Abstract
Prolonged hypertension is the leading cause of heart failure. Failing hearts show reduced peroxisome proliferator-activating receptor activity and enhanced nuclear factor κB activity, which together modify cardiac inflammation and fibrosis. In vitro studies suggest that phytochemicals alter peroxisome proliferator-activating receptor and nuclear factor κB activity, but the capabilities of a phytochemical-rich diet are less understood. Grapes contain an array of commonly consumed dietary phytochemicals. In Dahl Salt-Sensitive hypertensive rats, we previously showed that dietary provision of whole table grape powder (3% w:w) for 18 weeks reduced blood pressure, cardiac hypertrophy, and diastolic dysfunction. The hypothesis tested here is that in this model, phytochemical provision from whole grape powder impacts cardiac peroxisome proliferator-activating receptor and nuclear factor κB activity and their related gene transcripts. Grape-fed rats had enhanced peroxisome proliferator-activating receptor-α and peroxisome proliferator-activating receptor-γ DNA binding activity but reduced nuclear factor κB DNA binding activity. RT-PCR revealed that grape-fed rats showed up-regulated mRNA for peroxisome proliferator-activating receptor-α, peroxisome proliferator-activating receptor-γ co-activator-1α, peroxisome proliferator-activating receptor-γ, and the cytosolic nuclear factor κB inhibitor, inhibitor κBα. By contrast, grape-fed rats showed down-regulated mRNA for tumor necrosis factor-α and transforming growth factor-β1. Finally, grape-fed rats showed significantly reduced cardiac tumor necrosis factor-α and transforming growth factor-β protein expression, increased inhibitor κBα expression, and reduced cardiac fibrosis. In the Dahl-Salt Sensitive rat, chronic intake of grapes altered cardiac transcripts related to peroxisome proliferator-activating receptor and nuclear factor κB that may be significant to the observed diet-associated cardioprotection.
Keywords: anthocyanin, antioxidant, hypertrophy, hypertension, inflammation
Introduction
Prolonged hypertension is a prevalent and significant contributor to morbidity and mortality from heart failure. The DASH(Dietary Approaches to Stop Hypertension) clinical trials provided evidence that diets rich in fruits and vegetables reduced blood pressure[1, 2]. Animal models of hypertension permit mechanistic appraisals of the interaction of diet and disease. One recent study in spontaneously hypertensive rats modeled the DASH diet using added nutrients, but failed to show an effect on blood pressure[3]. Therefore, a whole-food approach rather than altered nutrients alone may be a more appropriate dietary intervention to alter hypertension and related pathologies, including heart failure.
Previous studies by our group showed that in Dahl Salt-Sensitive rats fed with a high salt diet, dietary intake of whole table grape powder significantly reduced blood pressure but did not prevent the development of hypertension. Still, grape intake significantly reduced cardiac hypertrophy and cardiac lipid peroxide formation. In addition, grape-fed rats had improved diastolic dysfunction and cardiac output. Interestingly, the benefits of grape powder were not entirely related to blood pressure reduction, because comparable blood pressure reduction by vasodilator hydralazine failed to match the cardioprotective effects of grape treatment[4]. However, cardiac-specific mechanisms of these effects remain unknown.
The current project focuses on cardiac cell signaling related to transcription factors PPAR and NF-κB. PPARs are nuclear receptor transcription factors which impact cell metabolism, cell differentiation and inflammation. PPAR agonist drugs are used clinically to address hyperlipidemia and/or insulin resistance, but ancillary anti-inflammatory effects have also been observed[5, 6]. Importantly, PPAR isoform mRNA and/or protein is deficient in hypertrophied, failing hearts[7, 8], but early treatment with PPAR agonists reduces hypertension-related cardiac pathology[9, 10]. However, the risks and benefits of PPAR-targeted drugs in human heart failure are controversial[11, 12] but is of current clinical interest given the widespread use of PPAR agonists in cardiac patients.
Transcription factor NF-κB activity is enhanced by oxidative stress. Active NF-κB promotes inflammation by promoting the transcription of various pro-inflammatory genes including cell adhesion molecules, inflammatory cytokines, and chemokines. Cardiac NF-κB activity is positively correlated with heart failure progression[13, 14], and inhibition of NF-κB activity limits heart failure progression[15, 16]. PPAR and NF-κB have been described as physiological antagonists; PPAR activation reduces NF-κB activation, and active NF-κB reduces PPAR/DNA binding. In heart failure models, PPAR agonists reduce cardiac NF-κB activity and reduce morbidity and mortality[17, 18]. However, the effect of phytochemical-rich diets upon cardiac PPAR and NF-κB activity is poorly understood.
In our current model, bioavailable grape phytochemicals may alter cardiac PPAR and/or NF-κB activity. Grapes are a source of diverse phytochemicals, but are particularly rich in pigment-conferring anthocyanins. In vitro studies show that anthocyanin-rich extracts can activate PPARs in varied experimental models[19–21]. If the grape diet altered cardiac PPAR activity, it could also limit cardiac NF-κB activity and associated cardiac inflammation and fibrosis. We then tested the hypothesis that dietary grape powder supplementation, which reduces Dahl-SS rat diastolic heart failure pathogenesis[4], is also associated with increased cardiac PPAR activity, decreased NF-κB activity, and reduced cardiac expression of cytokines and growth factors relevant to human heart failure pathogenesis.
Methods
Animal Care and Diets
Five week old Dahl-Rapp Salt-Sensitive rats (Harlan, Indianapolis, IN) were acclimated for one week on AIN-76a powdered diet (Research Diets, New Brunswick, NJ). Afterwards, rats were randomly assigned to one of five treatments (n = 12 each). Low Salt diet (“LS”, AIN-76a with 2.8% added carbohydrate, glucose:fructose 1:1), Low Salt diet + grape powder (“LSG”, AIN-76a with 3.0% w/w added grape powder), High Salt diet with 6% added NaCl (“HS”, AIN-76a with 2.8% w/w added carbohydrate), High Salt Diet + grape powder (“HSG”, AIN-76a with 3.0% w/w added grape powder), or High Salt Diet + hydralazine (“HSH”, 20mg/kg body weight/day, in drinking water). Hydralazine dose was based upon findings in the Dahl-SS rat[22], to obtain a similar % reduction in systolic blood pressure as that observed previously with our grape powder[4]. Diet composition and grape powder phytochemical profile are detailed in tables in the online-supplement table S1 and table S2 via http://hyper.ahajournals.org. Hydralazine-fortified drinking water was made every two days, with concentration adjusted dynamically based upon weekly changes in water intake and body weight. Rats were fed 20g of powdered diet/head/day. Ad libitum intake of AIN diet averages 19–21 grams of AIN powder/day in the Dahl-SS rat[23], so provision of 20 grams/day ensured complete daily consumption. For the high salt diets, NaCl was added directly to the food hopper and mixed carefully with the daily ration of powdered diet. Rats were housed three/cage in 12h light:12h dark cycles, and water was provided ad libitum. This project was approved by the Animal Care and Use Committee at the University of Michigan.
Blood Pressure and Echocardiography Measures
During the 18 week study, blood pressure was measured bi-monthly in conscious, restrained rats by the IITC Mark 12 photoelectric/oscillometric tail cuff system (IITC Life Sciences, Woodland Hills, CA) using the unit and method we previously described[4] and validated against telemetric approaches[23]. A run was accepted if at least six of eight repeated measures were adequate (having detectable pulses and free of gross artifacts). The average was then calculated as the mean systolic value for that time point.
Echocardiography was performed after 18 weeks of diet treatment. Anesthesia was induced with 4% isoflurane and maintained with 1% isoflurane. All measurements were made in accordance with the conventions of the American Society of Echocardiography, and were conducted by the same trained, blinded research animal sonographer. Two-dimensionally guided M-mode recordings and Doppler tissue imaging were acquired as we described previously[23].
Cardiac Tissue Fractionation
Rats were anesthetized with 4% isoflurane and sacrificed by guillotine. Hearts were harvested, washed in phosphate-buffered saline, blotted, and weighed. The left ventricle was minced, flash frozen in liquid nitrogen, and stored at −80°C. Frozen cardiac tissue was subjected to nuclear and cytosolic fractionation by the method of Li et al.[24] using a NE-PER Nuclear Extraction Kit (Pierce, Rockford, IL). Complete fractionation and validation protocol is described in the online-supplement via http://hyper.ahajournals.org. Protein concentration of both fractions was measured by the BCA Assay (Pierce, Rockford IL).
Transcription Factor DNA Binding Assays
Once successful fractionation was confirmed, PPAR-α, PPAR-γ, and NF-κB activity were determined in nuclear extracts using Transcription Factor DNA Binding assays (Cayman Chemical, Ann Arbor MI) according to manufacturer’s protocol. In this assay, a specific, proprietary oligonucleotide containing PPAR response elements (PPREs) or κB response elements is immobilized onto the bottom of a 96-well plate. If present in the nuclear extract, PPAR isoforms and NF-κB-element p65 bind to the well-bound oligonucleotide PPREs or κB elements, respectively. Binding is then detected by addition of specific primary antibodies directed against the individual PPAR isoforms or against the p65 subunit of NF-κB. A secondary antibody conjugated to horseradish peroxidase is added to enable colorimetric detection by reaction with substrate TMB/hydrogen peroxide and subsequent color development measured at 450 nm. Values are expressed as optical density relative to total protein in the respective nuclear extract.
RT-PCR
Total RNA from minced left ventricle was isolated with the RNeasy™ Fibrous Tissue Midi Kit (Qiagen, Valencia CA, USA) following the manufacturer’s protocol. First strand cDNA synthesis was accomplished with the RT2 First Stand Kit (SABiosciences, Frederick MD). The relative abundance of eleven mRNA transcripts was compared using a custom RT2 Profiler PCR Array™ (SABiosciences). Relative expression was normalized relative to the average ΔCt of four housekeeping genes (P1 large ribosomal protein, hypoxanthine guanine phosphoribosyl transferase, ribosomal protein L13A, and lactate dehydrogenase), which were confirmed to be unaffected by treatment.
Cardiac Fibrosis, TNF-α, TGF-β, and IκBα Expression
Collagen component hydroxyproline was measured in left ventricle homogenates as a quantitative index of total tissue fibrosis using a method we described previously[4] and in the on-line supplement via http://hyper.ahajournals.org. The amount of hydroxyproline was calculated using the standard curve and expressed as µg/mg total protein. For TNF-α and TGF-β, ELISAs were conducted on left ventricle cytosolic fractions (n = 9 group) using commercial kits(R&D Systems, Minneapolis, MN) according to manufacturers’ instructions. Results are expressed relative to total protein. For the IκBα immunoblot, cytosolic fractions (50 µg) were mixed with reducing SDS sample buffer, denatured for five minutes at 95°C, resolved by electrophoresis on pre-cast NuPAGE™ 10% Bis-Tris polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and subsequently transferred onto PVDF membranes using a Novex Mini-Cell (Invitrogen). Blocking and antibody incubation steps were accomplished using the vacuum-based, SNAP i.d. Protein Detection System (Millipore, Billerica, MA, USA) using the ECL-specific blocking reagents and ECL-chemiluminescence detection system (GE Healthcare, Piscataway, NJ, USA). Primary antibody dilution was 1:100 for IκBα (SantaCruz Biotechnology) and 1:5,000 for β-actin (SantaCruz Biotechnology). PVDF membranes were exposed to CL-XPosure film (Pierce, Rockford, IL, USA), and band densities were analyzed using UN-SCAN-IT Gel software version 6.1 (Silk Scientific, Orem, UT, USA).
Statistics
Pair-wise comparisons of mRNA transcript were determined ±SD using the ΔΔCT method of Livak[25], using the PCR Array data analysis web portal of SABiosciences. All other endpoints were expressed ±SEM and compared using a one-way ANOVA to compare all five groups, or two-way ANOVA to compare four groups (LS, LSG, HS, HSG). Pair-wise, two-tailed comparisons were accomplished with Bonferonni post-hoc tests. Analysis was conducted with SPSS, version 16.0(SPSS, Chicago, IL), and a p value <0.05 was considered statistically significant.
Results
Blood Pressure and Cardiac Remodeling
Data for these detailed functional measures are included in the on-line supplement via http://hyper.ahajournals.org. Neither HSH nor HSG prevented the development of hypertension, but both significantly reduced systolic blood pressure relative to HS (on-line supplement figure S3). The first statistically significant decrease versus HS was detected at six weeks of treatment. The difference between LS and LSG was not statistically significant at any time point.
Compared to LS and LSG, HS and HSH were associated with cardiac hypertrophy and increased cardiac hydroxyproline content, an index of collagen content and fibrosis (on-line supplement table S4). However, these effects were attenuated in HSG. In contrast, LSG had no effect on heart weight and hydroxyproline content. HS and HSH had increased relative wall thickness (RWT), but HSG reduced RWT. Mild or early diastolic dysfunction is commonly characterized by altered filling velocities, measured by the ratio of early filling velocity (E wave) to late filling velocity (A wave). HS and HSH showed increased E/A elevation which was significantly attenuated in HSG. Prolonged isovolumetric relaxation time (IVRT) indicates increased myocardial stiffness due to fibrosis [26]. IVRT increased significantly in HS, but was significantly reduced in HSG. Ejection fraction was not significantly altered by high-salt feeding, which is expected in this rat model; the Dahl-SS rat is a model of diastolic dysfunction rather than systolic dysfunction. However, cardiac index reflects cardiac contractile efficiency by measuring the volume of blood pumped per minute (stroke volume × heart rate), per unit of body weight, and therefore reflects both diastolic and systolic function. Cardiac index was significantly lower in HS and HSH but was significantly improved in HSG. Collectively, these findings indicate that changes in cardiac geometry, diastolic parameters, and cardiac function are improved by the grape-containing diet, but not by the vasodilator hydralazine.
Transcription Factor Activity
Because HSH did not impact cardiac remodeling, fibrosis or function, the subsequent mechanistic studies only compared four groups (LS, LSG, HS and HSG). In LSG, both PPAR-α and PPAR-γ activity were increased as compared to LS (Table 1). HS showed reduced PPAR-α and PPAR-γ activity compared to LS. However, HSG displayed enhanced PPAR-α and PPAR-γ activity compared to HS. The conserved increase in nuclear extract PPAR binding in both LSG and HSG relative to their respective salt controls could suggest a specific effect of bioavailable grape phytochemicals and/or their metabolites upon PPAR activity. Compared to LS, LSG showed reduced NF-κB activity but HS had sharply increased NF-κB activity. However, HSG showed reduced NF-κB activity compared to HS.
Table 1.
Cardiac PPARα, PPARγ and NF-κB Activity
| Transcription Factors | LS | LSG | HS | HSG |
|---|---|---|---|---|
| PPAR-α | 0.8±0.02 | 1.2±0.03* | 0.6±0.02† | 0.8±0.02‡ |
| PPAR-γ | 0.35±0.06 | 0.45±0.03* | 0.25±0.03† | 0.35±0.04‡ |
| NF-κB | 0.07±0.03 | 0.04±0.02* | 0.16±0.04† | 0.10±0.03‡ |
In O.D./microgram protein ± SEM, n = 9 per group,
p<0.05 vs. LS.
p<0.05 vs. LS, LSG.
p<0.05 vs. HS, LSG.
RT-PCR
Compared to LS, LSG showed increased mRNA for PPAR-α, PPARγ, and PGC-1α, which would support the greater PPAR activity observed in the transcription factor activity ELISA (Table 2). Compared to LS, LSG also showed increased mRNA for IκBα and reduced mRNA for pro-inflammatory TNF-α. HS showed decreased PPAR-α, PGC-1α, and IκBα mRNA, increased NF-κB (p=0.08), and increased mRNA for pro-inflammatory cytokines and growth factors. These results are aligned with increased NF-κB activity observed in the transcription factor activity ELISA. In contrast, HSG showed increased PPAR-α, PPARγ, and PGC-1α mRNA, which would support enhanced PPAR activity. In addition, HSG showed increased IκBα mRNA and decreased mRNA related to multiple pro-inflammatory cytokines and growth factors, which would support reduced NFκB activity observed in the transcription factor activity ELISA. As with the transcription factor activity results, the conserved transcriptional effects in both LSG and HSG suggest specific effects from bioavailable grape phytochemicals and/or their metabolites.
Table 2.
RT-PCR
| Gene Symbol |
Gene Name | Fold Regulation by Salt |
Fold Regulation by Grape |
|
|---|---|---|---|---|
| HS (v LS) | LSG (v LS) | HSG (v HS) | ||
| PPAR-α | PPAR-α | −6.86* | 1.44* | 2.91* |
| PPAR-γ | PPAR-γ | 1.17 | 1.52* | 1.71* |
| PGC-1α | PPAR-γ coactivator 1α | −3.62* | 1.56* | 3.65* |
| NF-κB | Nuclear factor κ B | 1.28 | 1.02 | 1.14 |
| IκBα | Inhibitor kappa Bα | −2.31* | 1.3* | 3.48* |
| TNF-α | Tumor necrosis factor-α | 3.64* | −1.56* | −1.92* |
| IL-6 | Interleukin-6 | 1.23 | 1.06 | −1.75* |
| IL-1β | Interleukin-1β | 1.64* | −1.1 | −5.06* |
| TGF-β1 | Transforming growth factor-β1 | 2.62* | −1.21 | −2.06* |
| ICAM | Intercellular Adhesion Molecule | 1.26* | −1.17 | −1.62* |
N = 4 per group. Fold regulation, comparison via ΔΔCT method.
p at least <0.05 vs. respective salt control.
Cardiac TNF-α, TGF-β, and IκBα Expression
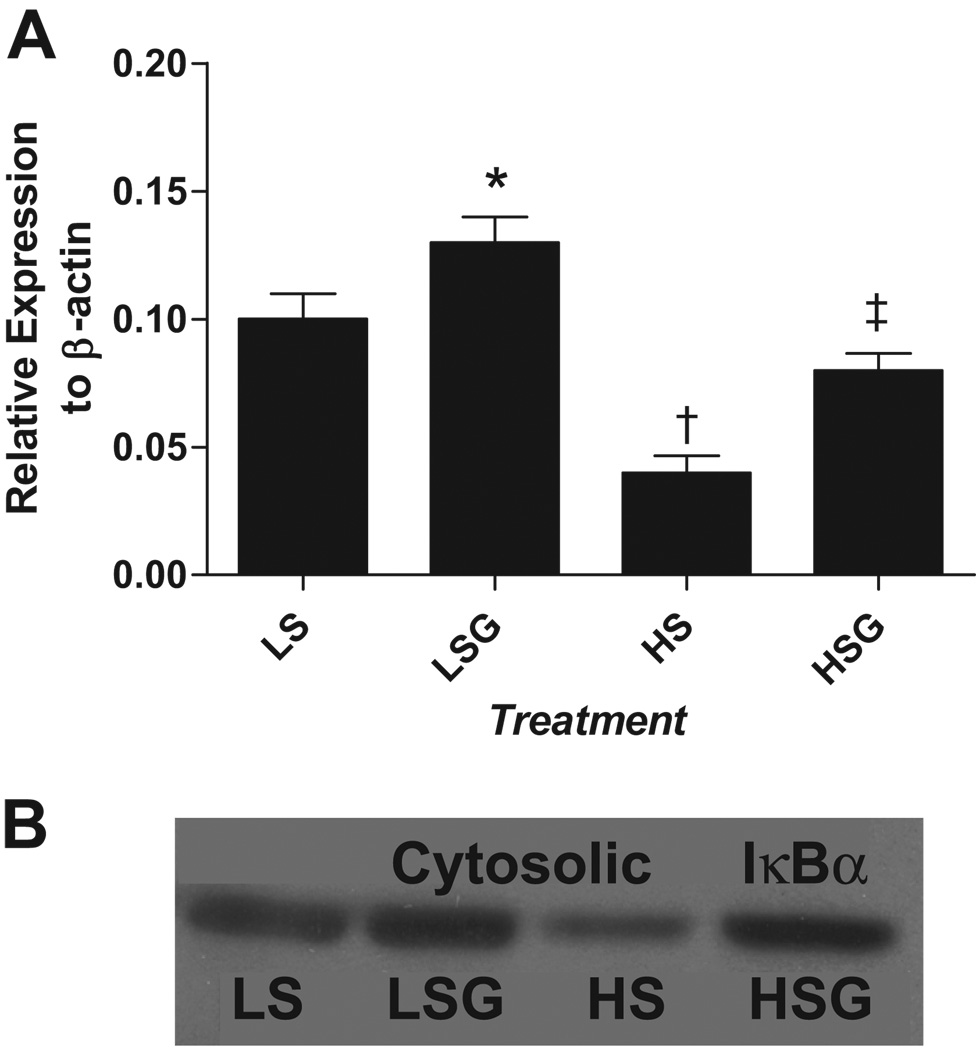
Results show that compared to LS, LSG had slightly reduced TNF-α and TGF-β expression which was not statistically significant (Table 3). In contrast, HS had sharply increased TNF-α and TGF-β expression over that observed in LS and LSG. Finally, compared to HS, HSG had reduced TNF-α and TGF-β expression. Immunoblot revealed that relative to LS, LSG had increased IκBα expression, while HS showed reduced IκBα expression (Figure 1a–b). Compared to HS, HSG had elevated IκBα expression. IκBα elevation in both LSG and HSG relative to their respective salt controls supports a conserved effect of grape-enriched diets upon IκBα expression.
Table 3.
Cardiac Protein Expression
| Proteins | LS | LSG | HS | HSG |
|---|---|---|---|---|
| TNF-α | 0.14±0.03 | 0.11±0.03 | 0.75±0.08† | 0.54±0.07‡ |
| TGF-β | 0.13±0.03 | 0.15±0.05 | 0.47±0.06† | 0.36±0.04‡ |
TNF-α and TGF-β quantified by ELISA relative to standard curve. Expressed as mean pg/mg total protein ± SEM, n = 9 per group.
p<0.05 vs. LS.
p<0.05 vs. LS, LSG, and HSG.
p<0.05 vs. LS, LSG, and HS.
Figure 1. a–b. IκBα Expression.
Mean ± SEM, n = 9 per group, quantitatively expressed relative to β-actin(1a) with a representative Western Blot(1b). (LS) low-salt diet; (LSG) low salt + grape powder diet; (HS) high-salt diet; (HSG) high salt + grape powder diet. * At least p < 0.05 vs. LS; † at least p < 0.05 vs. LS, LSG and HSG; ‡ at least p< 0.05 vs. HS and LSG.
Discussion
In summary, chronic intake of grapes altered cardiac transcripts related to PPAR and NF-κB activation that may be significant to the diet-associated cardioprotection in Dahl-SS rats. Grape-fed groups had enhanced PPAR-α and PPAR-γ activity, but reduced NF-κB activity. In addition, results support grape-associated up-regulation of PPAR-α, PPAR-γ co-activator-1α, PPAR-γ, and NF-κB inhibitor IκBα mRNA and down-regulation of TNF-α and TGF-β mRNA. Finally, grape intake was associated with significantly reduced cardiac TNF-α and TGF-β protein expression, increased IκBα expression, and reduced cardiac fibrosis.
Beyond Blood Pressure
Grape-related benefits may derive from indirect effects on cardiac pathology from reduced blood pressure. HSG effects on systolic blood pressure were early and sustained in HSG; these data support the previous findings of vasodilation from of grape product consumption. However, equivalent blood pressure by hydralazine did not translate to a similar reduction in cardiac fibrosis and remodeling. These observations may suggest that mechanisms beyond a systemic depressor effect are participating in the observed phenotypes.
The lack of depressor effect in LSG as compared to LS suggests that depressor effects are largely observed in hypertensive subjects. Despite the perceived absence of a hemodynamic effect in LSG, numerous mRNA/protein changes occurred in LSG hearts which suggest cardiac bioavailability of grape constituents and specific molecular effects of grape consumption. In this manner, LSG is perhaps the most intriguing group for revealing possible molecular alterations following grape consumption.
PPAR Activity Opposes NF-κB Activity
The interaction of PPAR activity and NF-κB-associated inflammation is a focus of this work. NF-κB is present in the cytosol in an inactive form complexed to an inhibitory kappaB (IκB) monomer. Various stimuli, including ischemia, free radicals, and cytokines activate NF-κB by inducing phosphorylation of its cytoplasmic inhibitor IκBα. NF-κB contributes to heart failure pathogenesis because it regulates genes/proteins important for disease progression including cytokines (e.g. TNF-α), interleukins (e.g., IL-1β, IL-6), growth factors (e.g. TGF-β), and adhesion molecules (e.g. ICAM).
PPAR and NF-κB interact in several ways to oppose their respective activities. First, PPAR activation increases IκBα transcription[6]. Furthermore, PPARs physically interact with NF-κB via its Rel homology domain that mediates interaction with IκBα[5]. Finally, nuclear NF-κB can inhibit PPAR binding to genomic PPREs, thereby reducing PPAR transcriptional activity and the expression of PPAR-related transcripts[5]. Current results show that grape intake increased IκBα expression, which would likely reduce NF-κB activity. However, further study is needed to examine if other potential mechanisms, independent of altered IκBα expression, contribute to the observed reduced NF-κB activity by grape intake.
Studies with PPAR agonists confirm the inverse association of PPAR activity with NF-κB activity. In Dahl-SS rats, PPAR-α agonist fibrate inhibited cardiac hypertrophy and hemodynamic dysfunction and improved survival[27]. Fibrate treatment also decreased NF-κB activity and the expression of NF-κB related target genes. In stroke-prone, spontaneously hypertensive rats, PPARγ agonist pioglitazone reduced cardiac NF-κB activity, cardiac fibrosis, and expression of NF-κB-related transcripts like TNF-α[28].
NF-κB Activity Impacts Heart Failure
HSG showed reduced NF-κB transcripts TNF-α and TGF-β. Human heart failure is correlated with increased plasma levels of TNF-α and TGF-β, and ex vivo analysis of explanted failing hearts reveals increased cardiac TNF-α[29] and TGF-β[30]. TNF-α may be directly involved in the progression of heart failure by exerting direct negative inotropic effects and by triggering apoptosis in cardiomyocytes[31]. In humans, elevated plasma TNF-α correlates with heart failure trajectory[32]. Cardiac TGF-β is up-regulated by increased work load and provokes the hypertrophic and pro-inflammatory cardiac gene expression[33]. Other genes affected by HSG in Table 2 would also contribute to reduced local fibrosis and inflammation, including ICAM and IL-6.
Of note, unlike HSG group PPAR activity, which parallels that of healthy rats (LS, LSG), increased expression of NF-κB related genes/proteins in salt-fed rats were not completely prevented in HSG hearts. That is, expression was significantly reduced compared to HS rats, but still did not approximate those found healthy rats (LS, LSG). Indeed, these results mirror those for altered systolic blood pressure, cardiac fibrosis and function which also did not match those of healthy rats. We expect that multiple factors impact fibrosis and related hemodynamic function such as neurohormone activation and oxidative stress. In addition, TNF-α and TGF-β expression are also altered by a number of redox related transcription factors like AP-1 and ets-1, which may also have increased activity in this model and may be unaffected by grape intake. Thus while PPAR activation can reduce NF-κB activation, this relationship did not completely limit pluripotent pro-fibrotic or pro-inflammatory pathogenesis in this model.
PPAR Activity Impacts Heart Failure
While the consequences of NF-κB activity are clear, the phenotypic sequelae of PPAR activity can depend upon which PPAR isoforms are activated. In hypertensive rats, PPAR-α activation reduces cardiac hypertrophy[27, 34, 35] improves diastolic and systolic function, and prolongs lifespan[27, 36]. However, in stroke-prone spontaneously hypertensive rats, PPAR-γ agonist pioglitazone did not alter blood pressure or cardiac hypertrophy, but did reduce NF-κB activity, fibrosis and a cardiac inflammation[28]. When isoform-specific agonists were directly compared in post-infarction rats[36], PPAR-α activation dose-dependently improved cardiac output, myocardial contractility, and diastolic relaxation and reduced cardiac hypertrophy and fibrosis. However, PPAR-γ activation exacerbated cardiac dysfunction. Therefore, experimental models appear to support the premise that PPAR-α agonism is beneficial for the heart while the effects of PPAR-γ agonism appear to vary.
Phytochemical Bioavailability and Candidate Effectors
Effects of grape phytochemicals upon cardiac transcription or cell signaling would likely require tissue uptake of phytochemical and their enterohepatic-conjugated metabolites. Many grape-derived phytochemicals may participate in the observed effects, including anthocyanins, flavanols, flavonols, and the stilbene resveratrol. Anthocyanins are the largest group of water-soluble pigments in the plant kingdom and are widely distributed in the human diet. Anthocyanins can increase PPAR activity in vitro and in vivo, and table grape powder may have impacted cardiac PPAR activity due in part to the content of anthocyanins, which average over 50% of the total flavonoids in table grapes[37] and in the particular powder used here[4]. Our group previously showed that intake of anthocyanin-rich tart cherry powder elevated liver PPAR-α and PPAR-γ mRNA[38]. In diabetic mice, diets enriched with anthocyanin-rich mulberry extract increased liver and adipose tissue expression of PPAR-γ and PPAR-α[39]. Macrophages exposed to anthocyanins showed increased PPAR-γ expression and PPAR-related transcriptional activity[21]. As such, the current results are in agreement with others using purified anthocyanins, anthocyanin-rich extracts, or anthocyanin-rich whole foods.
Regarding altered PPAR activity, bioavailable grape anthocyanins (and perhaps other phytochemicals) may modulate cell signaling components including phosphoinositide 3-kinase, Akt/PKB, tyrosine kinases, protein kinase C, and MAP kinases. For example, bioavailable phytochemicals, their enterohepatic metabolites, and their intracellular metabolites may interact with sulfhydryl moieties or binding sites on kinase proteins and alter secondary protein structure and activity. The exact kinase signaling pathways involved in the observed grape-related effects are unknown and require further investigation.
Regarding altered NF-κB activity, bioavailable grape phytochemicals, including non-anthocyanin compounds, may act directly as antioxidants and thereby reduce oxidative stress and NF-κB activity. However, conjugated phytochemical metabolites present in the heart have a reduced ability to donate hydrogen and to scavenge damaging radicals as compared to their non-conjugated parent compounds. Also, concentrations of these metabolites in tissues are much lower (1000 fold or more) than endogenous antioxidant compounds like glutathione, superoxide dismutase, or catalase. As such, grape diet effects upon NF-κB activity are likely mediated by altered kinase signaling and gene transcription/translation rather than by direct antioxidant ability.
Alternative Hypotheses and Study Limitations
Diet-mediated protection in HSG may also involve reduced neurohormonal or biomechanical sources of cardiac oxidative stress. Local cardiac oxidative stress is generated by increased cardiac renin-angiotensin-aldosterone (RAAS) system activation, increased norepinephrine, and from local inflammation. Furthermore, pressure/volume overload increases cardiac work and cardiac metabolism, increasing the opportunity for lost free electrons and oxidative stress. Should grape intake impact local neurohormones or cardiac work, grape intake may then indirectly affect redox-regulated NF-κB activity.
As related to PPAR activity, grape intake may have indirectly altered the formation of endogenous PPAR ligands like free fatty acids or eicosanoids. Also, the current study did not explore the activation of the PPAR-β/δ isoform, which is a recent target for pharmaceutical development and of growing clinical interest. Finally, grape phytochemicals other than anthocyanins may be involved in altered cell signaling and transcription factor activity, or they may participate synergistically. In summary, physiologically relevant phytochemical intake increased cardiac PPAR activity and decreased NF-κB activity, fibrosis, and inflammation in a rat model of salt-sensitive hypertension and diastolic dysfunction.
Perspectives.
The current results suggest that a phytochemical-rich diet imparts specific molecular effects within heart tissue which confer a degree of cardioprotection against hypertension-associated diastolic dysfunction. The pathogenesis of this rat model is particularly relevant to hypertensive heart failure pathogenesis in African Americans and in the elderly, particularly elderly women. A fruit and vegetable-rich DASH-style diet was recently shown to be inversely related to human heart failure[40]. Given the disappointing clinical results with vitamin and mineral supplement trials, phytochemical-rich whole foods may be a critical component of a DASH-style diet for reducing hypertension-associated heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH-NHLBI award F019691(Seymour) and NIH-NIA AG013283(Bolling). Pilot funding and grape powder were also provided by an unrestricted grant from the California Table Grape Commission1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The California Table Grape Commission did not contribute to the study design or analysis of study results.
Disclosures
None
REFERENCES
- 1.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 3.Doyle L, Cashman KD. The effect of nutrient profiles of the Dietary Approaches to Stop Hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br J Nutr. 2003;89:713–724. doi: 10.1079/BJN2003833. [DOI] [PubMed] [Google Scholar]
- 4.Seymour EM, Singer AA, Bennink MR, Parikh RV, Kirakosyan A, Kaufman PB, Bolling SF. Chronic intake of a phytochemical-enriched diet reduces cardiac fibrosis and diastolic dysfunction caused by prolonged salt-sensitive hypertension. J Gerontol A Biol Sci Med Sci. 2008;63:1034–1042. doi: 10.1093/gerona/63.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 6.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 7.Goikoetxea MJ, Beaumont J, Gonzalez A, Lopez B, Querejeta R, Larman M, Diez J. Altered cardiac expression of peroxisome proliferator-activated receptor-isoforms in patients with hypertensive heart disease. Cardiovasc Res. 2006;69:899–907. doi: 10.1016/j.cardiores.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Schupp M, Kintscher U, Fielitz J, Thomas J, Pregla R, Hetzer R, Unger T, Regitz-Zagrosek V. Cardiac PPARalpha expression in patients with dilated cardiomyopathy. Eur J Heart Fail. 2006;8:290–294. doi: 10.1016/j.ejheart.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Ichihara S, Noda A, Nagata K, Obata K, Xu J, Ichihara G, Oikawa S, Kawanishi S, Yamada Y, Yokota M. Pravastatin increases survival and suppresses an increase in myocardial matrix metalloproteinase activity in a rat model of heart failure. Cardiovasc Res. 2006;69:726–735. doi: 10.1016/j.cardiores.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Saka M, Obata K, Ichihara S, Cheng XW, Kimata H, Nishizawa T, Noda A, Izawa H, Nagata K, Murohara T, Yokota M. Pitavastatin improves cardiac function and survival in association with suppression of the myocardial endothelin system in a rat model of hypertensive heart failure. J Cardiovasc Pharmacol. 2006;47:770–779. doi: 10.1097/01.fjc.0000211791.22411.0d. [DOI] [PubMed] [Google Scholar]
- 11.Stafylas PC, Sarafidis PA, Lasaridis AN. The controversial effects of thiazolidinediones on cardiovascular morbidity and mortality. Int J Cardiol. 2009;131:298–304. doi: 10.1016/j.ijcard.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Campbell IW. The clinical significance of PPAR gamma agonism. Curr Mol Med. 2005;5:349–363. doi: 10.2174/1566524053766068. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Sen S. Role of the NF-kappaB signaling cascade and NF-kappaB-targeted genes in failing human hearts. J Mol Med. 2005;83:993–1004. doi: 10.1007/s00109-005-0691-z. [DOI] [PubMed] [Google Scholar]
- 14.Purcell NH, Molkentin JD. Is nuclear factor kappaB an attractive therapeutic target for treating cardiac hypertrophy? Circulation. 2003;108:638–640. doi: 10.1161/01.CIR.0000085362.40608.DD. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol. 2008;375:637–649. doi: 10.1016/j.jmb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frantz S, Hu K, Bayer B, Gerondakis S, Strotmann J, Adamek A, Ertl G, Bauersachs J. Absence of NF-{kappa}B subunit p50 improves heart failure after myocardial infarction. FASEB J. 2006;20:1918–1920. doi: 10.1096/fj.05-5133fje. [DOI] [PubMed] [Google Scholar]
- 17.Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol. 2004;43:1481–1488. doi: 10.1016/j.jacc.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Smeets PJ, Teunissen BE, Planavila A, de Vogel-van den Bosch H, Willemsen PH, van der Vusse GJ, van Bilsen M. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPARalpha and PPARdelta. J Biol Chem. 2008;283:29109–29118. doi: 10.1074/jbc.M802143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao ES, Tseng TH, Lee HJ, Chan KC, Wang CJ. Anthocyanin extracted from Hibiscus attenuate oxidized LDL-mediated foam cell formation involving regulation of CD36 gene. Chem Biol Interact. 2009;179:212–218. doi: 10.1016/j.cbi.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Xia M, Liu C, Guo H, Ye Q, Hu Y, Zhang Y, Hou M, Zhu H, Ma J, Ling W. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008;83:176–184. doi: 10.1016/j.lfs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, Li Y, Chi D, Yu X, Zhao T, Han P, Xia X, Ling W. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 22.Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, Kaneko T, Liu J, Ando K, Fujita T. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension. 2006;48:225–231. doi: 10.1161/01.HYP.0000232617.48372.cb. [DOI] [PubMed] [Google Scholar]
- 23.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–668. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Dedman JR, Kaetzel MA. Nuclear Ca2+/calmodulin-dependent protein kinase II in the murine heart. Biochim Biophys Acta. 2006;1763:1275–1281. doi: 10.1016/j.bbamcr.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Tarmonova L, Shutov AM, Chernysheva EV. [Factors influencing left ventricular diastolic function in elderly patients with chronic heart failure] Klin Med (Mosk) 2007;85:26–29. [PubMed] [Google Scholar]
- 27.Ichihara S, Obata K, Yamada Y, Nagata K, Noda A, Ichihara G, Yamada A, Kato T, Izawa H, Murohara T, Yokota M. Attenuation of cardiac dysfunction by a PPAR-alpha agonist is associated with down-regulation of redox-regulated transcription factors. J Mol Cell Cardiol. 2006;41:318–329. doi: 10.1016/j.yjmcc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Diep QN, Amiri F, Benkirane K, Paradis P, Schiffrin EL. Long-term effects of the PPAR gamma activator pioglitazone on cardiac inflammation in stroke-prone spontaneously hypertensive rats. Can J Physiol Pharmacol. 2004;82:976–985. doi: 10.1139/y04-094. [DOI] [PubMed] [Google Scholar]
- 29.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 30.Aharinejad S, Krenn K, Paulus P, Schafer R, Zuckermann A, Grimm M, Abraham D. Differential role of TGF-beta1/bFGF and ET-1 in graft fibrosis in heart failure patients. Am J Transplant. 2005;5:2185–2192. doi: 10.1111/j.1600-6143.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 31.Kubota T, Miyagishima M, Frye CS, Alber SM, Bounoutas GS, Kadokami T, Watkins SC, McTiernan CF, Feldman AM. Overexpression of tumor necrosis factor- alpha activates both anti- and pro-apoptotic pathways in the myocardium. J Mol Cell Cardiol. 2001;33:1331–1344. doi: 10.1006/jmcc.2001.1393. [DOI] [PubMed] [Google Scholar]
- 32.Bolger AP, Anker SD. Tumour necrosis factor in chronic heart failure: a peripheral view on pathogenesis, clinical manifestations and therapeutic implications. Drugs. 2000;60:1245–1257. doi: 10.2165/00003495-200060060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Parker TG, Packer SE, Schneider MD. Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990;85:507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battling obesity & hypertension. Does the DASH diet really reduce blood pressure? AWHONN Lifelines. 2002;6:21–23. doi: 10.1111/j.1552-6356.2002.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 35.Ogata T, Miyauchi T, Sakai S, Irukayama-Tomobe Y, Goto K, Yamaguchi I. Stimulation of peroxisome-proliferator-activated receptor alpha (PPAR alpha) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin Sci (Lond) 2002;103 Suppl 48 doi: 10.1042/CS103S284S. 284S–288S. [DOI] [PubMed] [Google Scholar]
- 36.Linz W, Wohlfart P, Baader M, Breitschopf K, Falk E, Schafer HL, Gerl M, Kramer W, Rutten H. The peroxisome proliferator-activated receptor-alpha (PPAR-alpha) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacol Sin. 2009;30:935–946. doi: 10.1038/aps.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantos E, Espin JC, Tomas-Barberan FA. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J Agric Food Chem. 2002;50:5691–5696. doi: 10.1021/jf0204102. [DOI] [PubMed] [Google Scholar]
- 38.Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. 2008;11:252–259. doi: 10.1089/jmf.2007.658. [DOI] [PubMed] [Google Scholar]
- 39.Park MY, Lee KS, Sung MK. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-alpha, PPAR-gamma, and LPL mRNA expressions. Life Sci. 2005;77:3344–3354. doi: 10.1016/j.lfs.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 40.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169:851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.