Abstract
Background and Purpose
Stroke survivors are at high risk for falling. Identifying physical, clinical, and social factors that predispose stroke patients to falls may reduce further disability and life-threatening complications, and improve overall quality of life.
Methods
We used 5 biennial waves (1998–2006) from the Health and Retirement Study to assess risk factors associated with falling accidents and fall-related injuries among stroke survivors. We abstracted demographic data, living status, self-evaluated general health, and comorbid conditions. We analyzed the rate ratio (RR) of falling and the OR of injury within 2 follow-up years using a multivariate random effects model.
Results
We identified 1174 stroke survivors (mean age±SD, 74.4±7.2 years; 53% female). The 2-year risks of falling, subsequent injury, and broken hip attributable to fall were 46%, 15%, and 2.1% among the subjects, respectively. Factors associated with an increased frequency of falling were living with spouse as compared to living alone (RR, 1.4), poor general health (RR, 1.1), time from first stroke (RR, 1.2), psychiatric problems (RR, 1.7), urinary incontinence (RR, 1.4), pain (RR, 1.4), motor impairment (RR, 1.2), and past frequency of ≥3 falls (RR, 1.3). Risk factors associated with fall-related injury were female gender (OR, 1.5), poor general health (OR, 1.2), past injury from fall (OR, 3.2), past frequency of ≥3 falls (OR, 3.1), psychiatric problems (OR, 1.4), urinary incontinence (OR, 1.4), impaired hearing (OR, 1.6), pain (OR, 1.8), motor impairment (OR, 1.3), and presence of multiple strokes (OR, 3.2).
Conclusions
This study demonstrates the high prevalence of falls and fall-related injuries in stroke survivors, and identifies factors that increase the risk. Modifying these factors may prevent falls, which could lead to improved quality of life and less caregiver burden and cost in this population.
Keywords: falling, risk factors, fall-related injury, stroke
Falls and fall-related injuries count among the most common complications after stroke, and bear high morbidity and mortality rates.1 Stroke survivors, especially those with locomotor deficits, are at high risk for falls. The rate of falling among stroke survivors ranges from 25% to 44%.2–4
Falls are a frequent problem in the elderly, resulting in reduced mobility and independence.5 Up to 90% of all geriatric injuries are caused by falls,6 augmenting health care economical burden. Among the sequelae of falling, hip fracture is the most common; it leads to serious disability and loss of overall health. The risk of hip fracture in stroke patients is 4-fold higher compared to the general population.7 There are several retrospective or prospective studies focusing on falls and their risk factors in subjects after a stroke.4,8–13 However, longitudinal data derived from large cohorts are required to obtain population-based estimates of fall frequency and to reliably identify risk factors for falls among stroke survivors. The aim of this study was to identify such risk factors in a nationwide representative elderly population cohort studied in the Health and Retirement Study (HRS).
Materials and Methods
We used the HRS database to identify risk factors associated with falling and subsequent injuries among subjects with a history of stroke. The HRS is a national longitudinal study of the economic, health, marital status, and family status of Americans who were age 50 years or older and noninstitutionalized at the time of their first interview. The study is conducted every 2 years by the Institute for Social Research at the University of Michigan and the National Institute on Aging. The data are collected using a mixed-mode design of telephone and face-to-face interviews. Proxy interviews are obtained when study participants are unable to respond for themselves. The full description of HRS study design including design history, sample size, and response rates has been published by Heeringa and Connor.14 HRS uses a national area probability sample of US households with supplemental over samples of Blacks, Hispanics, and residents of the state of Florida. Based on the 1991 Current Population Survey, ≈19.2% of US households were expected to be eligible for HRS. The survey response rate in HRS varies between 70% and 81% across included cohorts and the retention rates ranged from 82% to 86% through the 2006 interview wave.14,15
We used 5 biennial interview waves in HRS from 1998 (4th interview wave) to 2006 (8th interview wave). From 1998 to 2004 (4th to 7th interview waves), we selected participants aged 65 years or older with a history of stroke. History of new and recurrent stroke was ascertained with the following questions: “Has a doctor ever told you that you had a stroke” and “Since previous wave, has a doctor told you that you had another stroke?” Subjects who were not available or were institutionalized during the following survey-year were excluded from the analysis. This resulted in a cohort size of 1117 subjects. We abstracted demographic data (age, gender, and race), living arrangement, self-evaluated general health, self-reported medical conditions diagnosed by a physician (diabetes mellitus, cancer, lung disease, psychiatric problems, memory loss, neurological/sensory conditions), and other self-reported health problems (urinary incontinence, motor impairment, impaired vision, impaired hearing, pain, falling in the past 2 years, number of falls, injury attributable to a previous fall, and fractured hip since the last interview). We also recorded the time interval from first stroke onset to the time of interview and assessed whether the participant had multiple stroke events. In addition, we abstracted mode of interview (face-to-face or telephone interview) and respondent (self or proxy). Outcome variables (ie, frequency of fall and injury attributable to fall that required medical attention) were abstracted from the 2000 to 2006 consecutive waves (5th to 8th interview wave).
General health score ranged from 1 to 5, with 1 being excellent health and 5 being poor health. Motor impairment was determined as reduced large muscle function, with a score ranging from 0 to 4 (0 being the highest mobility and 4 the least mobility). The subjects were scored as excellent, very good, good, fair, poor, or legally blind or deaf for hearing and vision assessments. We classified a subject as having vision or hearing impairment if eye sight or hearing was rated as “poor” or worse. Neurological comorbidity was recorded if subjects reported multiple sclerosis, cerebral palsy, epilepsy, Parkinson disease, amyotrophic lateral sclerosis, seizures, or neuropathy representing neurological conditions that increase the risk for falling.16
Statistical Analysis
Baseline characteristics of the cohort are presented as percent and mean±SD for categorical and continuous variables. We estimated the 2-year frequency of falling, incidence of fall-related injury, and incidence of broken hip using a multivariate random effects model with a Poisson distribution (for frequency) and binomial distribution (for incidence) to account for repeated observations within subjects. To identify risk factors for frequency of falling and incidence of fall-related injury, we estimated the rate ratio (RR) and OR, with their 95% CI of individual risk factors after adjusting for baseline age, gender, and time from first stroke event. We also utilized a model (full model) that included age, gender, time from first stroke event, living arrangements, medical and health-related factors, and self-evaluated general health factors. Only factors with P<0.2 after adjusting for baseline age, gender, and time duration from the first stroke associated with at least one of the outcome variables were included in the full model. The same multivariate random effects models were used for all the analyses. We considered P<0.05 to be statistically significant.
Results
We identified 1174 subjects with a history of stroke who were interviewed in the HRS 2 to 5 times (2407 observations) between 1998 and 2004. The mean age±SD of the subjects was 74.4±7.2 years; 53% were female, 81% were white, and 58% lived as a couple. The baseline characteristics of the subjects are described in Table 1. The most common medical conditions diagnosed by a physician were diabetes mellitus (28%) and psychiatric problems (20%). The most common self-reported conditions were urinary incontinence (28%), pain (37%), and past injuries attributable to fall (15%). Median large muscle function score, used as an assessment of motor impairment, was 2 (range, 0–4). At the time of the last follow up, 8% of subjects had >1 stroke.
Table 1. Characteristics of Stroke Survivors Interviewed in the HRS.
| Demographics | N | (%) |
|---|---|---|
| Age, yr | ||
| 65–69 | 367 | 31% |
| 70–74 | 270 | 23% |
| 75–79 | 243 | 21% |
| 80–84 | 173 | 15% |
| 85+ | 121 | 10% |
| Gender | ||
| Male | 550 | 47% |
| Female | 624 | 53% |
| Race | ||
| White | 956 | 81% |
| Black | 191 | 16% |
| Other | 27 | 2% |
| Living arrangements | ||
| Live with couple | 679 | 58% |
| Live with other | 187 | 16% |
| Live alone | 308 | 26% |
| General health (self-evaluated) | ||
| Excellent | 30 | 3% |
| Very good | 142 | 12% |
| Good | 311 | 26% |
| Fair | 386 | 33% |
| Poor | 305 | 26% |
| Comorbid conditions | ||
| Parkinson disease, multiple sclerosis, amyotrophic lateral sclerosis, epilepsy, etc | 13 | 1% |
| Diabetes mellitus | 334 | 28% |
| Cancer | 180 | 15% |
| Lung disease | 176 | 15% |
| Psychiatric problem | 238 | 20% |
| Memory problem | 83 | 7% |
| Urinary incontinence | 326 | 28% |
| Impaired vision | 160 | 14% |
| Impaired hearing | 133 | 11% |
| Pain | 434 | 37% |
| Past injury due to fall | 175 | 15% |
| Multiple episodes of stroke | 90 | 8% |
| Time from first stroke, yr | ||
| 0–2 | 428 | 36% |
| 2–4 | 327 | 28% |
| 4–6 | 189 | 16% |
| 6–8 | 172 | 15% |
| 8–10 | 58 | 5% |
| Survey procedures | ||
| Phone interview | 758 | 64% |
| Proxy respondent | 179 | 15% |
The 2-year risks of falling, subsequent injury, and broken hip attributable to falls were 46% (CI, 43%–48%), 15% (CI, 13%–16%), and 2.1% (CI, 1.6%–2.8%), respectively. The 2-year average frequency of fall was 0.92 (CI, 0.84–0.99). The distribution of frequency of falls is shown in the Figure.
Figure.
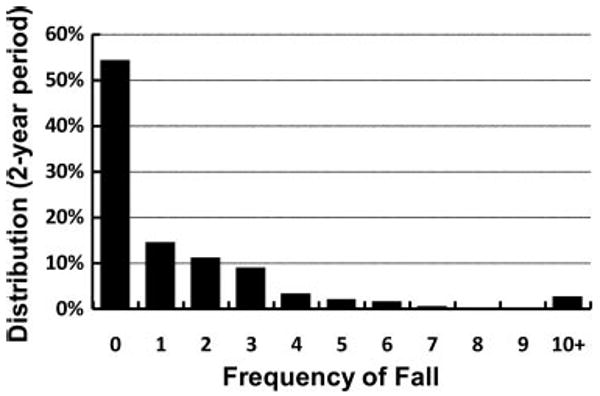
Distribution of the number of falls in a 2-year period among stroke survivors interviewed in HRS.
Table 2 presents factors associated with increased frequency of falling after adjusting for age, gender, living arrangement, poor general health, time from first stroke, psychiatric problems, urinary incontinence, pain, motor impairment, and past frequency of falling. For living arrangement, subjects living with household members were at higher risk than those living alone (RR, 1.4; CI, 1.1–1.7). For past frequency of falling, having fallen 1 to 2 times increased the likelihood of subsequent falls by 30%, and having fallen 3 or more times increased the likelihood by 20%. When all the factors were combined in the same model (full model), we found that age, living arrangement, time from first stroke, past injury attributable to fall, psychiatric problems, urinary incontinence, pain, motor impairment, and past frequency of falling remained associated with increased frequency of falling for the subsequent 2-year period; direction of these associations remained similar to those observed for the age, gender, and time of first stroke only in the adjusted models, although the strength of the association was smaller in magnitude.
Table 2. RR Associated With Frequency of 2-Year Risk of Falling for Stroke Survivors Interviewed in HRS.
| Risk Factor | Age, Gender, and Time From First Stroke Adjusted RR (95% CI), P | Full Model RR (95% CI), P |
|---|---|---|
| Age per 5-year increase | 1.0 (0.98–1.1), 0.15 | 1.1 (1.0–1.2), 0.01 |
| Female | 1.1 (0.93–1.3), 0.27 | 1.1 (0.90–1.3), 0.46 |
| Race | ||
| White | Reference, P=0.30 | Reference, P=0.48 |
| Black | 0.84 (0.66–1.1) | 0.88 (0.70–1.1) |
| Other | 0.83 (0.47–1.5) | 0.87 (0.51–1.5) |
| Living arrangements | ||
| Live with others | Reference, P=0.001 | Reference, P=0.002 |
| Live with spouse | 1.2 (0.98–1.5) | 1.2 (1.0–1.5) |
| Live alone | 0.87 (0.7–1.1) | 0.91 (0.75–1.1) |
| Comorbid conditions* | ||
| General health score per unit increase (1–5) | 1.1 (1.0–1.2), 0.001 | 1.0 (0.97–1.1), 0.42 |
| Time from first stroke, per 2-year interval | 1.2 (1.1–1.2), <0.0001 | 1.1 (1.1–1.2), <0.0001 |
| Past injury from fall | 0.97 (0.86–1.1), 0.60 | 0.82 (0.73–0.92), 0.002 |
| Psychiatric problems | 1.7 (1.5–2.0), <0.0001 | 1.6 (1.4–1.8), <0.0001 |
| Memory problems | 1.2 (0.96–1.4), 0.13 | 1.1 (0.93–1.4), 0.22 |
| Urinary incontinence | 1.4 (1.2–1.5), <0.0001 | 1.3 (1.1–1.4), <0.0001 |
| Impaired vision | 0.97 (0.84–1.1), 0.71 | 0.97 (0.84–1.1), 0.73 |
| Impaired hearing | 1.1 (0.9–1.2), 0.50 | 0.93 (0.81–1.1), 0.34 |
| Pain | 1.4 (1.3–1.6), <0.0001 | 1.2 (1.1–1.4), 0.0003 |
| Motor impairment per unit increase (0–4) | 1.2 (1.1–1.2), <0.0001 | 1.1 (1.1–1.2), <0.0001 |
| Multiple strokes | 1.0 (0.82–1.2), 0.99 | 0.91 (0.75–1.1), 0.34 |
| Past frequency of falling | ||
| 0 | Reference, P<0.0001 | Reference, P<0.0001 |
| 1–2 | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) |
| ≥3 | 1.2 (1.1–1.3) | 1.1 (0.98–1.3) |
Comorbid conditions are assessed for the past 2 years for each interview wave.
Table 3 presents factors associated with fall-related injuries. After adjusting for age, gender, and time from first stroke, fall-related injuries were associated with higher age, female gender, poor general health, motor impairment, pain, injury after a previous fall, psychiatric problems, urinary incontinence, impaired hearing, multiple strokes, and past frequency of falling. We did not observe that memory problems, impaired vision, time since stroke, and living arrangements were associated with fall-related injury risk. After including all the factors together, only previous injury from a fall, pain, motor impairment, and past frequency of falling remained associated with risk of injury. Direction of these associations was similar to those observed from age, gender, and times from first stroke only in adjusted models, although the strength of the association was reduced. Although living arrangements were not found to have a statically significant association with risk of injury, the odds of having injury attributable to falling for subjects who were living alone were higher when compared to those living with other household members within the 2 models for fall-related injury. Other medical conditions not associated with either outcome were neurological conditions other than stroke, diabetes mellitus, cancer, and lung disease (data not shown).
Table 3. OR for 2-Year Risk of Fall-Related Injury for Stroke Survivors Interviewed in HRS.
| Risk Factor | Age, Gender, and Time From First Stroke Adjusted OR (95% CI), P | Full Model OR (95% CI), P |
|---|---|---|
| Age per 5-year increase | 1.1 (1.1–1.3), 0.004 | 1.1 (0.99 –1.1), 0.07 |
| Female | 1.8 (1.4–2.3), <0.0001 | 1.5 (1.2–2.0), 0.003 |
| Race | ||
| White | Reference, P=0.058 | Reference, P=0.09 |
| Black | 0.63 (0.43–0.92) | 0.67 (0.46–0.98) |
| Other | 0.96 (0.42–2.2) | 0.99 (0.44–2.2) |
| Living arrangements | ||
| Live with others | Reference, P=0.22 | Reference, P=0.10 |
| Live with spouse | 1.2 (0.85–1.8) | 1.2 (0.85–1.8) |
| Live alone | 1.4 (0.96–2.0) | 1.5 (1.0–2.2) |
| Comorbid conditions* | ||
| General health score per unit increase (1–5) | 1.2 (1.0–1.3), 0.02 | 0.95 (0.83–1.1), 0.60 |
| Time from first stroke per 2-year interval | 1.1 (0.99–1.2), 0.07 | 1.1 (0.96–1.2), 0.15 |
| Past injury from fall | 3.2 (2.4–4.2), <0.0001 | 2.2 (1.6–2.8), <0.0001 |
| Psychiatric problems | 1.4 (1.1–1.9), 0.02 | 0.95 (0.70–1.3), 0.91 |
| Memory problems | 1.4 (0.87–2.3), 0.16 | 1.2 (0.75–2.0), 0.31 |
| Urinary incontinence | 1.4 (1.1–1.8), 0.02 | 1.0 (0.77–1.3), 0.62 |
| Impaired vision | 1.0 (0.72–1.4), 0.92 | 0.88 (0.62–1.2), 0.47 |
| Impaired hearing | 1.6 (1.1–2.3), 0.01 | 1.3 (0.92–1.9), 0.10 |
| Pain | 1.8 (1.4–2.3), <0.0001 | 1.4 (1.0–1.8), 0.02 |
| Motor impairment per unit increase (0–4) | 1.3 (1.2–1.4), <0.0001 | 1.2 (1.0–1.3), 0.002 |
| Multiple strokes | 3.2 (2.4–4.2), <0.0001 | 1.4 (0.89–2.1), 0.15 |
| Past frequency of falling | ||
| 0 | Reference, P<0.0001 | Reference, P=0.0004 |
| 1–2 | 1.9 (1.4–2.5) | 1.3 (0.95–1.8) |
| 3+ | 3.1 (2.3–4.2) | 2.0 (1.4–2.8) |
Co-morbid conditions are assessed for the past two years for each interview-wave.
Discussion
Risk of Falling
With a 2-year incidence of 46%, 15%, and 2.1% observed here in a longitudinal study of a large nationwide cohort, falls, fall-related injuries, and hip fractures represent serious long-term complications for stroke survivors. Nyberg and Gustafson2 reported 36% in-hospital fall accidents among 135 patients who were admitted to rehabilitation wards after cerebrovascular accidents. A prospective, population-based stroke incidence study conducted in Auckland, New Zealand, reported 37% of 1104 interviewed subjects had at least 1 falling accident within 6 months after stroke.11 Fall-related injuries greatly increase inpatient hospitalization costs17 and cause disability. Thus, it is vital to identify stroke survivors at high risk.
Impaired motor or sensory function or balance deficits predict falls.9 Motor deficits are common after stroke (50%–85% of stroke survivors18). Spasticity in paretic muscles can affect balance,19 but also antispasticity medications that reduce axial muscle tone or affect vigilance can increase fall risk.7,9,19,20 Our results support an association between motor impairment and falls. Improving motor dysfunction through specific interventions may therefore reduce risk of falling after stroke.
Depression has been linked to fall risk in previous studies, although the mechanisms of this association are not fully understood. In the general geriatric population, depression is an independent risk factor for falling, with an OR of 2.2.21 Affecting 30% to 50% of stroke survivors,22 depression likely increases fall risk in this population. Our data support this association. Reasons for this association may be fear of falling,23 self-underestimation of abilities,24 or impaired cognition.11,12 The combination of gait, balance disturbance, and cognitive impairment may exponentially increase fall risk,10,13 possibly reflecting higher cognitive load if a subject walks with impairment. Dual-task gait training methods combining motor and cognitive tasks may address this risk.25
In contrast to previous reports on stroke populations,26 visual impairment was not identified as a predictor for falling. Coding in HRS was limited to ocular visual abnormalities, not including visual–spatial deficits that have been associated with fall risk.3 Our study therefore may have lacked sufficient power to identify an association of this disorder with falls and fall injury.
In our data, the frequency of falling was higher among stroke survivors living with household members as compared with those living alone. It is possible that falls were under-represented in stroke survivors living alone, related to reluctance to report falls or to anosognosia.24 However, it is also possible that lesser functional deficits and impairment in activities of daily living, and hence less fall risk, is present among those who do not need a caregiver's assistance.
Previous falls were a prominent risk factor for falling, consistent with several other datasets on stroke populations.11 Other risk factors, not evaluated here because data were unavailable, are sedative, hypnotic,12 and antidepressant medications. Selective serotonin reuptake inhibitors are associated with increased fall risk,26,27 although this may merely reflect the association between falls and depression. Comorbidities such as epileptic seizures, urinary infection, and heart disease are reported to increase the risk of falling.10,12,13,22,26 Specifically treating these conditions may reduce fall risk.26–28
Risk of Fall-Related Injuries
The current dataset suggests several risk factors associated with fall-related injuries. Several studies have shown that after stroke, the risk for experiencing a hip fracture is 4-fold increased, especially in patients with hemiplegia.29 Most fractures are caused by falls onto the paretic side. Patients with hemiparesis not only have a higher risk of falling toward the paresis side but also show higher degrees of osteoporosis on the same side.30 In the first year after stroke, the paretic femoral neck loses up to 14% of bone mass.31 The intact leg is also affected, likely attributable to immobility and reduced weight-bearing.32 Vitamin D deficiency attributable to reduced exposure to sunlight may contribute further.33 Preventive therapies such as bisphonates and vitamins or mechanical hip protectors are important preventive measures.30
Our data suggest a 50% to 80% higher risk of fall-related injuries in woman, whereas fall risk itself was not affected by gender. The disproportionate increase in risk of injury attributable to fall among women may be related to higher prevalence of osteoporosis in women after menopause compared with men.34
Based on a large, representative cohort that was prospectively studied over several years, this study emphasizes the importance of falls as one of the most frequent complications in chronic stroke survivors. The frequency of this complication mandates fall prevention in every neuro-rehabilitative program. Based on the diversity of risk factors identified here and elsewhere, an optimal therapeutic strategy may require a multimodal, individually adjusted protocol.
We have found that some predictors were significantly associated for only 1 of the outcome variables; furthermore, some of the variables were either significantly associated for the age, gender, and time from first stroke adjusted models or the full models. The discordance between results for outcome variables can be explain by the small number of injury events as compared to the frequency of falls (lower statistical power for injury), and the larger measurement error in frequency of falling as compared to injury (bias toward the null for frequency of falling). Differences between the conservative adjusted models and the full model can be either attributable to underadjustment or overadjustment. We present both approaches for a more complete appraisal of the associations. One factor that might be worth mentioning is “past injury” attributable to fall. This factor is not associated with frequency of 2-year risk of falling in the conservative adjusted model (RR, 0.97; CI, 0.86–1.1; P=0.60), but it is in the full model (RR, 0.82; 95 CI, 0.73–0.92; P=0.0018). This situation may be explained by the high association with other factors such as past frequency of falling. In addition, this variable is strongly associated with 2-year risk of fall-related injury.
Study Limitations on Self-Reported Data
Our cohort included only noninstitutionalized individuals and may therefore have underestimated fall risk by exclusion of subjects with severe disabilities living in institutionalized care who might be at higher risk for falls.
Self-reports and proxy reports generally bear the risks of recall bias and may lack precision or under-report disability.35 In a national prospective (British Regional Heart Study) published by Walker et al,36 the investigators discovered that patients over-recall (25%) and under-recall (11%) doctor-diagnosed stroke events. Interestingly, the general practice record also showed an under-report of 23% and over-report of 5% for strokes.
Boele van Hensbroek et al37 validated a triage instrument identifying risk factors for recurrent falls in elderly patients. The instrument measures frequency of falls in the past 12 month. They found good construct validity between risk factors and frequency of falls. Kanten et al38 examined the concordance of various fall-reporting methods among residents of nursing homes; they found that self-report frequency of falling overestimates the frequency of falling obtained from chart review. Chen et al39 studied the validity of self-reported bone fractures among postmenopausal women. The self-reported fractures were adjudicated by reviewing the medical record for the subjects participated in the study. The investigators observed a higher confirmation rates among self-reports as compared to proxy reports. Factors such as fraction site, age, and number of falls in the past year were significantly related to the validity of fracture reports. Further research is needed to ascertain the validity of self-report frequency of falling and injury in elderly population.
However, one systematic assessment of the reliability of self-reporting in the elderly population showed substantial to moderate agreement with medical records depending on the item reported, eg, 98% for the incidence of stroke and 91% for fracture.40 Moreover, in a recent published article by Glymour et al,15 the reliability of self-reported strokes events was studied in HRS. The authors concluded a nonsystematic misreporting of stroke incidence in HRS, making it a valuable dataset for stroke surveillance.
In the HRS, motor dysfunction is heterogeneously reported. Although several self-reported motor test batteries were included to cover motor function, HRS interviewers did not ask about easier tasks if the subject was able to perform more challenging ones.41 We used the large muscle function scores to assess motor impairment because it was available for all subjects included here and therefore minimized the measurement error. Validity of HRS physical functioning measures is discussed in detail by Fonda and Herzog.41 Information about stroke subtypes and severity, medications with side effects on gait and balance, neurological examinations, and neuroimaging studies was not available, limiting the number of fall-related hypotheses that could be explored here. Future prospective data collections are necessary to address these shortcomings.
Conclusion
Any fall, fall-related injury, or the fear thereof may adversely affect quality of life for the stroke survivor and increase caregiver burden and cost.42 The patient at risk for falling is likely to walk less and be less mobile, which accelerates osteoporosis, thereby further increasing risk of fall-related injuries. Immobility also leads to deconditioning, which potentiates cardiovascular and cerebrovascular risk factors (obesity, diabetes, metabolic syndrome), leading to further increased risk of cerebrovascular events and impairment in a dangerous vicious cycle.43 It is important to break this cycle by modification of risk factors, development of specific training and management protocols,43 and informing patients and caregivers about frequency and morbidity associated with falls after stroke.
Footnotes
Disclosures: None.
References
- 1.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: A multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg L, Gustafson Y. Fall prediction index for patients in stroke rehabilitation. Stroke. 1997;28:716–721. doi: 10.1161/01.str.28.4.716. [DOI] [PubMed] [Google Scholar]
- 3.Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: Factors associated with high risk. Arch Phys Med Rehabil. 2002;83:329–333. doi: 10.1053/apmr.2002.29623. [DOI] [PubMed] [Google Scholar]
- 4.Ugur C, Gucuyener D, Uzuner N, Ozkan S, Ozdemir G. Characteristics of falling in patients with stroke. J Neurol Neurosurg Psychiatry. 2000;69:649–651. doi: 10.1136/jnnp.69.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: Prevalence and associated factors. Age Ageing. 1988;17:365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- 6.Aizen E, Shugaev I, Lenger R. Risk factors and characteristics of falls during inpatient rehabilitation of elderly patients. Arch Gerontol Geriatr. 2007;44:1–12. doi: 10.1016/j.archger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Smith RD, Fordham RJ. Economics of fall prevention programs: Evidence and research priorities. Exp Rev Pharmacoeconomic Outcomes Res. 2001;1:59–67. doi: 10.1586/14737167.1.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age Ageing. 2008;37:270–276. doi: 10.1093/ageing/afn066. [DOI] [PubMed] [Google Scholar]
- 9.Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87:554–561. doi: 10.1016/j.apmr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: Depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 11.Kerse N, Parag V, Feigin VL, McNaughton H, Hackett ML, Bennett DA, Anderson CS, Auckland Regional Community Stroke Study G Falls after stroke: Results from the Auckland regional community stroke (arcos) study, 2002 to 2003. Stroke. 2008;39:1890–1893. doi: 10.1161/STROKEAHA.107.509885. [DOI] [PubMed] [Google Scholar]
- 12.Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM, Gustafson Y. Risk factors for falling in home-dwelling older women with stroke: The women's health and aging study. Stroke. 2003;34:494–501. [PubMed] [Google Scholar]
- 13.Yates JS, Lai SM, Duncan PW, Studenski S. Falls in community-dwelling stroke survivors: An accumulated impairments model. J Rehabil Res Dev. 2002;39:385–394. [PubMed] [Google Scholar]
- 14.Heeringa SG, Connor J. Technical description of the health and retirement study sample design. Hrs/Ahead documentation report. 1995 [Google Scholar]
- 15.Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors? Evidence from the health and retirement study. Stroke. 2009;40:873–879. doi: 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 16.Stolze H, Klebe S, Zechlin C, Baecker C, Friege L, Deuschl G. Falls in frequent neurological diseases–prevalence, risk factors and aetiology. J Neurol. 2004;251:79–84. doi: 10.1007/s00415-004-0276-8. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo JA, Friedkin R, Williams CS, Nabors J, Acampora D, Tinetti ME. Health care utilization and costs in a medicare population by fall status. Med Care. 1998;36:1174–1188. doi: 10.1097/00005650-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gresham GE, Kelly-Hayes M, Wolf PA, Beiser AS, Kase CS, D'Agostino RB. Survival and functional status 20 or more years after first stroke: The Framingham study. Stroke. 1998;29:793–797. doi: 10.1161/01.str.29.4.793. [DOI] [PubMed] [Google Scholar]
- 19.Koski K, Luukinen H, Laippala P, Kivela SL. Physiological factors and medications as predictors of injurious falls by elderly people: A prospective population-based study. Age Ageing. 1996;25:29–38. doi: 10.1093/ageing/25.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 21.Stalenhoef PA, Diederiks JP, Knottnerus JA, Kester AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: A prospective cohort study. J Clin Epidemiol. 2002;55:1088–1094. doi: 10.1016/s0895-4356(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 22.Wiart L. Post-cerebrovascular stroke depression. Encephale. 1997;23(Spec No 3):51–54. [PubMed] [Google Scholar]
- 23.van Haastregt JC, Zijlstra GA, van Rossum E, van Eijk JT, Kempen GI. Feelings of anxiety and symptoms of depression in community-living older persons who avoid activity for fear of falling. Am J Geriatr Psychiatry. 2008;16:186–193. doi: 10.1097/JGP.0b013e3181591c1e. [DOI] [PubMed] [Google Scholar]
- 24.Barrett AM, Eslinger PJ, Ballentine NH, Heilman KM. Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology. 2005;64:693–699. doi: 10.1212/01.WNL.0000151959.64379.1B. [DOI] [PubMed] [Google Scholar]
- 25.Yang YR, Wang RY, Chen YC, Kao MJ. Dual-task exercise improves walking ability in chronic stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1236–1240. doi: 10.1016/j.apmr.2007.06.762. [DOI] [PubMed] [Google Scholar]
- 26.Kallin K, Lundin-Olsson L, Jensen J, Nyberg L, Gustafson Y. Predisposing and precipitating factors for falls among older people in residential care. Public Health. 2002;116:263–271. doi: 10.1038/sj.ph.1900849. [DOI] [PubMed] [Google Scholar]
- 27.Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int. 1999;9:269–275. doi: 10.1007/s001980050147. [DOI] [PubMed] [Google Scholar]
- 28.Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Falls among frail older people in residential care. Scand J Public Health. 2002;30:54–61. [PubMed] [Google Scholar]
- 29.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 30.Poole KE, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke: Time to think about protection? Stroke. 2002;33:1432–1436. doi: 10.1161/01.str.0000014510.48897.7d. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: Bone adaptation after decreased mechanical loading. Bone. 2000;27:701–707. doi: 10.1016/s8756-3282(00)00374-4. [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med. 2005;165:1743–1748. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- 33.Gloth FM, III, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin d deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA. Estrogens, the menopause, and osteoporosis. Bone. 1996;19:185S–190S. doi: 10.1016/s8756-3282(96)90163-5. [DOI] [PubMed] [Google Scholar]
- 35.Sinoff G, Ore L. The Barthel activities of daily living index: Self-reporting versus actual performance in the old-old (> or = 75 years) J Am Geriatr Soc. 1997;45:832–836. doi: 10.1111/j.1532-5415.1997.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 36.Walker MK, Whincup PH, Shaper AG, Lennon LT, Thomson AG. Validation of patient recall of doctor-diagnosed heart attack and stroke: A postal questionnaire and record review comparison. Am J Epidemiol. 1998;148:355–361. doi: 10.1093/oxfordjournals.aje.a009653. [DOI] [PubMed] [Google Scholar]
- 37.Boele van Hensbroek P, van Dijk N, van Breda GF, Scheffer AC, van der Cammen TJ, Lips P, Goslings JC, de Rooij SE. The carefall triage instrument identifying risk factors for recurrent falls in elderly patients. Am J Emerg Med. 2009;27:23–36. doi: 10.1016/j.ajem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Kanten DN, Mulrow CD, Gerety MB, Lichtenstein MJ, Aguilar C, Cornell JE. Falls: An examination of three reporting methods in nursing homes. J Am Geriatr Soc. 1993;41:662–666. doi: 10.1111/j.1532-5415.1993.tb06741.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: Results from the women's health initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 40.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonda SJ, Herzog AR. Documentation of physical functioning measured in the health and retirement study and the asset and health cynamics among the oldest old study. 2004 [Google Scholar]
- 42.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–451. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: A randomized, controlled trial. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]


