Abstract
Myc is activated in many tumours, yet, paradoxically, stimulates differentiation in mammalian epidermis. To test whether the epidermis responds differently to different levels of Myc, we treated K14MycER transgenic mice with a range of concentrations of the inducing agent, 4-hydroxy-tamoxifen (4OHT). Proliferation was stimulated at all levels of Myc activity; sebocyte differentiation was stimulated at low and intermediate levels; and interfollicular epidermal differentiation at intermediate and high levels. Mutational inactivation of the Myc p21 activated kinase 2 (PAK2) phosphorylation sites increased Myc activity and further enhanced epidermal differentiation. We conclude that Myc induced differentiation acts as a fail-safe device to prevent uncontrolled proliferation and neoplastic conversion of epidermal stem cells expressing high levels of Myc.
Keywords: differentiation, Myc, oncogene, stem cells
INTRODUCTION
The most common forms of cancer are basal cell carcinoma and squamous cell carcinoma, non-melanoma tumours of the epidermis. They are believed to arise when epidermal stem cells acquire oncogenic lesions that result in uncontrolled proliferation and impaired differentiation (Janes & Watt, 2006). The mechanisms that control epidermal stem cell properties are therefore of considerable interest from the perspectives of cancer prevention and control.
50% of squamous cell carcinomas arising in patients who have undergone long-term immunosuppression have v-myc myelocytomatosis viral oncogene homologue (Myc) amplification (Watt et al, 2008). In healthy epidermis, Myc is detected at low levels in those regions, such as the basal layer of the interfollicular epidermis (IFE) and bulb of growing hair follicles (HFs), where proliferation normally occurs (Watt et al, 2008). Epidermal stem cells tend to divide infrequently (Watt & Jensen, 2009). Myc activation in the epidermis stimulates proliferation, and maintenance of stem cell quiescence requires expression of two proteins, B Lymphocyte-induced Maturation Protein-1 (Blimp1) and leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1), which negatively regulate Myc (Watt et al, 2008; Watt & Jensen, 2009).
Blimp1 is expressed by stem cells in the sebaceous gland (SG) (Horsley et al, 2006), while Lrig1 is a marker of stem cells in the IFE (Jensen & Watt, 2006; Jensen et al, 2009). Overexpression of Myc in the epidermis not only stimulates proliferation, as in virtually all cell types (Eilers & Eisenman, 2008), but also, surprisingly, stimulates differentiation. The lineages that are expanded on Myc activation are those that are normally controlled by Blimp1 and Lrig1, namely SG and IFE (Arnold & Watt, 2001; Waikel et al, 2001; Watt et al, 2008). These observations have led to the hypothesis that Myc-induced differentiation is a fail-safe device to prevent uncontrolled proliferation, and thus tumour formation, by the epidermal stem cell compartment (Jensen & Watt, 2006; Jensen et al, 2009; Watt & Jensen, 2009).
In a recent study, cells in a range of adult somatic mouse tissues were shown to respond differently to different levels of Myc activation (Murphy et al, 2008). Low levels of Myc were found to drive proliferation and tumour formation, whereas high levels triggered the apoptotic Alternative Reading Frame of Ink4a/CDKN2A locus (ARF)/p53 tumour suppressor pathway. Although the epidermis is resistant to Myc induced apoptosis, epidermal terminal differentiation involves loss of proliferative ability and, in the case of the IFE, shares a number of characteristics of apoptosis, including activation of transglutaminase and caspases (Watt et al, 2008). Thus, the epidermis might respond differently to different levels of Myc activity, with differentiation being a selective response to high Myc levels (Watt et al, 2008).
In order to examine epidermal responsiveness to different levels of Myc, we used K14MycER transgenic mice (Arnold & Watt 2001; Frye et al, 2003; Gebhardt et al, 2006; Frye et al, 2007). The keratin14 promoter targets transgene expression to the basal layer of the IFE, periphery of the SG and outer root sheath of the HF; transgenes are thus expressed by the known epidermal stem cell and committed progenitor compartments (Watt & Jensen, 2009). The C-terminus of the Myc transgene is fused to the ligand binding domain of a mutant estrogen receptor (ER); binding of ER by heat shock proteins in the cytoplasm renders Myc inactive, until on treatment with 4-hydroxy-tamoxifen (4OHT), the protein translocates to the nucleus and becomes transcriptionally active (Gandarillas & Watt, 1997).
We previously demonstrated that when a β-cateninER chimeric protein is expressed in the epidermis, different levels of β-catenin activity are achieved with different 4OHT concentrations (Silva-Vargas et al, 2005). We therefore devised the strategy of using different doses of 4OHT to achieve different levels of Myc activation in K14MycER mice. This provides a more refined approach to controlling Myc levels than that of comparing homozygous and heterozygous transgenic mice (Murphy et al, 2008).
RESULTS AND DISCUSSION
Differential activation of MycER by different 4OHT concentrations
We treated keratinocyte lines established from K14MycER epidermis overnight with 4OHT concentrations ranging from 10 to 200 nM, and examined nuclear accumulation of MycER by immunofluorescence staining with an anti-ER antibody. Previously, 100 nM 4OHT was shown to cause nuclear accumulation of MycER protein and stimulate differentiation (Gandarillas & Watt, 1997). In ≥90% of MycER cells cultured without 4OHT, there was no nuclear staining, indicating a low level of transgene leakiness, as reported previously (Arnold & Watt, 2001; Frye et al, 2003). The proportion of ER-positive nuclei increased approximately 3.5-fold on addition of 4OHT (up to 35% of cells), with a peak at 25 nM. Higher concentrations of 4OHT did not increase the proportion of ER-positive nuclei further (Fig 1A, B).
Figure 1. 4OHT dose-dependent induction of Myc.
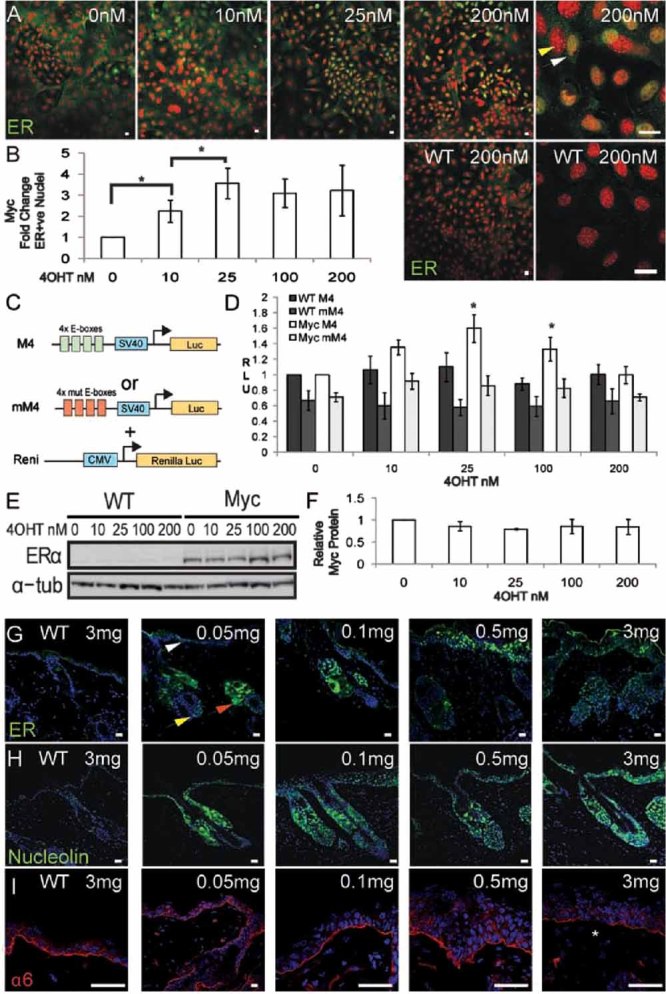
Transgene-negative (WT) and K14MycER (Myc) keratinocytes and mice were treated with the 4OHT concentrations (nM) or doses (mg) indicated.
- A. Anti-ER staining of immortalized K14MycER and WT keratinocytes. Yellow arrowhead: negative nucleus, white arrowhead: positive nucleus.
- B. Fold change in ER-positive nuclei relative to untreated K14MycER cells. Data are mean ± SEM of three independent experiments. Asterisks: p<0.05.
- C. Constructs used in luciferase assays.
- D. Cells were transfected with the constructs indicated. Firefly luciferase was normalized against R. luciferase activity. Relative light units (RLUs) are shown. Mean ± SEM of six experiments performed in duplicate. Asterisks: p<0.05 relative to WT cells transfected with M4 treated with same 4OHT concentration.
- E. Western blot probed with anti-ER or, as loading control, α-tubulin (tub) antibody.
- F. Quantitation of ER protein by densitometry, showing mean ± SEM of at least three blots.
- G–I. Dorsal skin of WT and K14MycER mice treated with 4OHT doses indicated, stained with antibodies to ER (G), nucleolin (H) or α6 integrin (I). White arrow: positive IFE cells; red arrow: positive SG cells; yellow arrow: positive HF bulb cells. Asterisk in (I): focal loss of α6. Scale bars: 10 µm (A, G-H, I, 0.05 mg), 50 µm (I, other doses).
To confirm that there was dose-dependent activation of Myc, we transfected wild type and K14MycER keratinocytes with a synthetic Myc reporter (M4) in which luciferase is expressed under the control of a SV40 promoter enhanced by four Myc specific E-boxes, or mM4, a reporter in which the E-boxes have been mutationally inactivated (Laherty et al, 1997). To control for transfection efficiency, M4 and mM4 were co-transfected with a Renilla luciferase reporter constitutively expressed under the control of a cytomegalovirus (CMV) promoter (Fig 1C). Cells transfected with mM4 had less basal luciferase activity than cells transfected with M4 (Fig 1D). Wild type cells showed some M4-dependent luciferase activity, attributable to endogenous Myc, but this was not affected by 4OHT (Fig 1D). When K14MycER cells transfected with M4 were treated with 10 or 25 nM 4OHT, luciferase activity increased, maximal activity being observed at 25 nM. 200 nM 4OHT did not result in any induction relative to untreated cells (Fig 1D). The bell-shaped dose response curve was not attributable to changes in total MycER protein (Fig 1E, F), nor to a decrease in the proportion of cells with nuclear MycER (Fig 1A, B).
To examine whether different 4OHT concentrations resulted in different levels of Myc activation in vivo, we treated adult mouse dorsal epidermis with the following 4OHT doses dissolved in 100 µl acetone: 0.05, 0.1, 0.25, 0.5, 1.0, 1.5 and 3.0 mg. Skin was examined 4 days later. Previously we applied 1 mg 4OHT in 200 µl ethanol to stimulate differentiation (Arnold & Watt, 2001; Frye et al, 2003). In K14MycER skin treated with acetone or WT skin treated with any 4OHT dose, no nuclear ER labelling was observed (Fig 1G and data not shown). At the lowest 4OHT dose, 0.05 mg, we observed scattered positive nuclei in the SG (red arrow), bulb of the HF (yellow arrow) and IFE (white arrow). The number of positively stained cells in all locations increased progressively with increasing concentrations of 4OHT (Fig 1G and data not shown).
To examine whether there was a dose-dependent change in expression of Myc target genes, we stained epidermal sections with antibodies to nucleolin and the α6 integrin subunit (Fig 1H, I). Nucleolin expression is positively regulated by Myc, whereas α6 integrin expression is repressed (Frye et al, 2003; Gebhardt et al, 2006). The number of nucleolin positive cells increased with increasing 4OHT dose (Fig 1H). α6 staining was unaffected by low concentrations of 4OHT, but was reduced and discontinuous at the highest concentration, 3 mg (Fig 1I, asterisk).
We conclude that, both in culture and in vivo, different levels of Myc activity in K14MycER expressing keratinocytes can be achieved by varying the 4OHT concentration to which the cells are exposed.
Different levels of Myc activity differentially affect epidermal proliferation and differentiation
There was no discernable effect of 4OHT on wild type skin, nor of acetone on K14MycER skin (Fig 2A, data not shown; Arnold & Watt, 2001; Frye et al, 2003). However, the consequences of Myc activation in K14MycER skin differed according to the dose of 4OHT applied (Fig 2B–D).
Figure 2. Epidermal response to different Myc levels.
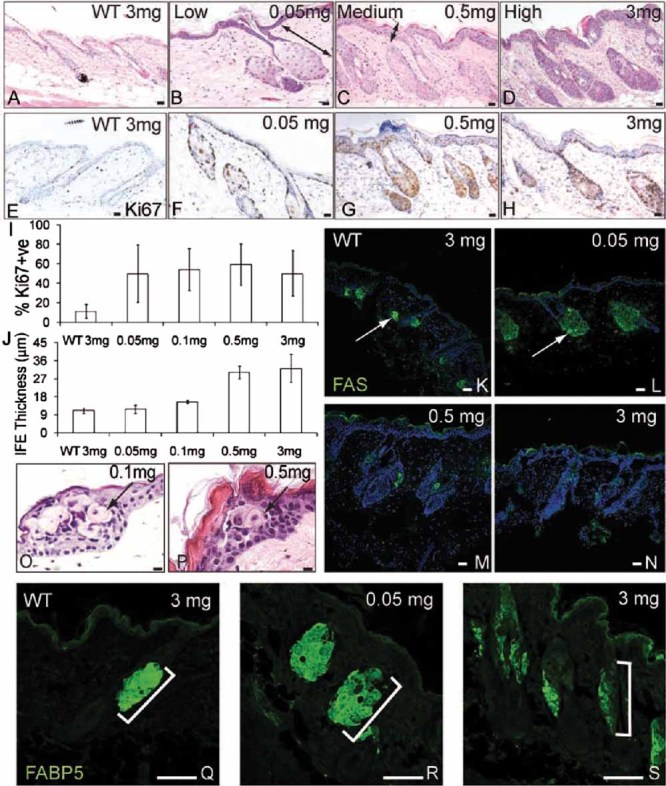
K14MycER and transgene negative (WT) dorsal skin was treated with the 4OHT doses indicated.
- A–D. Haematoxylin and eosin (H&E) staining. Doubleheaded arrows: SG enlargement (B); IFE thickening (C).
- E–H. Ki67 immunostaining.
- I. % Ki67 positive cells ± SEM in IFE basal layer.
- J. Maximum IFE thickness (µm) ± SEM.
- K–N, Q–S. Immunofluorescence staining for FAS (green, K–N) with DAPI nuclear counterstain (blue, K–N) or FABP5 (Q–S). Arrows indicate FAS expression. Brackets demarcate SG.
- O,P. H&E staining of IFE. Arrows show ectopic sebocytes. Scale bars: 10 µm (A–H, K–P), 75 µm (Q–S).
Ki67 labelling was used to evaluate proliferation (Fig 2E–I). In wild type telogen epidermis and untreated K14MycER epidermis there were small numbers of positive cells in the IFE basal layer and periphery of the SG (Fig 2E, I and data not shown). At all doses of 4OHT, the IFE basal layer became highly proliferative (Fig 2F–I and data not shown), while proliferation in the SG and HF increased with increasing dose (Fig 2G, H). At all 4OHT doses, IFE proliferation was largely confined to the basal layer of transgene positive cells (Fig 2F–H).
Low 4OHT doses led to no (0.05 mg) or a small (0.1 mg) increase in IFE thickness, whereas medium and high doses triggered marked thickening (Fig 2B–D, F–H, J). The thickening of the IFE reflected an increased number of terminally differentiating cell layers rather than expansion of the basal compartment (Fig 2A–H and data not shown).
SGs have an outer layer of undifferentiated, proliferative cells. As cells move towards the centre of the gland, they undergo terminal differentiation and are identified by their large size and pale cytoplasm (Lo Celso et al, 2008). In back skin the long axis of a normal SG is parallel to its associated HF (Fig 2A). We have previously reported that on Myc activation, the SG becomes greatly enlarged and expands into the adjacent HF, occupying the region below the infundibulum and above the base of the HF (Arnold & Watt, 2001; Frye et al, 2003). By measuring the maximum width and length of SG from micrographs, we determined that there was a variable, 3–10-fold increase in total SG area at 4OHT concentrations of 0.05 to 3 mg (Fig 2B–D and data not shown).
While the SG was enlarged at all 4OHT concentrations, different 4OHT concentrations differentially influenced the number of differentiated cells that expressed fatty acid synthase (FAS) (Fig 2K–N) and fatty acid binding protein 5 (FABP5) (Fig 2Q–S) (Lo Celso et al, 2008). The FAS positive area of individual SG was increased at low 4OHT doses (Fig 2K, L) but FAS was almost undetectable at the highest doses (Fig 2M, N). There was also an expansion of FABP5 expression at low 4OHT doses (Fig 2Q, R) and a reduction at higher doses (Fig 2S). The appearance of rare differentiated sebocytes in the IFE (Arnold & Watt, 2001; Braun et al, 2003) was stimulated at all 4OHT concentrations (Fig 2O, P and data not shown), suggesting that the loss of differentiated sebocytes in the SG at high 40HT concentrations reflected an expansion of the undifferentiated sebocyte compartment rather than a block in sebocyte terminal differentiation.
We conclude that different levels of Myc activity have different effects on epidermal proliferation and differentiation. Proliferation is stimulated at all levels. Low doses stimulate sebocyte terminal differentiation while at high doses the undifferentiated cells of the SG expand. IFE differentiation is unaffected at low 4OHT doses, but is stimulated at doses of 0.1 mg and above.
Mutation of the PAK2 phosphorylation sites enhances Myc activity
Myc activity is regulated by Myc binding partners and post-translational modifications (Eilers & Eisenman, 2008). p21 activated kinase 2 (PAK2)-mediated phosphorylation of Myc on three C-terminal residues (T358, S373 and T400), reduces the affinity of Myc for MYC associated factor X (Max), decreases the DNA binding affinity of Myc/Max heterodimers and decreases Myc stability (Huang, 2004; Huang et al, 2004). PAK2 is active in the epidermal stem cell compartment and acts downstream of ras-related C3 botulinum toxin substrate (Rac1) to negatively regulate Myc induced differentiation (Benitah et al, 2005; Watt et al, 2008). We tested whether mutational inactivation of the Myc PAK2 phosphorylation sites increased Myc-induced differentiation by generating K14MycAER transgenic mice in which the three Myc residues targeted by PAK2 were replaced by alanines. The level of transgene-encoded Myc protein was similar in skin from the K14MycAER and K14MycER lines examined (Fig 3A).
Figure 3. Mutation of PAK2 phosphorylation sites enhances Myc activity.
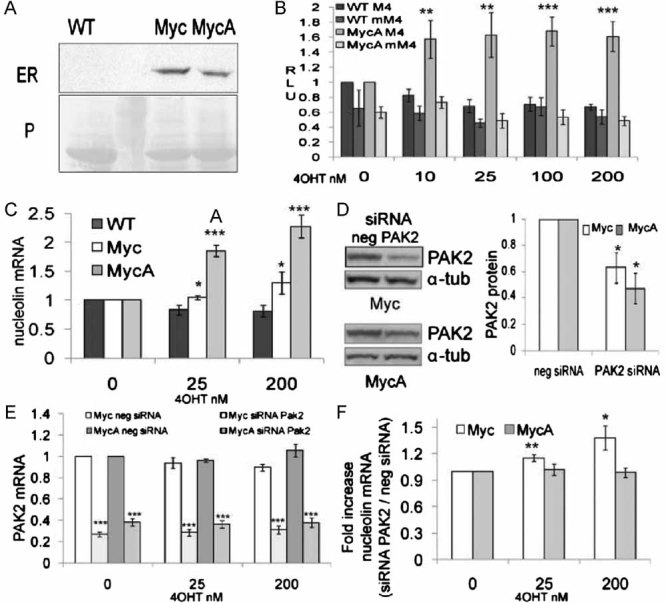
WT: transgene-negative; Myc: K14MycER; MycA: K14MycAER.
- Western blot of dorsal skin lysates probed with anti-ER. Equal protein loading was verified by Ponceau staining (P). Second lane from left: molecular mass markers.
- Luciferase assays performed as in Fig 1C, D. **p<0.01, ***p<0.005 relative to WT cells transfected with M4 treated with same 4OHT concentration.
- Q-PCR of nucleolin mRNA. *p<0.05, ***p<0.005 relative to WT cells at same 4OHT concentration.
- Western blotting of PAK2 and α-tubulin (α-tub; loading control) in cells transfected with PAK2 or control (neg) siRNAs. Densitometric analysis of three blots ± SEM is shown. Asterisks: *p<0.05 relative to neg cells.
- Q-PCR of PAK2 mRNA levels in cells transfected with control (neg) or PAK2 siRNA. ***p<0.005 relative to negative control siRNA transfected cells, treated with the same 4OHT concentration.
- Fold increase in nucleolin expression in cells transfected with PAK2 siRNA relative to control siRNA. *p<0.05, **p<0.01 relative to 0 nM 4OHT treated cells. (C, E, F) GAPDH was used for normalization. (B, C, E, F) Data are mean ± SEM from 5 (B, E, F) or 4 (C) experiments.
Cultured keratinocyte lines established from K14MycAER mice were transfected with the luciferase reporters shown in Fig 1C and treated with different concentrations of 4OHT. Maximal stimulation of luciferase activity was observed at 10 nM 4OHT and was sustained at all doses tested (Fig 3B). Thus maximal activity was achieved at a lower 4OHT concentration than in K14MycER cells and there was no tailing off of activity at 200 nM (cf Fig 1D, Fig 3B).
To confirm that MycAER was more effective in activating Myc target genes, we used quantitative PCR to measure nucleolin mRNA levels (Fig 3C). Nucleolin was induced in a dose-dependent manner in MycER and MycAER cells, but mRNA levels were higher in MycAER cells at both 4OHT concentrations tested (Fig 3C).
To examine whether the enhanced transcriptional activity of MycA was attributable to insensitivity to PAK2, we transfected K14MycER and K14MycAER keratinocytes with a short interfering RNA (siRNA) targeting PAK2 or a control siRNA (neg). Western blotting revealed a 40–60% reduction in PAK2 protein (Fig 3D). A 70% reduction in PAK2 mRNA levels was detected by Q-PCR in K14MycER and a 60% reduction in K14MycAER cells, both in the presence and absence of 4OHT (Fig 3E).
Myc-induced upregulation of nucleolin was not observed in K14MycER cells transfected with negative control siRNA, possibly reflecting stress-induced PAK2 activation (data not shown). However, when PAK2 was knocked down, MycER-induced activation of nucleolin was restored (Fig 3F). K14MycAER cells were insensitive to PAK2 inhibition, and upregulated nucleolin to the same extent as non-transfected cells in the presence of control or PAK2 siRNA (Fig 3F).
Mutation of the PAK2 phosphorylation enhances Myc induced differentiation in culture
To test whether MycA was more potent than wild type Myc in inducing keratinocyte differentiation in culture, we examined the colony forming efficiency (CFE) of K14MycER and K14MycAER keratinocytes treated with 4OHT for 8–12 days (Fig 4A). The CFE of K14MycER keratinocytes increased slightly in 20 nM 4OHT but at 2 and 200 nM was the same as in untreated cells. In contrast, the CFE of K14MycAER cells was dramatically reduced by 4OHT concentrations of 20 nM and above (Fig 4A). We also compared the area of the largest colonies formed by K14MycER and K14MycAER keratinocytes in the presence of 0 or 200 nM 4OHT. Colony size was not affected by Myc activation, but was markedly reduced on activation of MycA (Fig 4C, D).
Figure 4. Differential responses of cultured keratinocytes to activation of Myc and MycA.
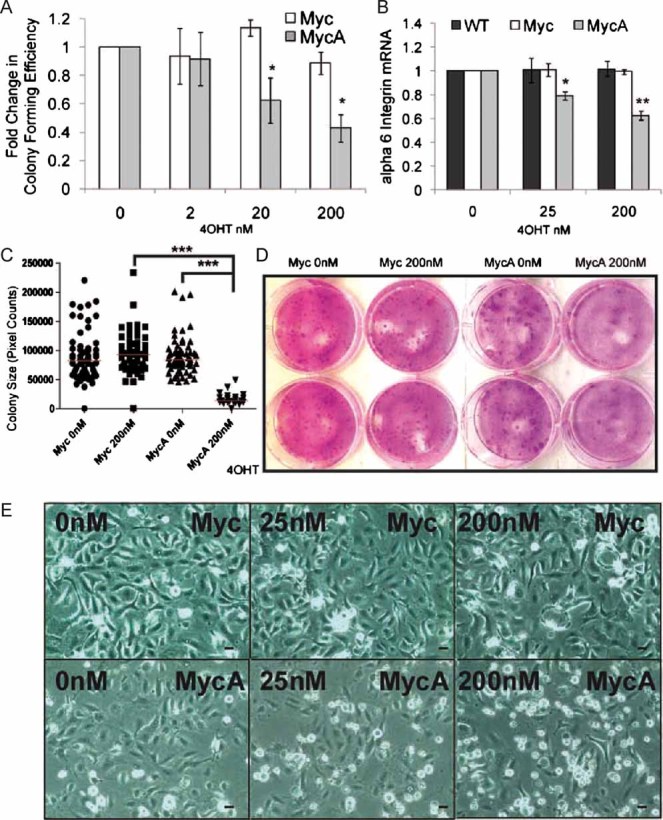
- Mean fold change in CFE ± SEM in keratinocytes cultured for 8 days in the presence or absence of 4OHT. Data pooled from triplicate dishes in three independent experiments. *p<0.05 relative to Myc expressing cells treated with same 4OHT concentration.
- Q-PCR of α6 integrin mRNA normalized to GAPDH. Data are mean ± SEM of three experiments. *p<0.05, **p<0.01 relative to WT treated with same 4OHT concentration.
- Size of largest colonies after 8 days in 0 or 200 nM 4OHT. At least 21 colonies from triplicate dishes are shown. Red bars: median values. ***p<0.005.
- Dishes of keratinocytes in CFE experiments, fixed after 8 (Myc) or 12 (MycA) days.
- Phase contrast micrographs of keratinocytes in unsupplemented Keratinocyte Serum Free Medium (KSFM) treated with 4OHT for 24 h. Scale bars: 10 µm.
As a further readout of Myc induced differentiation, we examined integrin-mediated adhesion (Frye et al, 2003; Gebhardt et al, 2006). α6 integrin mRNA levels were unchanged when K14MycER cells were treated for 16 h with 4OHT (Fig 4B). In contrast, α6 integrin mRNA was downregulated in K14MycAER cells (Fig 4B). Consistent with this, K14MycAER cells exhibited reduced cell-substrate adhesion in response to 4OHT, whereas K14MycER cells did not (Fig 4E).
PAK2 insensitive mutant Myc enhances epidermal differentiation
We next examined the response of K14MycAER mice to the 4OHT doses tested on K14MycER mice. Like K14MycER mice, K14MycAER mice exhibited increased proliferation, IFE thickening and SG enlargement (Fig 5A–J). Proliferation was stimulated at all 4OHT doses and, in the IFE, was largely confined to the basal layer (Fig 5E–G). However, IFE thickening was more pronounced at a low 4OHT dose (0.1 mg 4OHT) in K14MycAER than K14MycER mice (Fig 5H). Focal loss of α6 integrin expression was observed in K14MycAER mice (data not shown), consistent with the Q-PCR data from cultured keratinocytes (Fig 4B).
Figure 5. Epidermal response to MycA.
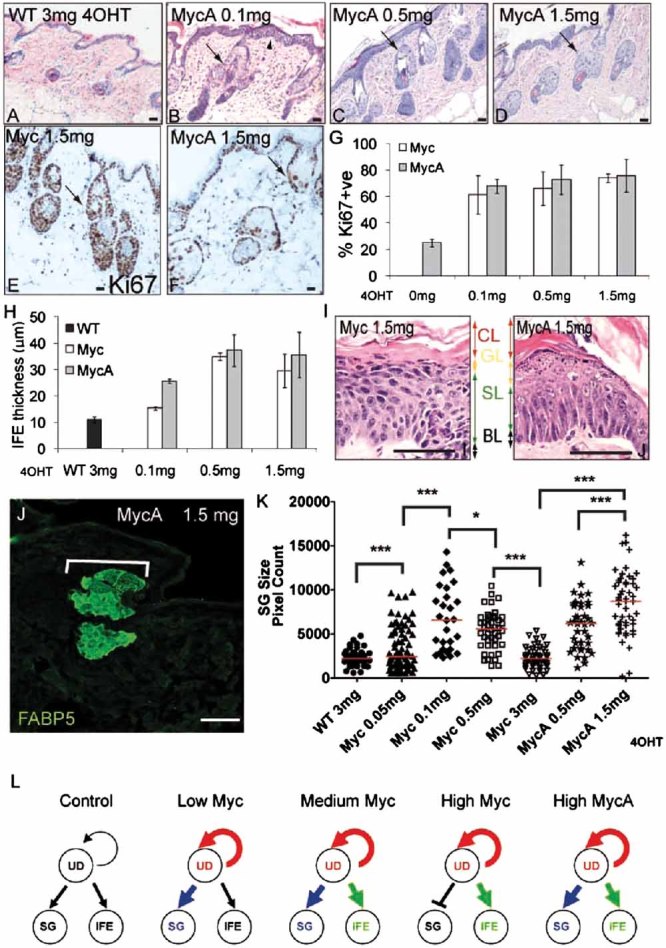
WT: transgene negative; Myc: K14MycER; MycA: K14MycAER. 4OHT doses are indicated.
- A–D. H&E stained sections of back skin. Arrowhead in (B) shows IFE thickening. Arrows (B–D): differentiated sebocytes.
- E,F. Ki67 labelling of back skin. Arrows: SG.
- G. % Ki67 positive IFE basal layer cells ± SEM.
- H. Maximum IFE thickness (µm) ± SEM.
- I. IFE basal (BL), spinous (SL), granular (GL) and cornified (CL) layers; 8 days, 0.5 mg 4OHT.
- J. FABP5 staining. Bracket: SG.
- K. Scatterplot of FABP5 positive area of individual SG; at least 28 SGs per condition. Red bar: median. *p<0.05, ***p<0.005.
- L. Summary of effects of Myc and MycA on proliferation of undifferentiated keratinocytes (UD) and differentiation into SG and IFE. Thickened arrows indicate stimulation relative to control, untreated epidermis. Scale bars: 10 µm (A–F), 50 µm (I), 75 µm (J).
Activation of MycA increased the proportion of cells in the later stages of IFE terminal differentiation to a greater extent than Myc, an effect that was particularly pronounced at 8 days (Fig 5I). This is the longest time for which K14MycAER mice could be treated, because the epidermis became overtly fragile and flaky, and mice exhibited weight loss and dehydration. At 8 days the granular layer was expanded in K14MycAER mice and the granular cells failed to flatten (Fig 5I). This is reminiscent of the effects of overexpressing active cofilin in reconsituted human epidermis, supporting the model that LIM domain kinase (LIMK)-mediated downregulation of Myc plays a role in granular layer compaction (Honma et al, 2006). In K14MycAER mice, the combined granular and cornified layers constituted 62% of IFE thickness, compared to 40% in K14MycER mice.
Whereas Myc and MycA activation were equally effective in enlarging the SG, in K14MycAER skin terminally differentiated sebocytes were preserved at the highest 4OHT concentrations (Fig 5J). This was quantitated by measuring the area of individual SG that stained positive for FABP5 (Fig 5J, K). In K14MycER mice the FABP5 positive areas increased at 0.05 and 0.1 mg 4OHT, then decreased at higher doses (also see Fig 2Q–S). In contrast, FABP5 labelling in K14MycAER skin was further enhanced by 1.5 mg compared to 0.5 mg 4OHT (Fig 5K). Rare IFE sebocytes were seen at all 4OHT concentrations in K14MycAER skin, as in K14MycER skin (data not shown).
We conclude that epidermal keratinocytes respond differently to different levels of Myc activation (Fig 5L). Proliferation was stimulated at all levels, whereas there was progressive accumulation of differentiated cell layers in the IFE with increasing 4OHT concentration. In K14MycER mice accumulation of differentiated sebocytes within the SG was greater at low and intermediate 4OHT doses than at high doses. In contrast, activation of MycAER preserved SG differentiation even at high 4OHT doses. The phenotype of K14MycAER mice suggests that PAK2 mediated phosphorylation serves as a negative regulator of Myc induced differentiation but not proliferation, highlighting the importance of the Rac1/PAK2/Myc axis in regulating epidermal differentiation (Benitah et al, 2005; Watt et al, 2008).
Our observations support the hypothesis that Myc-induced IFE differentiation is a fail-safe mechanism to prevent uncontrolled proliferation of the stem cell compartment (Jensen & Watt, 2006; Watt et al, 2008). Low levels of Myc stimulate proliferation and enable stem cell expansion, while high levels trigger accumulation of terminally differentiated cells and thereby prevent neoplastic conversion to squamous cell carcinoma. Our results support the conclusion that in somatic tissues Myc must be activated at a low level to avoid engaging tumour suppression mechanisms: apoptosis in the case of tissues such as pancreas (Murphy et al, 2008) and terminal differentiation in the epidermis. Our studies also raise the intriguing possibility that in squamous cell carcinomas with Myc amplification, the oncogene may not only be driving proliferation but also stimulating some cells to undergo differentiation.
MATERIALS AND METHODS
An expanded version of Materials and Methods can be found under Supporting Information
The paper explained
PROBLEM
Myc is a classic oncogene that is frequently upregulated in a wide variety of tumours. It is therefore surprising that in the epidermis, high levels of Myc promote differentiation rather than tumour formation. We set out to test the hypothesis that the epidermal response to Myc is dependent on the level of Myc activity.
RESULTS
We were able to modulate Myc activity in a transgenic mouse model by using different doses of the transgene-inducing agent, 4-hydroxy-tamoxifen (4OHT).
We found that low levels of Myc activity stimulated proliferation while higher levels stimulated differentiation into sebaceous glands and interfollicular epidermis. By preventing negative regulation of Myc via Pak2 phosphorylation, we achieved a more potent stimulation of epidermal differentiation.
IMPACT
Many epithelial tumours contain areas of proliferation and areas of differentiation, and the degree of differentiation is used as a predictor of prognosis. Our studies raise the intriguing possibility that in tumours with Myc amplification, the oncogene is not only driving proliferation but also stimulating differentiation.
Transgenic mice
K14MycER transgenic mouse founder line 2184C.1 (Arnold & Watt, 2001) and K14MycAER founder line 6972.C3 were used. Mice were treated once with the stated doses of 4OHT dissolved in 100 µl acetone and harvested 4 days later, except when stated. All experiments were subject to Cancer Research (CR)-UK ethical review and performed under the terms of a UK Government Home Office licence.
Keratinocyte culture
Primary and spontaneously immortalized keratinocytes were cultured essentially as described previously (Silva-Vargas et al, 2005). To determine CFE, 103 keratinocytes (passage 7–10), were seeded per well in triplicate wells of 6-well plates. To examine Myc-induced cell rounding, 4 × 105 cells were seeded per well in 6 well plates (Silva-Vargas et al, 2005) overnight without feeders. The cells were transferred to complete Keratinocyte Serum Free Medium (KSFM) for 24 h and then to unsupplemented KSFM ± 4OHT for an additional 24 h.
siRNA transfection
4 × 105 cells were seeded per well in 6 well plates overnight. Adherent keratinocytes were transfected with 2 µl of 20 µM siRNA and 2 µl lipofectamine 2000 in unsupplemented KSFM for 4 h, then cultured in complete KSFM for 2 days before harvesting. Qiagen All stars negative control siRNA 1027280 and Qiagen Mm_Pak2_1 HP siRNA SI01368647 siRNA oligos were used.
Q-PCR
Keratinocytes were either starved for 16 h in unsupplemented KSFM and then transferred to unsupplemented KSFM ± 4OHT for 5 h or treated with unsupplemented KSFM ± 4OHT for 16 h without prior starvation. Quantitative PCR was performed as described (Frye et al, 2003).
Immunohistochemistry
Antibodies against the following proteins were used: FAS (IBL), c-myc (N-262 sc-764, Santa Cruz), ERα (MC-20 sc-542, Santa Cruz), α6 integrin (GoH3, Serotec), Nucleolin (A300-711A, Bethyl Laboratories), PAK2 (A301-263A, Bethyl Laboratories), actin (AC-40, Sigma), Ki67 (Neomarkers, Fremont, CA), FABP5 (BAF1476, R&D Systems). AlexaFluor 488- or 555-conjugated goat anti-rabbit, anti-mouse or anti-rat IgG (Invitrogen Corporation; Paisley, UK) were used to detect primary antibodies. Slides were mounted in Slow Fade Gold reagent (Invitrogen).
Tissue samples were either fixed overnight in neutral buffered formalin or 4% PFA and embedded in paraffin or else frozen, unfixed, in OCT compound (Miles). Cultured keratinocytes were fixed in 4% PFA and stained with 4′-6-diamidino-2-phenylindole (DAPI) and anti-ER. Slides were viewed on a Olympus DMI6000 or Nikon confocal scanning microscope.Quantitation of Ki67 labelling, IFE thickness and SG differentiation was performed as described in the Supporting Information.
Western blotting
Proteins were extracted from whole skin in radioimmunoprecipitation (RIPA) buffer containing a protease inhibitor cocktail (Roche) using a homogenizer (Power Gen 500, Fisher Scientific). Proteins were extracted from cultured keratinocytes by scraping into ice cold (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid) (HEPES) lysis buffer (10mM HEPES pH 8, 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.2% NP-40, Roche protease inhibitor cocktail). Proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF, NEN) or nitrocellulose (Amersham) membranes essentially as described previously (Arnold & Watt, 2001; Silva-Vargas et al, 2005).
Luciferase assays
Keratinocytes were seeded without feeders in 48-well collagen-coated plates at a density of 2.5 × 104 cells per well and allowed to adhere overnight. Adherent keratinocytes were transfected with 1 µl lipofectamine 2000, mixed with 1.15 µg of pGL2-M4 or pGL2-mutantM4 firefly luciferase reporter plasmids (kindly provided by Robert Eisenman; Laherty et al 1997) and 0.1 µg pCMV-RL R. luciferase reporter in unsupplemented KSFM medium for 4 hours. Keratinocytes were incubated in complete KSFM overnight, then transferred to unsupplemented KSFM containing 0–200 nM 4OHT in ethanol for 24 h. Keratinocytes lysates were assayed using the Promega dual luciferase reporter assay kit according to the manufacturer's instructions and analysed on a Glomax luminometer (Promega). Luciferase values were normalized to R. luciferase activity to adjust for transfection efficiency.
Statistics
Statistical analysis was performed using the unpaired Student's t-test.
Acknowledgments
FMW gratefully acknowledges financial support from the European Science Foundation, Cancer Research UK, the Wellcome Trust and the Medical Research Council, the University of Cambridge and Hutchison Whampoa Ltd. We thank the CRUK core facilities of the Cambridge Research Institute and London Research Institute for excellent technical assistance.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
FMW conceived the project, wrote the paper and carried out quantitative analysis; CMB generated the MycA mice and performed in vivo experiments; MAB examined dose responsiveness in vivo and in vitro; DLC analysed Myc dependent transcription and the effects of PAK2.
For more information:
Oncomine – Cancer profiling database
Cancer Research UK
http://www.cancerresearchuk.org/
Fiona Watt's Laboratory:
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc's broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS ONE. 2007;2:e 763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Gardner C, Li ER, Arnold I, Watt FM. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130:2793–2808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- Gandarillas A, Watt FM. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997;11:2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt A, Frye M, Herold S, Benitah SA, Braun K, Samans B, Watt FM, Elsasser HP, Eilers M. Myc regulates keratinocyte adhesion and differentiation via complex formation with Miz1. J Cell Biol. 2006;172:139–149. doi: 10.1083/jcb.200506057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Benitah SA, Watt FM. Role of LIM kinases in normal and psoriatic human epidermis. Mol Biol Cell. 2006;17:1888–1896. doi: 10.1091/mbc.E05-12-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. Stress signaling and Myc downregulation: implications for cancer. Cell Cycle. 2004;3:593–596. [PubMed] [Google Scholar]
- Huang Z, Traugh JA, Bishop JM. Negative control of the Myc protein by the stress-responsive kinase Pak2. Mol Cell Biol. 2004;24:1582–1594. doi: 10.1128/MCB.24.4.1582-1594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci USA. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- LoCelso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, Watt FM. Characterization of bipotential epidermal progenitors derived from human sebaceous gland: contrasting roles of c-Myc and β-catenin. Stem Cells. 2008;26:1241–1252. doi: 10.1634/stemcells.2007-0651. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. β-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nat Rev Cancer. 2008;8:234–242. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Jensen KB. Epidermal diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


