.3 | 1,474 (57.6%) | 307 | 15.0 |
|
| ||||||
| Clinic location | n=10,404 | n=3106 | ||||
| Urban | 5535 (53.2%) | 1608 | 21.7 | 1,590 (51.2%) | 362 | 13.4 |
| Rural | 4869 (46.8%) | 1086 | 18.0 | 1,516 (48.8%) | 404 | 15.0 |
Denotes enrolment data
IQR=interquartile range
Table 3.
Predictors of HIV-exposed and HIV-infected children becoming lost to follow-up: Unadjustedμ and Adjusted Hazard Ratios (HR) and 95% Confidence Intervals (CI)
| HIV Exposed | HIV Infected | |||
|---|---|---|---|---|
| Unadjusted HR (95% CI) |
Adjusted HRμ (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
| Gender (male vs. female) | 1.00(0.89-1.12) | - | 1.10 (0.95-1.26) | - |
| Age per year increase* | 0.87 (0.85-0.89) % missing: 0% |
0.90 (0.85-0.96) | 0.96 (0.94-0.99) % missing: 0% |
0.93 (0.86-1.00) |
|
Orphaned at enrolment
(yes vs. no) |
0.31 (0.26-0.37) | 1.57 (1.23-1.64) | 0.83(0.70-0.98) | 1.09 (0.75-1.60) |
|
Attending urban clinic
(yes vs. no)* |
1.24(1.11-1.39) | 0.93(0.83-1.04) | 1.06 (0.92-1.22) | - |
|
Severely low weight for
height (z ≤−3 vs.>-3)* |
2.10 (1.68-2.61) % missing:0% |
1.69 (1.25-2.28) | 3.82(2.74-5.32) % missing: 25% |
1.61(0.66-3.93) |
|
Severely immune-
suppressed (per age cate- gory)* |
1.35(1.04-1.76) % missing: 86% |
- | 1.83(1.46-2.30) % missing: 32% |
2.17 (1.51-3.12) |
|
CDC clinical stage(B/C vs.
A) |
0.59 (0.56-0.70) % missing: 0% |
1.41(1.14-1.74) | 1.21 (1.02-1.43) % missing: 30% |
0.85 (0.59-1.23) |
|
Enrolled 2005-2006
(vs. <2005) |
0.62(0.56-0.69) | 0.68(0.58-0.80) | 0.85(0.68-1.06) | 0.80(0.43-1.48) |
|
Enrolled≥2007
(vs. <2005) |
0.62 (0.55-0.70) | 0.71(0.60-0.85) | 0.70(0.55-0.91) | 0.92 (0.47-1.84) |
|
Received food supplemen-
tation(yes vs. no)* |
0.52(0.37-0.72) | 0.58(0.32-1.04) | 0.09 (0.02-0.38) | - |
|
Became HIV-infected
(yes vs. no)* |
0.22(0.19-0.25) | 0.26(0.21-0.32) | - | - |
|
Receiving antiretrovirals
(yes vs. no)* |
0.47(0.41-0.55) % missing: 0% |
1.56 (1.23-1.99) | 0.94 (0.81-1.08) % missing: 0% |
- |
Completeness of data for cross-sectional variables are presented in Table 1. Completeness of data for longitudinal variables (age, weight for height, immune suppression, clinical stage) are presented as a proportion of the expected frequency of measures as per clinical algorithms for HIV-exposed and HIV-infected children, respectively,pre-imputation.
Longitudinal variables from enrolment until last visit using all available data.
The variables used in the full model are those that were significant in the univariable models(p<0.05). Some univariately significant variables are not included in the multi-variable models because the missing-data lead to non-estimable regression co-efficients.i.
We included all LTFU events for each subject and accounted for the intra-patient clustering effect by using the sandwich estimator of the standard errors of the regression coefficients [21]. Point estimates of incidence rates adjusting for length of follow-up were computed and the confidence intervals constructed using exact binomial limits. All analyses were performed in R software using the “survival” and “epiR” packages.
Results
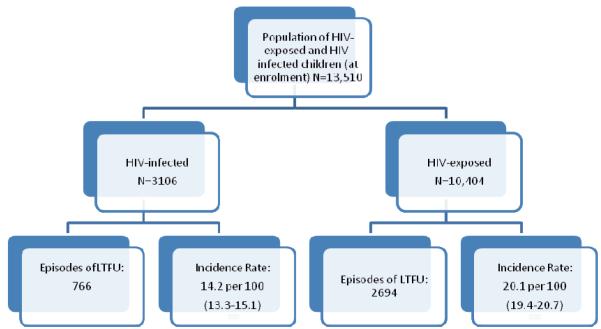
There were 13,510 children eligible for analysis, comprising 3106 children who were HIV-infected at enrolment, and 10,404 children who were HIV-exposed at enrolment. Both groups were evenly distributed by sex, with a median year of enrolment of 2007 (Table 1). Compared to the children who were HIV-exposed at enrolment, HIV-infected children were older (median years 5.51 vs. 0.25), more likely to be orphans (42% vs. 11%), more likely to have ever received cART (57% vs. 21%), and somewhat more likely to have ever received food supplementation (4.6% vs. 3.2%). Of the children who received CD4 testing within the first 3 visits, half of both the HIV-infected and HIV-exposed children were severely immune suppressed at enrolment. While 42% of the HIV-infected children were CDC Stage B or C at enrolment, only 13% of the HIV-exposed children were this advanced disease stage.
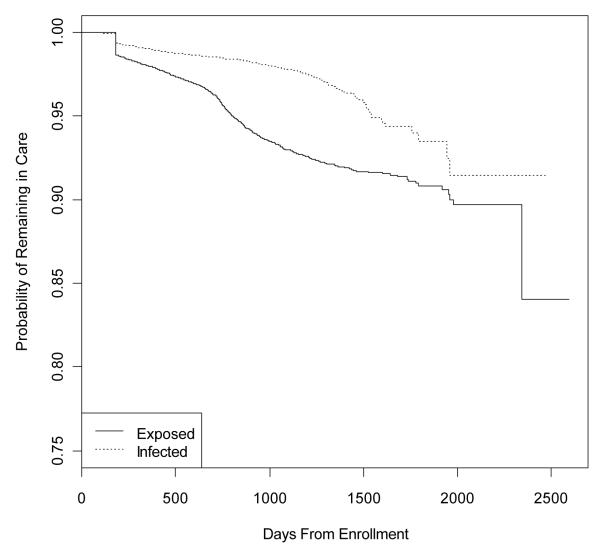
The median time of follow-up for the HIV-infected group was 552.5 days. The median follow-up time for the HIV-exposed was 373.0 days. The median time to first loss to follow-up or censoring among the HIV-infected group was 438 days; among the HIV-exposed, it was 297.5 days.
There were 766 and 2,694 episodes of LTFU among HIV-infected and HIV-exposed children during 5,400 and 13,436 child-years of follow-up, respectively. There were 2,596 children who had only 1 episode of LTFU, 312 had 2, and 72 had 3 or more episodes. The overall incidence rate (IR) (95% confidence interval, CI) of LTFU was 18.4 (17.8-18.9) per 100 child-years. The rate of LTFU among all HIV-infected children was 14.2 per 100 (13.3-15.1). Pre-cART use, 15.2 (13.8-16.7) HIV-infected children became LTFU, compared to 14.1 (13.1-15.8) post-cART. The HIV-exposed children had a higher rate of LTFU, at 20.1 per 100 (19.4-20.7) (Table 2).
Table 2.
Incidence rates of loss follow-up among HIV-infected and HIV-exposed children attending USA-ID-AMPATH Partnership clinics
| Population | Incidence rate per 100 child-years |
95% Confidence Interval |
|---|---|---|
|
| ||
|
Entire HIV-exposedand infected popu-
lationaged 0-13 years |
18.4 | 17.8-18.9 |
|
| ||
| HIV-exposed | ||
| From enrolment(all) | 20.1 | 19.4-20.7 |
|
From enrolment and prior to con-
firmed HIV-infection |
20.7 | 19.7-21.7 |
|
| ||
| HIV-infected | ||
| From enrolment (all) | 14.2 | 13.3-15.1 |
| HIV-infected-cART | 15.2 | 13.8-16.7 |
| HIV-infected on cART | 14.1 | 13.1-15.8 |
As summarized in detail in Table 3, in unadjusted analysis HIV-exposed children were more likely to become LTFU if they were severely low weight for height, severely immune suppressed, or if they were attending an urban clinic. Also unadjusted, they were less likely to become LTFU if they were orphaned, older (per year), if they received food supplementation, or were subsequently found to be HIV-infected. In multivariable analysis, it was determined that having severely low weight for height (AHR: 1.69, 95%CI: 1.25-2.28), being CDC Stage B/C (AHR: 1.41, 95% CI: 1.14-1.74), and having received cART (AHR: 1.56, 95% CI: 1.23-1.99) were independent risk factors for becoming LTFU among the HIV-exposed children. Becoming HIV-infected was protective against becoming LTFU (AHR: 0.26, 95%CI: 0.21-0.32), as was enrolling in later time periods, and older age (AHR: 0.90, 95% CI: 0.85-0.96). Although it did not reach statistical significance in multivariable analysis due to the low number of events, there was a strong trend suggesting that receiving food supplementation may also have been protective against LTFU among the HIV-exposed children (AHR: 0.58, 95% CI: 0.32-1.04),
In unadjusted analysis among the HIV-infected children, those who became LTFU were more likely to be severely immune-suppressed, and severely low weight for height. Orphans, older children (per year), those who received food supplementation, and those who enrolled in later time periods were less likely to become LTFU. In adjusted analyses, only the effect of severe immune-suppression (AHR: 2.17, 95% CI: 1.51-3.12) remained strongly predictive of becoming LTFU.
Discussion
These data indicate a substantial rate of LTFU among these HIV-affected children. Particularly high in HIV-exposed children, obvious clinical indicators such as severely low weight for height, advanced clinical disease, and severe immune suppression should be red flags to clinicians that a child is at risk for disappearing from care. These data highlight several important issues and opportunities for intervention.
These analyses confirm that losses to follow-up from HIV programs are an extremely important clinical issue among children in sub-Saharan Africa. Of every 100 children seen in AMPATH clinics, 18 will become LTFU. Even among those on cART, 14% have become LTFU, raising the spectre of drug resistance and further disease progression. Other centers have reported that approximately 10% of those on cART are becoming LTFU [5-8], while among HIV-infected children not on cART, and children whose last known serostatus was HIV-exposed, rates of LTFU are reported to be much higher (30%-40%) [15, 16]. Our data for HIV-infected children on cART, and the HIV-exposed children, are consistent with these other reports. In contrast however, our rate of LTFU for the HIV-infected prior to cART initiation is considerably lower. It is unknown if this is because of our peer outreach program or some other, as yet unmeasured, factor.
Secondly, it is unclear what happens to children who become LTFU, and this issue is the subject of a dedicated prospective evaluation by our group. We do know that among adults, approximately 50% of those LTFU will in fact be deceased [22, 23]. Given our findings that the risk of LTFU is most strongly associated with severe immune suppression, advanced clinical disease, and severely low weight for height, we hypothesize that a substantial portion of LTFU children will actually be deceased. Epidemiologically, the true mortality rate among children who are LTFU remains unknown. Our finding that severely low weight for height is associated with an increased of LTFU complements data from Malawi [15] and South Africa [16] suggesting that malnutrition is associated with not returning to clinic. We postulate that our finding that antiretroviral use is associated with a higher risk of LTFU among HIV-exposed children is due to confounding, with markers of illness and disease, and also with calendar time. Specifically, cART use in HIV-exposed children could suggest that the clinician has a high degree of suspicion that the child is actually HIV-infected and is at an advanced enough clinical stage that antiretroviral treatment is warranted. Because they are so sick, they would also therefore be at independently higher risk of LTFU. The other possibility is that, as can be seen from Tables 1 and 3, the risk of becoming LTFU is higher in the early period of the program. In a stratified sub-analysis, we did find that HIV-exposed children were more likely to receive cART in this early period (data not presented). However, we did adjust in the multivariable models for both disease stage and calendar time among the HIV-exposed children, and cART use remained predictive of LTFU.
Our third key finding is that there appear to be opportunities for intervention. Although our statistical power was limited in assessing the impact of food supplementation, our data suggest that providing food supplementation could reduce LTFU. The AMPATH food program is a partnership between the World Food Program and other non-governmental organizations [24]. Food supplementation may potentially help as an incentive to continue care and treatment and/or as an adjunct to cART; both of these mechanisms are currently unproven and need rigorous evaluation but these data indicate that this might be a way of retaining pediatric patients in care and subsequently optimizing their health. The specific impact of food supplementation on clinical outcomes needs to be more closely evaluated. A second opportunity for intervention arises from the substantial number of HIV-exposed children who become LTFU. This suggests a number of possible issues. The first is that HIV-exposed children not found to be HIV-positive may be healthier, and may therefore be less likely to continue to seek healthcare. However, as our data indicate that those who are malnourished and those who are sicker are more likely to become LTFU, the healthier LTFU population may only account for a small proportion of the lost HIV-exposed children. A second possibility is that children found to be HIV-infected are systematically better cared for by the healthcare system. The HIV care system is specifically geared for the HIV-infected patient, and these data are perhaps telling us that our pediatric care programs need to be expanded to the broader needs of children rather than specifically focus on needs associated with HIV infection. Indeed the high rate of LTFU suggests a lost opportunity from the perspective of child health in providing primary healthcare to these children. As HIV-exposed children who are orphaned at enrolment are 57% more likely to become LTFU, strengthening community and family supports may also support children to receive on-going healthcare.
Another opportunity for intervention arises from our fifth key finding, that severe immune suppression is an important risk factor for LTFU among HIV-infected children. Although more research is needed to determine the optimal time to initiate cART in older children, our data support the CHER Study [25] that indicate that cART should be initiated in infants as soon as they are diagnosed with HIV. Further, aggressive screening for HIV should be widely implemented, such as in well-child and immunization clinics, in order to identify HIV-infected children as early as possible and preferably prior to the emergence of symptoms.
There are a number of strengths to our analysis. The first is its large, geographically and ethnically diverse population, observed over a period of several years. This increases the likelihood that our findings could be generalized to the situation facing HIV-affected children elsewhere in sub-Saharan Africa, particularly East Africa. Secondly, we used time-updated measures of nutritional status, age, use of antiretrovirals, and immune suppression to more rigorously evaluate the impact of these factors on LTFU. Three, we evaluated LTFU in both HIV-exposed and HIV-infected children. This allowed for a more comprehensive evaluation of LTFU in an HIV-affected pediatric population. In so doing we identified several risk factors and opportunities for intervention that program planners and clinicians can use to improve pediatric retention in HIV care and treatment programs. A fourth important strength is that these findings are in the context of an active peer-led outreach program that aims to find patients who have missed scheduled visits within a short period of time. Fifth, our definition of LTFU does not include individuals known to have died.
There are also limitations to our analysis. First and foremost is that there were important proportions of missing data on several key factors which prevented us from fully investigating their effects, in spite of imputation. For example, while orphan status can change over time and was hypothesized to be a strong predictor of LTFU, we were not able to evaluate this variable in a longitudinal fashion because the data were only collected at enrolment. It is possible that the change in the hazard ratio of orphan status from protective to high risk is related to the inadequacy of investigating this effect as a fixed co-variate. There are also important proportions of missing values on immune status, disease stage, and weight adjusted for height Z-score (due to missing either weight or height) for HIV infected children even after imputations, which caused loss of statistical power in multivariable analysis. This is likely to be one of the reasons that several factors are significant predictors in univariable analysis but not in multivariable analysis (e.g. food supplementation), and why in the multivariable models some effects change substantially (i.e. different number of events and subjects at risk). This can also be seen from the widened confidence intervals of the hazard ratios in Table 3 (HIV-infected). A second limitation is that because these are observational data, incomplete recording of information by clinicians may have led to some random misclassification bias in the ascertainment of predictor variables. Third, resource constraints have forced the outreach program to initiate an outreach attempt in a prioritized manner, whereby the top priority are HIV-infected children on cART, followed by HIV-infected children not on cART, and then lastly HIV-exposed children. This may in part explain the higher LTFU among the HIV-exposed children. Fourth, our findings suggest that a large number of exposed children are not receiving HIV DNA testing, preventing both researchers and clinicians from being able to identify early on those children who are HIV-infected. Fifth, we were unable to reliably link the mother’s and the child’s treatment records and so are unable to explore the relationship between, for example, a mother’s probability of becoming lost to follow-up, and the child’s.
In conclusion, losses to follow-up among HIV-infected and HIV-exposed children are a major challenge to clinicians, epidemiologists, patients, and their care-givers in this East African setting. More than 20% of HIV-exposed children, and approximately 14% of HIV-infected children, are being LTFU from our program - and this in spite of an active outreach program, and a food distribution system to those who are food insecure or clinically malnourished. Further research is needed to understand the impact of food supplementation, earlier use of cART, and orphan status on pediatric retention in care. Qualitative research is needed to understand the perspective and needs of caregivers as they relate to maintaining children in care. Keeping patients in care is a crucial step in keeping them alive and healthy. Developing and providing interventions that assist patients to remain in care must be a top priority.
Figure 1.
Kaplan-Meier graph showing time to first loss to follow-up event among HIV-exposed and HIV-infected children
Figure 2.
Flowchart summarizing patients included in the analysis and their outcomes
Acknowledgements
We thank all the clinicians in all the AMPATH clinics, especially the Clinical Officers, Medical Officers, pediatricians, nutritionists, outreach workers, and social workers, for their dedication in caring for patients, and their attentiveness in accurately recording their patients’ data. We also thank all the data entry technicians, data managers, administrative and clerical staff, for enabling the collection, management, interpretation, and publication of these data.
AMPATH and the authors are particularly grateful to the Rockefeller Foundation for funding the development of the AMPATH Medical Records System, and the Kenyan Department of Leprosy, TB and Lung Disease (formerly the Kenyan National Leprosy and Tuberculosis Program) for their support. This research was supported in part by a grant to the USAID-AMPATH Partnership from the United States Agency for International Development as part of the President’s Emergency Plan for AIDS Relief (PEPFAR). This research was also supported by a grant from the National Institutes for Health in support of the East African International Epidemiologic Databases to Evaluate AIDS (IeDEA) Consortium.
References
- 1.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. Aids. 2007;21(9):1185–97. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moatti JP, I ND, Hammer SM, et al. Antiretroviral treatment for HIV infection in developing countries: an attainable new paradigm. Nature Medicine. 2003;9(12):1449–1452. doi: 10.1038/nm1203-1449. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . In: Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. W.H. Organization, editor. World Health Organization; Geneva: 2008. [Google Scholar]
- 4.UNAIDS . Report on the Global AIDS Epidemic. Joint United Nations Program on HIV/AIDS; Geneva: 2008. [Google Scholar]
- 5.Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49(5):523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 6.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. Jama. 2007;298(16):1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 7.Ellis J, Molyneux EM. Experience of anti-retroviral treatment for HIV-infected children in Malawi: the 1st 12 months. Ann Trop Paediatr. 2007;27(4):261–7. doi: 10.1179/146532807X245643. [DOI] [PubMed] [Google Scholar]
- 8.George E.e.a. Antiretroviral therapy for HIV-1 infected children in Haiti. Journal of Infectious Diseases. 2007;195(10):1411–8. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 9.Janssens B. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120(5):e1134–40. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 10.Kiboneka A, Wangisi J, Nabiryo C, et al. Clinical and immunological outcomes of a national paediatric cohort receiving combination antiretroviral therapy in Uganda. Aids. 2008;22(18):2493–9. doi: 10.1097/QAD.0b013e328318f148. [DOI] [PubMed] [Google Scholar]
- 11.Nyandiko WM, Ayaya S, Nabakwe E, et al. Outcomes of HIV-infected orphaned and non-orphaned children on antiretroviral therapy in western Kenya. J Acquir Immune Defic Syndr. 2006;43(4):418–25. doi: 10.1097/01.qai.0000243122.52282.89. [DOI] [PubMed] [Google Scholar]
- 12.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46(11):1751–60. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45(3):311–7. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . In: Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector - progress report. WHO, editor. World Health Organization; Geneva: 2008. [Google Scholar]
- 15.Ioannidis JP, Taha TE, Kumwenda N, et al. Predictors and impact of losses to follow-up in an HIV-1 perinatal transmission cohort in Malawi. 1999. pp. 769–775. [DOI] [PubMed]
- 16.Van Kooten Niekerk NK. The first 5 years of the family clinic for HIV at Tygerberg Hospital: family demographics, survival of children and early impact of antiretroviral therapy. Journal of Tropical Pediatriacs. 2006;52(1):3–11. doi: 10.1093/tropej/fmi047. [DOI] [PubMed] [Google Scholar]
- 17.Einterz RM, Kimaiyo S, Mengech HN, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82(8):812–8. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 18.Tierney WM, Rotich JK, Hannan TJ, et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Medinfo. 2007;12(Pt 1):372–6. [PubMed] [Google Scholar]
- 19.World Health Organization . Management of severe malnutrition: a manual for physicians and other senior health workers. World Health Organization; Geneva: 1999. [Google Scholar]
- 20.World Health Organization WHO Care Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children. 2007.
- 21.Wei LJ, Lin Y, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 22.Anglaret X, Toure S, Gourvellec G, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35(3):320–3. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 23.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. Jama. 2008;300(5):506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamlin J, Kimaiyo S, Lewis S, et al. Integrating nutrition support for food-insecure patients and their dependents into an HIV care and treatment program in Western Kenya. Am J Public Health. 2009;99(2):215–21. doi: 10.2105/AJPH.2008.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]