Abstract
Purpose
Guidelines for advanced life support (ALS) of cardiac arrest emphasize continuous and effective chest compressions as one of the main factors of cardiopulmonary resuscitation (CPR) success. The use of an automated load distributing chest compression device for CPR is promising but initial studies on survival show contradictory results. The aim of this study was to evaluate the effect of use of Autopulse™ on blood pressure (BP) in out-of-hospital cardiac arrest patients.
Methods
This prospective study included adult patients presenting with in refractory out-of-hospital cardiac arrest. Invasive arterial BP produced by AutoPulse™ was compared to BP generated by manual cardiopulmonary resuscitation (Active Compression Decompression). Systolic, diastolic and mean BP and end-tidal carbon dioxide were recorded before and after initiating up the automated band device for each patient. The comparison of diastolic BP produced by manual vs automated chest compressions was the primary end point.
Results
Hemodynamics are reported and analyzed in 29 patients. Median diastolic BP increased after starting Autopulse™ from 17[11–25] mmHg to 23[18–28] mmHg (p<0.001). Median systolic BP increased from 72[55–105] mmHg to 106[78–135] mmHg (p=0.02). Mean BP increased from 29[25–38] mmHg to 36[30–15] mmHg (p=0.002). On the other hand, ETCO2 did not increase significantly with Autopulse™ (21[13–36] vs 22[12–35] mmHg, p=0.80)
Conclusions
In patients with out-of-hospital cardiac arrest, the use of AutoPulse™ is associated with an increased diastolic BP compared to manual chest compressions. Until its benefit on survival is demonstrated, the increase of diastolic and mean BP is promising of better outcomes.
Keywords: Hemodynamics, cardiac arrest, resuscitation, out-of-hospital
INTRODUCTION
Guidelines for advanced life support (ALS) for cardiac arrest (CA) emphasize continuous and effective chest compressions as one of the main factors of cardiopulmonary resuscitation (CPR) success [1,2]. Manual chest compressions however, produce coronary and cerebral perfusion that is just 30% of normal values. [3]. Moreover, the effectiveness of manual compressions is uncertain, depending on caregiver’s efficacy and it deteriorates after one minute [4–6]. Studies performed in porcine and human models have shown an increased coronary perfusion pressure (a factor of survival after CPR [5]), when using the Autopulse™ band chest compression device [7,8]. First clinical studies on survival showed contradictory results [9,10]. To date, no studies have been conducted to evaluate its efficacy on hemodynamics in the prehospital setting. The aim of this study was to evaluate the effect of use of Autopulse™ on blood pressure (BP) in patients with out-of-hospital CA.
MATERIAL AND METHODS
This prospective study was carried out in the Emergency Medical Service (EMS) Department of two teaching hospitals in Paris area from January to December 2008. The institutional review board approved the study and waived the requirement for informed consent.
As previously described, the French EMS system has 2 types of ambulances: fire brigade squad are nearer to the patient and provides Basic Life Support (BLS) until a physician staffed ambulance arrives [11]. CPR is performed according to the European Resuscitation Council (ERC) guidelines until restoration of spontaneous circulation (ROSC) on scene or a decision to discontinue CPR efforts made by the physician [1]. Inclusion criterion was: adult patients with refractory out-of-hospital CA despite adequate CPR (no ROSC in the time interval during which the patient was intubated, had a IV line, received epinephrine and had an arterial catheter). All patients had first manual chest compression, then CPR with Autopulse™. The Autopulse™ automated chest compression system (Zoll®, Chelmsford, MA) is based on a load distributing band connected to a back-board containing a motor to retract the band [12]. The board was positioned under the patient. The length of the band automatically adjusted to the size and the shape of the patient. The device performs a constant 20% reduction in the anterior-posterior dimension under microprocessor control. Chest compression rate is 100 per minute in the continuous compression mode.
Patients presenting with CA were treated following standard ALS ERC guidelines 2005 [1]. All the patients were intubated and mechanically ventilated, received epinephrine and defibrillation if appropriate. Manual chest compressions were continued using active compression-decompression technique (Ambu cardiopump®), which is the standard CPR in both centres for years according to the French guidelines [13]. The Ambu cardiopump® device contains a force gauge which allows a control of the quality of chest compressions. A femoral arterial catheter was then inserted to monitor hemodynamics continuously [14]. Arterial catheters were femoral arterial catheters (Seldicath, 5F, 12 cm, Plastimed®, France) with fluid-flushed tubing to transducers (Sorensen Transpac III, Abbott Systems®) and a monitoring system (M-series CCT, Zoll®). The transducer was fixed by tape on the mid-axillary level. End-Tidal CO2 (ETCO2) was also measured continuously with a capnometer (M-series CCT, Zoll®) [15]. Patients were included at this stage. Three BP values (every 1 minute) were recorded. Then, Autopulse™ was started up and operated in the continuous mode. Three BP values were recorded again, during automated CPR (A-CPR). Diastolic BP produced by manual vs A-CPR were compared for each patient (primary objective). Systolic and mean BP were also compared, as well as ETCO2 (secondary objectives). A continuous print-out of curves was initiated at inclusion and followed until the end of the protocol.
Hemodynamic data were collected from the continuous print-out of curves. To improve the reliability of measurements, BP curves were digitalized and BP values were calculated from areas under the curve (image analysis software ImageJ). The diastolic BP values given by the monitor were not analyzed because they correspond most often to the nadir of the wave, which can be very different of the mean value of BP during diastole (Fig. 1). The diastole was defined as the last third of the wave cycle. Mean BP was calculated from the area under the curve of the whole cycle. Systolic BP was the peak pressure given by the monitor.
Fig. 1.
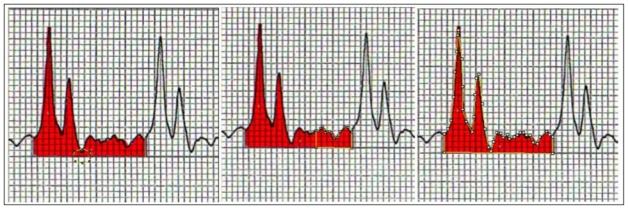
Nadir of the wave, which can be very different of the mean value of BP during diastole. The diastole was defined as the last third of the wave cycle. Mean BP was calculated from the area under the curve of the whole cycle.
Hypothesis was a 25% increase in diastolic BP with Autopulse™ when compared with manual CPR (primary end-point). The estimated number of patients was calculated from the analysis of a previous study, in which mean diastolic BP was 20 mmHg [16]. This calculation was based on a 25% (or 5 mmHg) increase of diastolic BP in the A-CPR group, a significance level of p < 0.05, a two-tailed analysis and a power of 90%. According to this calculation, a sample size of 28 patients would be necessary. The addition of a safety margin resulted in an estimate of 32 patients.
Data are expressed as median [IQR]. The study end-points were analyzed by the Wilcoxon rank sum test for paired data.
RESULTS
A total of 32 patients were included in the study. For 3 patients, BP curves could not be digitalized because of an inadequate scale set up, so hemodynamic data were analyzed from 29 patients. Mean age was 62±16 years. Most of them (n=23) presented with asystole, otherwise a pulseless electrical activity (n=4) or a ventricular fibrillation (n=2). They all received epinephrine before inclusion. Median time interval between CA and BLS start was 6[5–13] min and between CA and ALS start was 19[13–30] min. Median dose of epinephrine was 7[4–8] mg before inclusion. No ROSC was observed on scene. Diastolic BP on the whole increased after starting Autopulse™ from 17[11–25] mmHg to 23[18–28] mmHg (p<0.001) (Fig. 2). Systolic BP increased from 72[55–105] mmHg to 106[78–135] mmHg (p=0.02). Mean BP increased from 29[25–38] mmHg to 36[30–15] mmHg (p=0.002). On the other hand, ETCO2 did not increase significantly (21[13–36] vs 22[12–35] mmHg, p=0.80).
Fig. 2.
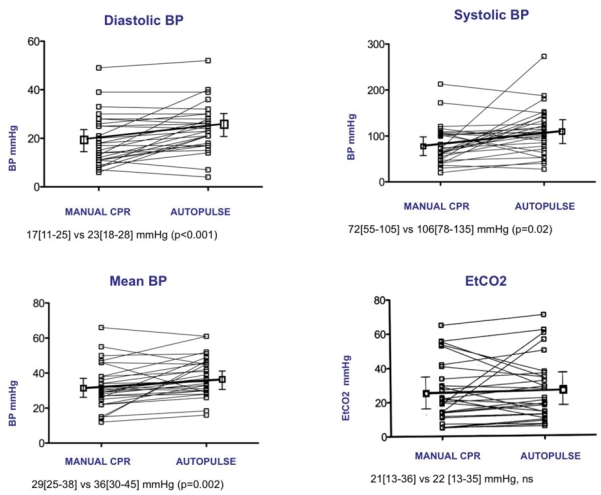
Systolic, diastolic, mean BP and End-Tidal CO2 under manual chest compressions and Autopulse™
DISCUSSION
The significant improvement of diastolic BP as well as systolic and mean BP, when using Autopulse™ compared to manual CPR in prolonged out-of-hospital CA are in accordance with previous studies on ICU terminally ill patients [8]. There is no recent evidence of new changes in CA management that improve prognosis a part from optimization of chest compressions. Recent guidelines indicated that chest compressions have to be performed continuously, which improves outcomes [17]. To date, the positive impact of the device on survivals has not been demonstrated.
The primary end point of the present study was an increase in diastolic BP, which reflects coronary flow. It has been demonstrated as a major determinant of survival among patients with CA in experimental models [19]. Beside an increase in diastolic BP, the present study showed improved systolic and mean BP. An improved mean BP may have a positive impact on cerebral flow and on neurological prognosis [17]. The mechanism of the device is the load distribution, compressing the entire chest and tacking advantage of the cardiac pump mechanism. To date, there is no clear explanation for the increased BP apart from the fact that distributing force over the anterior chest improves the effectiveness of chest compressions.
On the contrary, we did not observe any increase of the EtCO2, which is an indirect measurement of cardiac output and reflects the quality of CPR [2]. This result seems conflicting with the observed BP increase. However, ventilation modalities may affect the EtCO2 in a consequent way under mechanical cardiac massage [20]. For a large proportion of our patients, mechanical ventilation was really problematic after starting Autopulse™. The 20% reduction in the anterior-posterior dimension produced by the device with a rate of 100/min, hampers ventilation. Another important observation to mention is that if the majority of patients improved their hemodynamics, some patients did not or even get worse. It appears that there were both responders and non responders to the technique. There is no obvious relation with patient’s weight, comorbidities, initial rhythm, nor total dose of epinephrine in this population but the sample of non responders is small and does not allow to point out any common factor that could help predict who would be a non responder. This issue warrants further investigation. Our study has some limitations. For obvious reasons, the study could not be blinded, but the results are those given according to the assessment protocol, which is independent of attending physician’s observation. This before-after study has also the potential temporal bias related to the design. However, the study duration was only 5 minutes, and only 1 minute had elapsed between the 2 periods. Because the study is an observational one, all patients underwent first manual then A-CPR, without cross-over. This sequence is on the disadvantage of A-CPR because the elapsed time worsened the clinical conditions. Finally, the size of the study is limited which precludes any conclusions in term of morbidity and mortality. Nevertheless, the study was sufficiently powered regarding the primary end point.
CONCLUSION
In patients with out-of-hospital CA, a significant improvement on hemodynamics was observed during CPR when using the Autopulse™ automated chest compression device compared to the manual active compression-decompression CPR. Until its benefits on survival is demonstrated, the increase of diastolic and mean BP is promising of better outcomes in patients with out-of-hospital CA and may justify the use of this device in a modern strategy for ALS.
Acknowledgments
We would like to acknowledge the technical help of Bernard Cholley, MDPhD, in interpreting the data.
FUNDING SOURCES: French Society of Emergency Medicine fellowship.
Footnotes
ClinicalTrials: NTC000641069 http://clinicaltrials.gov/ct2/show/NCT00641069?term=NCT00641069&rank=1
Presented during the American Society of Anesthesiologists annual meeting, New Orleans, October 17–21, 2009.
No potential conflict of interest.
References
- 1.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council Guidelines for Resuscitation 2005, Section 4. Adult advanced life support. Resuscitation. 2005;67S1:S39–S86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 2.International Liaison Committee on Resuscitation. 2005 International Guidelines Conference on Cardiopulmonary Resuscitation and Emergency Care Science with Treatment Recommandations. Part 4: Advanced Life support. Resuscitation. 2005;67:213–247. doi: 10.1016/j.resuscitation.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Delguercio LR, Feins NR, Cohn JD, Coomaraswamy RP, Wollman SB, State D. Comparison of blood flow during external and internal cardiac massage in man. Circulation. 1965;31:171–180. doi: 10.1161/01.cir.31.4s1.i-171. [DOI] [PubMed] [Google Scholar]
- 4.Morley PT. Improved cardiac arrest outcomes: as time goes by? Crit Care. 2007;11:130. doi: 10.1186/cc5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa FJ, Ramalle-Gomara E, Lisa V, Saralegui I. The effect of rescuer fatigue on the quality of chest compressions. Resuscitation. 1998;37:149–152. doi: 10.1016/s0300-9572(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Halperin HR, Paradis N, Ornato JP, Zviman M, Lacorte J, Lardo A, Kern KB. Cardiopulmonary resuscitation with a novel chest compression device in a porcine model of cardiac arrest: improved hemodynamics and mechanisms. J Am Coll Cardiol. 2004;44:2214–2212. doi: 10.1016/j.jacc.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Timerman S, Cardoso LF, Ramires JA, Haleprin H. Improved hemodynamic performance with a novel chest compression device during treatment of in-hospital cardiac arrest. Resuscitation. 2004;61:273–80. doi: 10.1016/j.resuscitation.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Ong ME, Ornato JP, Edwards DP, Dhindsa HS, Best AM, Ines CS, Hickey S, Clark B, Williams DC, Powell RG, Overton JL, Peberdy MA. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA. 2006;295:2629–2637. doi: 10.1001/jama.295.22.2629. [DOI] [PubMed] [Google Scholar]
- 10.Hallstrom A, Rea TD, Sayre MR, Christenson J, Anton AR, Mosesso VN, Jr, Van Ottingham L, Olsufka M, Pennington S, White LJ, Yahn S, Husar J, Morris MF, Cobb LA. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA. 2006;295:2620–2628. doi: 10.1001/jama.295.22.2620. [DOI] [PubMed] [Google Scholar]
- 11.Gueugniaud PY, David JS, Chanzy E, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2006;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 12.Krep H, Marnier M, Breil M, Heister U, Fischer M, Hoeft A. Out-of-hospital cardiopulmonary resuscitation with Autopulse system: A prospective observational study with a new load-distributing band chest compression device. Resuscitation. 2007;73:86–95. doi: 10.1016/j.resuscitation.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Plaisance P, Lurie KG, Vicaut E, Adnet F, Petit JL, Epain D, Ecollan P, Gruat R, Cavagna P, Biens J, Payen D. A comparison of standard cardiopulmonary resuscitation and active compression-decompression resuscitation for out-of-hospital cardiac arrest. French Active Compression-Decompression Cardiopulmonary Resuscitation Study Group. N Engl J Med. 1999;31:569–575. doi: 10.1056/NEJM199908193410804. [DOI] [PubMed] [Google Scholar]
- 14.Duchateau FX, Ricard-Hibon A, Chollet C, Belpomme V, BenHellal A, Marty J. Feasibility of intra-arterial blood pressure monitoring in prehospital care. JEUR. 2003;16:123–126. [Google Scholar]
- 15.Cantineau JP, Lambert Y, Merckx P, Reynaud P, Porte F, Bertrand C, Duvaldestin P. End-tidal carbon dioxide during cardiopulmonary resuscitation in humans presenting mostly with asystole: a predictor of outcome. Crit Care Med. 1996;24:791–796. doi: 10.1097/00003246-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ducros L, Vicaut E, Soleil C, et al. Effect of the addition of vasopressin, or vasopressin plus nitroglycerine to epinephrine on arterial blood pressure during cardiopulmonary resuscitation in human. J Emerg Med. doi: 10.1016/j.jemermed.2010.02.030. in press. [DOI] [PubMed] [Google Scholar]
- 17.Ewy GA, Zuercher M, Hilwig RW, Sanders AB, Berg RA, Otto CW, Hayes MM, Kern KB. Improved neurological outcome with continuous chest compresions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116:2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RJ, Niemann JT. Manual vs Device-Assisted CPR. Reconciling Apparently Contradictory Results. JAMA. 2006;295:2661–2664. doi: 10.1001/jama.295.22.2661. [DOI] [PubMed] [Google Scholar]
- 19.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 20.Idris AH, Straples E, O’Brian DJ, Melker RJ, Del Duca KD, Falk JL. The effect of ventilation on acid-base balance and oxygenation in low blood-flow states. Crit Care Med. 1994;22:1827–1834. [PubMed] [Google Scholar]


