Abstract
Two novel SF5 analogs of the antimalarial agent mefloquine were synthesized in 5 steps and 10–23% overall yields and found to have improved activity and selectivity against malaria parasites. This work also represents the first report of SF5-substituted quinolines.
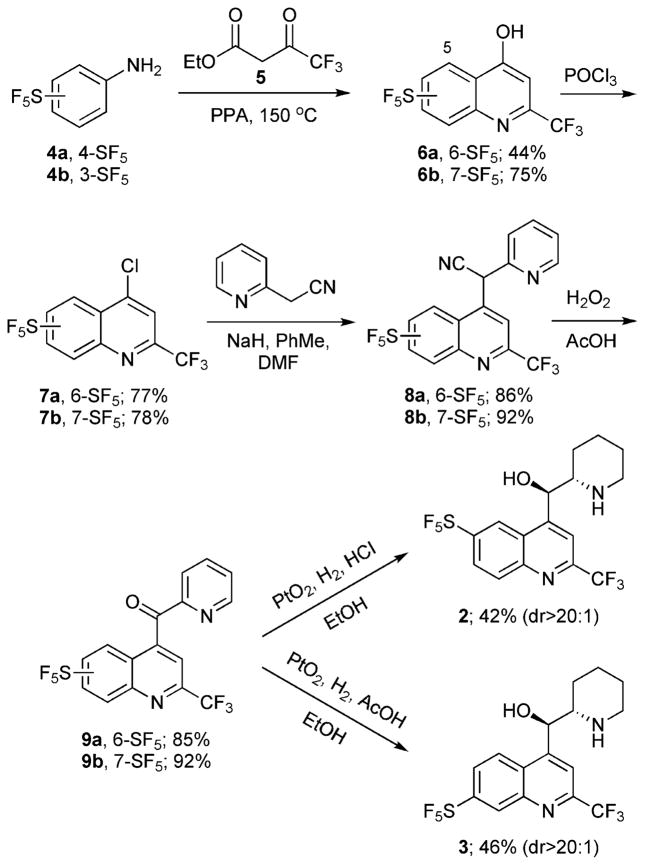
Malaria remains a major global health problem with approximately 300 million clinical cases and as many as 2.7 million casualties per year.1 One of the major factors contributing to the continued presence of malaria is the emergence of parasites that are resistant to one or more antimalarial compounds.2 Mefloquine (Fig. 1) is an orally-administered drug used as a prophylaxis and treatment for malaria, especially against chloroquine-resistant strains.3 It was until recently the drug of choice for U.S. military deployments in regions where malaria is endemic, primarily because its long half-life allows weekly administration. However, association of mefloquine with adverse neuropsychiatric effects, including anxiety, depression, halucinations and seizures4 has effectively curtailed its use. The ability of mefloquine to inhibit human P-glycoprotein and cross the blood–brain barrier has been suggested as an explanation for these adverse neurological events.5 We seek to reengineer the quinoline methanol scaffold to yield derivatives that, ultimately, should exhibit fewer adverse neurological effects but retain their antimalarial efficacy. In addition to optimizing the 4-position aminoalcohol moiety to reduce absorption through the blood–brain barrier, we were also interested in replacements of the CF3 groups in mefloquine with pentafluorosulfanyl (−SF5) substituents in order to probe slight perturbations of the electron density of the heteroaromatic scaffold.
Fig. 1.
Mefloquine (1).
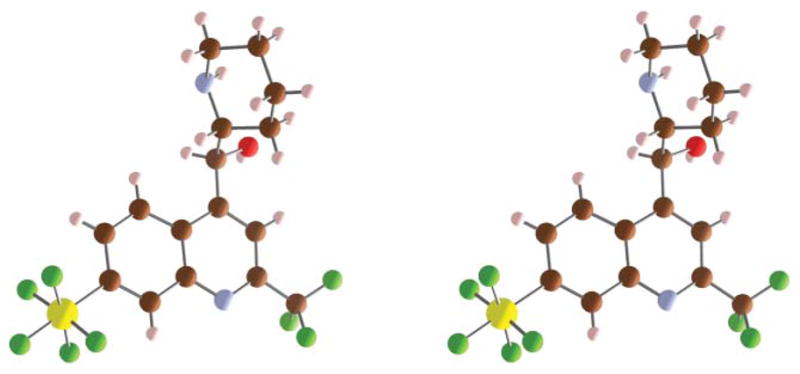
The physicochemical and pharmacological properties of small organic molecules are often significantly modified by the incorporation of fluorine atoms.6 While the synthesis of highly fluorinated and yet rigid octahedral SF5 derivatives is still emerging, recent studies are starting to exploit their unique potential in materials, pharmaceutical and agrochemical applications.7 The volume of the SF5 group is slightly less than that of a tert-butyl group, but considerably larger than CF3.8 The electronegativity of the SF5 function has been proposed to be as high as 3.65, vs. 3.36 for the CF3 group.9,10 In electrophilic aromatic substitutions, the SF5 group was found to have a Hammet σp value of 0.68 vs. σp = 0.54 for CF3.11 As our first foray into the chemistry and biology of SF5 derivatives, we are exploring the replacement of the CF3 groups in mefloquine with SF5 substituents. In agreement with the afore-mentioned parameters, the electron-density surface encoded with the electrostatic potential for 4-methyl-8-pentafluorosulfanyl-2-(trifluoromethyl)quinoline vs. 4-methyl-2,8-bis(trifluoromethyl)-quinoline in Fig. 2 shows higher steric crowding around the quinoline nitrogen, a slightly decreased electron density in the benzene ring, and a more positive nitrogen electrostatic charge (−0.64 vs. −0.66). In both model compounds, the nitrogen atoms are completely buried in between ortho- and peri-substituents.
Fig. 2.
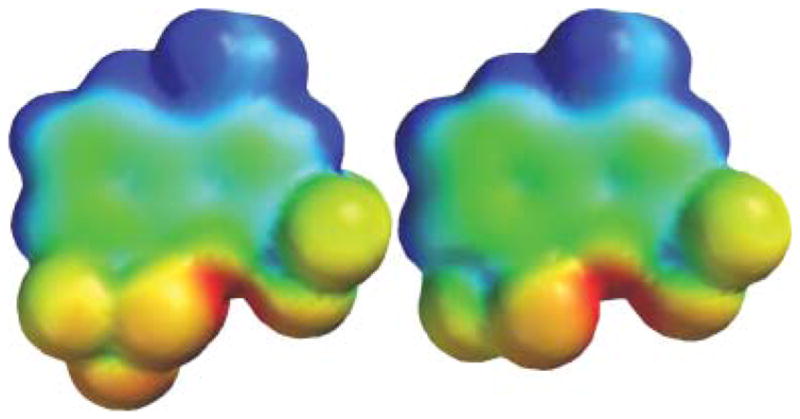
Electron-density surfaces/electrostatic potential maps calculated with Spartan 08 (HF/6–31G*) for 2 analogs of 1, 4-methyl-8-penta-fluorosulfanyl-2-trifluoromethylquinoline (left) and 4-methyl-2,8-bis-(trifluoromethyl)quinoline (right).
Other noteworthy features of the SF5 group include its remarkable chemical stability. Aromatic SF5 groups tolerate even harsh acidic conditions; their hydrolytic stability equals or exceeds that of the CF3 group.12
Despite their origins dating back half a century ago,13 only a limited number of aromatic pentafluorosulfanes have been prepared, and there is still a considerable need for practical synthetic routes. In particular, there are only a few heterocyclic derivatives, and there is no report in the literature on SF5 -containing quinolines. In this communication, we report efficient syntheses of 6-SF5 and 7-SF5 analogs of mefloquine, as well as the evaluation of their biological activities against malaria parasites.
The first synthesis of mefloquine was published in 1971,14 and since then, several other routes have been developed.15 A straightforward and high yielding synthesis is based on the oxidative decyanation of 2-(2,8-bis(trifluoromethyl)quinolin-4-yl)-2-(pyridin-2-yl)acetonitrile,16 and we selected this route to access analogs 2 and 3, which were each obtained in 5 steps from the commercially available amino-(pentafluorosulfanyl)-benzenes 4a and 4b (Scheme 1). Condensation of 4a and 4b with ethyl 4,4,4-trifluoroacetoacetate in the presence of polyphosphoric acid led to the 4-hydroxyquinolines 6a and 6b.17 In the conversion of 4b, only the desired 4-hydroxy-7-(pentafluorosulfanyl)quinoline 6b was isolated in 75% yield. The absence of the 5-pentafluorosulfanylquinoline isomer is probably due to the large steric demand of the SF5 group and/or electrostatic repulsion of the 4-oxygen substituent. Chlorination with phosphorus oxychloride gave the corresponding 4-chloroquinolines 7a and 7b in good yields. Subsequent nucleophilic aromatic substitution by 2-pyridylacetonitrile carbanion provided 8a and 8b. Exposure to a mixture of hydrogen peroxide and acetic acid then afforded the 4-quinolylketones 9a and 9b in excellent yields.
Scheme 1.
Synthesis of mefloquine analogs 2 and 3.
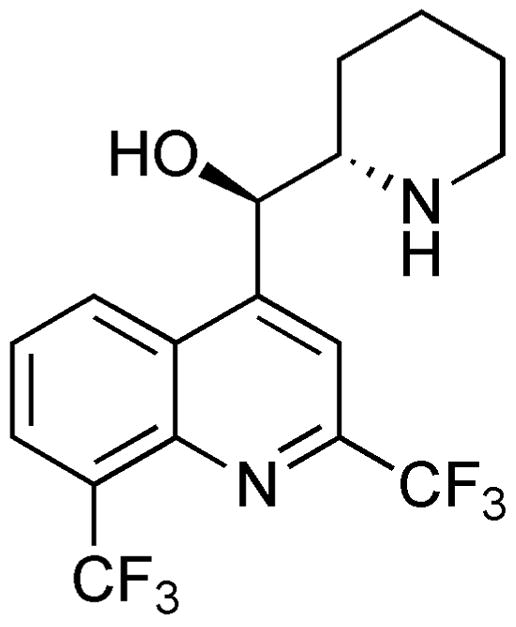
The concomitant reduction of the carbonyl and pyridyl groups in the presence of the quinoline moiety was achieved using catalytic hydrogenation under acidic conditions. This step of the synthesis proved to be problematic. After screening different solvents and acids, and varying hydrogen pressure and catalyst equivalents, optimal conditions for substrate 9a were found to be 0.4 equivalents of platinum oxide in ethanol containing hydrochloric acid, followed by recrystallization of crude 2 in MeOH. In contrast, 9b was best converted to target compound 3 in the presence of the milder acetic acid. Gratifyingly, both reactions were highly selective and afforded the desired anti-diastereomers. Furthermore, slow evaporation of a MeOH solution of 3 afforded needle-like crystals suitable for X-ray diffraction analysis (Fig. 3).18 The sulfur atom of the SF5 group is situated in an octahedral environment, and the disposition of the two stereocenters is anti as in mefloquine.
Fig. 3.
Stereoview of the X-ray structure of 3.
The antimalarial activities and selectivities of 2 and 3 were compared to 1 and mefloquine analogs in which the quinoline ring was substituted at the 6- and 7-positions with a trifluoromethyl group (10 and 11, Fig. 4).14,21 The 50% and 90% inhibitory concentrations (IC50 s and IC90 s) against four drug resistant strains of Plasmodium falciparum, and the LC50 s against a mammalian cell line were determined as previously described.20 Compound 2 exhibited generally equivalent or lower IC50 and IC90, and greater selectivity than its CF3 -congener 10 and mefloquine (Table 1). The IC50 and IC90 of 3 were generally equivalent to those of CF3 -analog 11 and mefloquine. These data demonstrate the effective biological mimicry as well as the considerable pharmaceutical potential of the CF3 –SF5 switch in quinoline containing antimalarials.
Fig. 4.
Structures of trifluoromethylated quinoline methanols 1019 and 1119 used as references in the biological assays.
Table 1.
Antimalarial activity and toxicity of selected quinoline methanols.20 The units are ng/mL for IC50, IC90 and LC50 data. The selectivity index (SI) is the ratio of the LC50 against RAW macrophages relative to the PfW2 IC50
| Analog | Pf W2 |
Pf D6 |
Pf C235 |
Pf C2A |
RAW LC50 | SI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |||
| 1 | 2.5 | 9.8 | 8.0 | 20 | 18 | 63 | 22 | 87 | 5064 | 2026 |
| 2 | 3.3 | 11 | 9.2 | 33 | 9.8 | 39 | 14 | 52 | 13740 | 4164 |
| 3 | 3.3 | 13 | 12 | 45 | 10 | 47 | 16 | 80 | ND | ND |
| 10 | 5.0 | 16 | 17 | 67 | 53 | 140 | 21 | 130 | ND | ND |
| 11 | 3.0 | 17 | 12 | 37 | 30 | 86 | 13 | 60 | ND | ND |
Conclusions
We have synthesized two novel pentafluorosulfanyl analogs of mefloquine and demonstrated their equivalent or improved biological activities vs. the parent drug and the corresponding C-6 and C-7 trifluoromethyl isomers. Further studies on other SF5-substituted analogs, in particular at the 8-position of the heterocyclic ring, are in progress and will be reported in due course. Our synthetic strategy also represents the first report on an SF5–quinoline construction, and thus expands the repertoire of pentafluorosulfanyl chemistry.22
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Experimental and spectra. CCDC reference number 735960. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b911483a
Contributor Information
Peter Wipf, Email: pwipf@pitt.edu.
Geoffrey S. Dow, Email: geoffrey.dow@us.army.mil.
Notes and references
- 1.World Health Organization. The World Health Report. World Heath Organization; Geneva, Switzerland: 1998. [Google Scholar]
- 2.Foley M, Tilley L. Pharmacol Ther. 1998;79:55. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]; Ridley RG. Nature. 2002;415:686. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 3.Trenholme GM, Williams RL, Desjardins RE, Frischer H, Carson PE, Rieckmann KH, Canfield CJ. Science. 1975;190:792. doi: 10.1126/science.1105787. [DOI] [PubMed] [Google Scholar]; Palmer KJ, Holliday SM, Brogden RN. Drugs. 1993;45:430. doi: 10.2165/00003495-199345030-00009. [DOI] [PubMed] [Google Scholar]; O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, Biagini GA, Bray PG, Gibbons P, Berry N, Winstanley PA, Mukhtar A, Bonar-Law R, Hindley S, Bambal RB, Davis CB, Bates M, Hart TK, Gresham SL, Lawrence RM, Brigandi RA, Gomez-delas-Heras FM, Gargallo DV, Ward SA. J Med Chem. 2009;52:1408. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 4.Tin F, Hlaing N, Lasserre R. Bull WHO. 1982;60:913. [PMC free article] [PubMed] [Google Scholar]; Weinke T, Trautmann M, Held T, Weber G, Eichenlaub D, Fleischer K, Kern W, Pohle HD. Am J Trop Med Hyg. 1991;45:86. doi: 10.4269/ajtmh.1991.45.86. [DOI] [PubMed] [Google Scholar]; Hennequin C, Bourée P, Bazin N, Bisaro F, Feline A. Arch Intern Med. 1994;154:2360. [PubMed] [Google Scholar]; Schlagenhauf P, Steffen R, Lobel H, Johnson R, Letz R, Tschopp A, Vranjes N, Bergqvist Y, Ericsson O, Hellgren U, Rombo L, Mannino S, Handschin J, Stürchler D. Trop Med Int Health. 1996;1:485. doi: 10.1046/j.1365-3156.1996.d01-85.x. [DOI] [PubMed] [Google Scholar]
- 5.Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Proc Natl Acad Sci U S A. 1989;86:695. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]; Riffkin CD, Chung R, Wall DM, Zalcberg JR, Cowman AF, Foley M, Tilley L. Biochem Pharmacol. 1996;52:1545. doi: 10.1016/s0006-2952(96)00556-4. [DOI] [PubMed] [Google Scholar]; Jones R, Kunsman G, Levine B, Smith M, Stahl C. Forensic Sci Int. 1994;68:29. doi: 10.1016/0379-0738(94)90376-x. [DOI] [PubMed] [Google Scholar]; Pham YT, Nosten F, Farinotti R, White NJ, Gimenez F. Int J Clin Pharmacol Ther. 1999;37:58. [PubMed] [Google Scholar]
- 6.For reviews: Isanbor C, O’Hagan D. J Fluorine Chem. 2006;127:303.Kirk K. J Fluorine Chem. 2006;127:1013.Bégué J, Bonnet-Delpon D. J Fluorine Chem. 2006;127:992.
- 7.Kirsch P, Hahn A. Eur J Org Chem. 2005:3095. [Google Scholar]; Geiser U, Schlueter JA, Wang HH, Kini AM, Williams JM, Sche PP, Zakowiez HI, VanZile ML, Dudek JD, Nixon PG, Winter RW, Gard GL, Ren J, Whangbo MH. J Am Chem Soc. 1996;118:9996. [Google Scholar]; St Clair TL, St Clair AK, Thrasher JS. 5,220,070. US Patent. 1993; Welch JT, Lim DS. Bioorg Med Chem. 2007;15:6659. doi: 10.1016/j.bmc.2007.08.012. [DOI] [PubMed] [Google Scholar]; Hansen JC, Savu PM. 5,286,352. US Patent. 1994; Nixon PG, Winter R, Castner DG, Holcomb NR, Grainger DW, Gard GL. Chem Mater. 2000;12:3108. doi: 10.1021/la040011w. [DOI] [PubMed] [Google Scholar]; Lim DS, Choi JS, Pak CS, Welch JT. J Pestic Sci. 2007;32(3):255. [Google Scholar]
- 8.Lentz D, Seppelt K. The SF5, SeF5 and TeF5 groups in organic chemistry. In: Akiba K, editor. Chemistry of Hypervalent Compounds. Wiley-VCH; New York: 1998. p. 295. [Google Scholar]
- 9.Sæthre LJ, Berrah N, Bozek JD, Børve KJ, Carroll TX, Kukk E, Gard GL, Winter R, Thomas TD. J Am Chem Soc. 2001;123:10729. doi: 10.1021/ja016395j. [DOI] [PubMed] [Google Scholar]; Wipf P, Henninger TC, Geib SJ. J Org Chem. 1998;63:6088. doi: 10.1021/jo981057v. [DOI] [PubMed] [Google Scholar]
- 10.See, however: Brant P, Berry AD, DeMarco RA, Carter FL, Fox WB, Hashmall JA. J Electron Spectrosc Relat Phenom. 1981;22:119.
- 11.Sheppard WA. J Am Chem Soc. 1962;84:3072. [Google Scholar]
- 12.Bowden RD, Comina PJ, Greenhall MP, Kariuki BM, Loveday A, Philp D. Tetrahedron. 2000;56:3399. [Google Scholar]
- 13.Sheppard WA. J Am Chem Soc. 1960;82:4751. [Google Scholar]; Sheppard WA. Aminoarylsulfur pentafluorides. 3,117,158. US Patent. 1964
- 14.Ohnmacht CJ, Patel AR, Lutz RE. J Med Chem. 1971;14:926. doi: 10.1021/jm00292a008. [DOI] [PubMed] [Google Scholar]
- 15.Adam S. Tetrahedron. 1989;45:1409. [Google Scholar]; Suresh Kumar M, Nageshwar YVD, Meshram HM. Syn Comm. 1996;26:1913. [Google Scholar]
- 16.Adam S. Tetrahedron. 1991;47:7609. [Google Scholar]
- 17.Pinder RM, Burger A. J Med Chem. 1968;11:267. doi: 10.1021/jm00308a017. [DOI] [PubMed] [Google Scholar]
- 18.The X-ray crystal structure of 3 is provided in the supporting information.
- 19.The relative configurations of 10 and 11 have not been assigned, but since a catalytic reduction was used, it is likely that they are also predominantly anti.
- 20.Dow GS, Chen Y, Andrews KT, Caridha D, Gerena L, Gettayacamin M, Johnson J, Li Q, Melendez V, Obaldia N, Tran TN, Kozikowski AP. Antimicrob Agents Chemo. 2008;52:3467. doi: 10.1128/AAC.00439-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KH, Hansch C, Fukunaga JY, Steller EE, Jow PYC, Craig PN, Page J. J Med Chem. 1979;22:366. doi: 10.1021/jm00190a007. [DOI] [PubMed] [Google Scholar]
- 22.This manuscript was reviewed by the Walter Reed Army Institute of Research and the U. S. Army Medical Research and Materiel Command, and there is no objection to its publication or dissemination. The opinions expressed herein are those of the authors and do not reflect the views or opinions of the Department of the Army or the Department of Defense.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.