Abstract
Type 2 diabetes is a global epidemic contributing to significant cardiovascular morbidity and mortality. The high prevalence of cardiovascular disease can largely be attributed to the metabolic syndrome with its multiple cardiovascular risk factors, including central obesity, hypertension, glucose intolerance, chronic inflammation, and dyslipidemia. The peroxisome proliferator-activated receptor-γ agonists, the thiazolidinediones, may potentially correct the inflammatory disarray, endothelial dysfunction, dyslipidemia, and plaque vulnerability associated with diabetic cardiovascular disease through their effects on insulin resistance and fat metabolism, yet they can also exacerbate congestive heart failure. This review summarizes basic science, animal, and human data on the effects of thiazolidinediones on cardiovascular disease.
Introduction
Type 2 diabetes has become an epidemic in the United States mainly due to an increase in obesity and sedentary lifestyles [1]. Diabetes is considered to be a cardiovascular disease equivalent by the National Cholesterol Education Program Adult Treatment Panel III. The risks of cardiovascular disease and coronary heart disease are increased two- to fourfold above the normal population [2]. The high prevalence of cardiovascular disease and mortality in patients with diabetes can largely be attributed to the high prevalence of the metabolic syndrome and insulin resistance with its multiple coronary heart disease risk factors, including central obesity, hypertension, glucose intolerance, chronic inflammation, and mixed dyslipidemia [3]. The thiazolidinediones, a class of drugs that work through peroxisome proliferator-activated receptor (PPAR)-γ agonism, are insulin sensitizers and have been shown to improve cardiac risk factors and decrease cardiovascular events. However, they have been shown to cause weight gain and fluid retention, leading to exacerbation of congestive heart failure. Thus, the question is raised: Are thiazolidinediones good or bad for the heart?
Pathophysiology of Diabetic Atherosclerosis
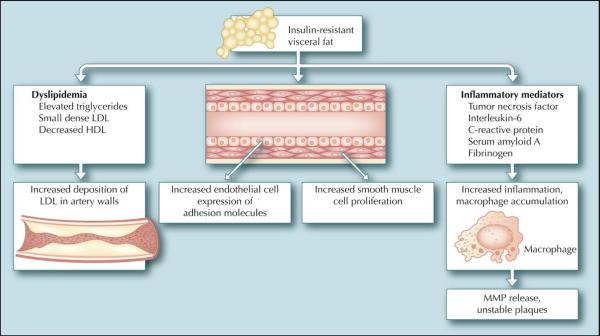
The pathophysiology of atherosclerosis is complex, involving multiple cellular elements and stages (Fig. 1) [4]. Diabetes has been shown to accelerate many of the pathways contributing to atherosclerosis. The state of insulin resistance often accompanying diabetes creates a metabolic environment fertile for the development of atherosclerotic plaques [5]. Although the exact mechanisms of insulin resistance are not fully understood, it has been well demonstrated that increased visceral fat is an important contributor to its development [6]. Breakdown of triglycerides into free fatty acids from insulin-resistant, dysfunctional fat cells contributes to the development of the dyslipidemia characteristic of diabetes. These free fatty acids stimulate liver production of triglycerides, apolipoprotein B, and very low density lipoprotein (VLDL), all of which have been shown to be atherogenic. Cholesterol ester transfer protein then facilitates the exchange of triglycerides from VLDL for cholesterol from either high-density lipoprotein (HDL) or low-density lipoprotein (LDL). This results in a small dense HDL particle that is easily removed from the circulation and a small dense LDL particle that easily enters and becomes trapped in the artery wall. Thus, the dyslipidemia of diabetes is characterized by elevated triglycerides, low HDL, and elevated small dense LDL and is driven by the underlying insulin resistance [7]. Not only do insulin-resistant fat cells create a dysfunctional lipid environment, they also produce a host of inflammatory mediators, including tumor necrosis factor-α, interleukin-6, soluble adhesion molecules, and resistin, which also contribute to the development of atherosclerosis. Downstream acute-phase reactants are elevated as well, including serum amyloid A, fibrinogen, and C-reactive protein. In recent years, inflammation has been shown to be a central contributor to the development of atherosclerosis [8].
Figure 1.
Pathophysiology of diabetic atherosclerosis. The state of insulin resistance often accompanying diabetes creates a metabolic environment fertile for the development of atherosclerotic plaques. Insulin-resistant fat cells contribute to dyslipidemia, an increased inflammatory milieu, and dysfunctional macrophages, endothelium, and smooth muscle cells. HDL—high-density lipoprotein; LDL—low-density lipoprotein; MMP—matrix metalloproteinase.
In addition, insulin resistance affects many cellular constituents of atherosclerosis, including vascular endothelial cells, vascular smooth muscle cells, and macrophages. The endothelium has proven to be a dynamic player in the development of atherosclerosis and insulin resistance renders it dysfunctional in many aspects [9]. Insulin-stimulated production of endothelial nitric oxide is significantly reduced, whereas release of the potent vasoconstrictor endothelin-1 is increased, contributing to the endothelial dysfunction characteristic of diabetes [10•,11]. Further, insulin resistance leads to an elevation in endothelial adhesion molecules, including vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin as well as chemokines including monocyte chemotactic protein-1, leading to an increase in monocyte/macrophage recruitment [10•,12]. Monocytes recruited to the area further release inflammatory cytokines as well as matrix metalloproteinases (MMPs), thus fueling the atherosclerotic process and contributing to unstable plaques. Further, endothelial release of plasminogen activator inhibitor-1 is increased and contributes to thrombosis and progression of the athero-sclerotic lesion [13]. Vascular smooth muscle cells are also affected by insulin resistance and demonstrate an increase in proliferation in response to hyperinsulinemia and increased growth factor expression. Thus, patients with diabetes have an increased rate of restenosis in response to vascular injury [14].
Modulation of Diabetic Atherosclerosis: The PPARs
It is well known that treating the multiple abnormalities associated with diabetes decreases atherosclerotic disease burden. Traditionally, this has been done by separately treating hypertension, hyperlipidemia, and hyperglycemia without affecting the underlying mechanism of insulin resistance. The PPAR-γ agonists, also known as the thiazolidinediones, are a promising class of medications that have been shown to potentially correct the inflammatory disarray, endothelial dysfunction, dyslipidemia, and plaque vulnerability associated with atherosclerosis through their positive effects on insulin resistance and fat metabolism [15,16].
PPAR-γ is a member of the nuclear receptor superfamily and is predominantly expressed in adipose tissue and to a lesser extent in muscle, vascular endothelial cells, vascular smooth muscle cells, and macrophage foam cells [15,17]. PPAR-γ is activated by several endogenous ligands, of which only a few are known and include polyunsaturated fatty acids, eicosanoids, and oxidized LDL [18]. Thus, the activity of PPAR-γ is largely affected by the presence or absence of activators as well as repressors. PPAR-γ, once activated, functions mainly to induce adipocyte differentiation; it does so by increasing the transcription of genes that promote fatty acid storage and fat cell redistribution from large insulin-resistant cells to smaller more insulin-sensitive cells [16,18]. This results in a net flux of fatty acids into the subcutaneous tissue and away from the viscera [6].
As has recently been reported throughout the literature, PPAR-γ agonism has a host of effects on multiple aspects of the atherosclerotic process. PPAR-γ is activated by the atherosclerotic process, specifically by oxidized LDL. The beneficial effects of PPAR-γ on foam cell formation were initially questioned because the activation of PPAR-γ increases expression of scavenger receptor CD36, which promotes the uptake of oxidized LDL into macrophages [19]. However, this effect seems to be offset by the down-regulation of the ApoB48 receptor, reducing the uptake of glycated LDL and triglyceride-rich remnant lipoproteins and the upregulation of adenosine triphosphate-binding cassette protein A1, which stimulates the efflux of cholesterol from the macrophage and thus the artery wall [9,16,20,21]. In addition, lipoprotein lipase activity in macrophages is decreased further by blunting the uptake of triglycerides and glycated LDL by macrophages. This shifting of triglyceride metabolism along with improvement in insulin sensitivity contributes to the normalization of LDL and HDL particle size, resulting in a more effective HDL particle and a less atherogenic LDL particle [10•].
PPAR-γ agonism also appears to affect multiple aspects of the inflammatory reaction associated with atherosclerosis. Early chemokine and adhesion molecule expression in the endothelium, including intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, are decreased, thus limiting monocyte/macrophage accumulation in the atherosclerotic lesion. Macrophage-derived inflammatory mediators are also independently decreased, all contributing to a potentially more stable plaque [22,23]. Additionally, there is a decrease in serum markers of inflammation, including C-reactive protein, leukocyte cell count, fibrinogen, tumor necrosis factor-α, soluble CD40 ligand, and serum amyloid A, suggesting overall anti-inflammatory effects of PPAR-γ activation [9,12,17,23].
Aside from decreasing endothelial cell adhesion molecules, PPAR-γ agonism decreases endothelial expression of endothelin-1 while increasing the release of nitric oxide through induction of endothelial nitric oxide synthase, thus blunting endothelial dysfunction [11,24,25]. There is also evidence that PPAR agonism antagonizes the effects of angiotensin II on the endothelium, contributing to a lowering of blood pressure [26]. Vascular smooth muscle cells are also affected by PPAR agonism. Chemoattractant vascular smooth muscle cell migration as well as proliferation is inhibited by PPAR activation. The growth-promoting effects of several mitogens, including insulin, basic fibroblast growth factor, angiotensin II, and platelet-derived growth factor, are blunted by PPAR-γ activation through inhibition of a mitogen-activated protein kinase-dependent signaling pathway and inhibition of the G1-S phase transition [14].
Acute plaque progression appears to be modulated by PPAR agonism as well. One well-described mechanism leading to acute plaque progression is the repeated rupture and thrombosis of plaque, which results in a gradually narrowing lumen. MMPs, which contribute to the weakening of the fibrous cap, are reduced in the setting of PPAR agonism, potentially lending to a more stable plaque [12]. Further, PPAR agonism decreases the production of plasminogen activator inhibitor-1, shifting the balance toward a more antithrombotic environment, potentially decreasing both plaque progression and incidence of myocardial infarction [27].
Are Thiazolidinediones Good for the Heart? What the Animal Models Show
Animal models studying the effects of PPAR agonism offer, for the most part, encouraging data to suggest that PPAR agonism limits development of atherosclerosis. Several murine models have shown that atherosclerosis is decreased in the presence of PPAR activation [9]. Several investigators have employed murine genetic models to decipher the complex effects of PPAR activation. It has been convincingly shown that PPAR-γ is absolutely necessary for survival because complete deficiency results in embryonic death with deficiencies in placental and systemic vasculature, lipodystrophy, and myocardial thinning [28]. Ablation of PPAR-γ in the muscle, adipose, or liver in adult mice results in increased free fatty acids in the circulation and the development of insulin resistance [18]. Thus, it is clear that some amount of PPAR-γ is necessary and contributes to correct lipid and glucose metabolism and healthy survival.
Murine gain-of-function models suggest that PPAR-γ is protective in the setting of diet-induced obesity and lends to improved insulin sensitivity and decreased free fatty acid production [29]. Adenoviral overexpression of PPAR-γ in the liver of mice increased expression of the insulin-sensitizing adipokine, adiponectin [30]. Similarly, pharmacologic PPAR-γ agonism in animal models has demonstrated an improvement in insulin sensitivity; however, this was at the expense of increased adipose tissue and fluid retention. In addition, PPAR-γ agonism has been shown to decrease macrophage recruitment to the arterial wall in vivo, resulting in a significant decline in inflammatory mediators. Improved vascular tone, likely through stimulation of nitric oxide production and reduction in endothelin-1 production, has been shown in several animal models to decrease endothelial dysfunction as well as angiotensin II and endothelin-1-induced hypertension [24,26]. Rabbit models of atherosclerosis have even suggested that in combination with a statin, pharmacologic PPAR-γ agonism may lead to regression of atherosclerotic plaques [31]. Further, PPAR-γ agonism, compared with placebo, led to a marked 60% to 80% reduction in lesion size in LDL receptor null mice fed a Western diet [32]. Transplantation of PPAR-γ null bone marrow into these mice resulted in a marked increase in atherosclerosis [33]. PPAR-γ agonism also has a beneficial effect following balloon injury, resulting in reduced smooth muscle cell proliferation and intimal hyperplasia [34]. In addition, PPAR-γ agonism has been shown to improve recovery of left ventricular systolic function in a porcine model of ischemia and reperfusion and to reduce infarct size when given prior to induction of ischemia in a rat model [35].
Are Thiazolidinediones Good or Bad for the Heart? Human Studies
The data from animal models suggest that PPAR-γ agonism is beneficial for the heart. In the current era, only full PPAR-γ agonists, the thiazolidinediones, are available for the treatment of human disease. The two agents currently available are rosiglitazone and pioglitazone. Troglitazone, a previously available agent, was removed from the market secondary to increased incidence of liver failure.
The thiazolidinediones were first used clinically for their beneficial effects on glucose metabolism. Insulin resistance can be decreased by almost 80% in patients treated with these PPAR-γ agonists [36]. Fasting plasma glucose, plasma insulin levels, and C-peptide of insulin are consistently lowered in treated patients [15]. In turn, glycosylated hemoglobin measurements are decreased by 1% to 1.5%.
There is a wealth of small studies available suggesting that these agents are beneficial, in addition, in the treatment of atherosclerosis and in the modification of risk factors that contribute to atherosclerosis. Both pioglitazone and rosiglitazone have been shown to decrease inflammatory markers in several small clinical trials consistent with animal model studies. Decreased serum measurements of MMP-9, tumor necrosis factor-α, serum amyloid A, C-reactive protein, soluble CD40 ligand, and fibrinogen, all of which have been shown to be elevated in the athero-sclerotic process, have been demonstrated [9,12,37].
The thiazolidinediones also have effects on lipid parameters; however, the effects vary between agents. Pioglitazone decreases triglycerides, increases HDL, and has a neutral effect on total cholesterol and LDL. Rosiglitazone decreases or increases triglycerides depending on the study, increases HDL, and increases total cholesterol in addition to LDL. Both agents shift LDL particles toward a less dense, less atherogenic lipoprotein [38–40]. In contrast, both agents increase lipoprotein(a), which potentially has proatherogenic tendencies. Perhaps the most rigorously controlled clinical trial, in which each patient was treated with both rosiglitazone and pioglitazone separated by a washout period, provides the most reliable information. In this study, pioglitazone decreased triglycerides by 52%, whereas rosiglitazone resulted in an increase of 13%. HDL was increased 5.2% by pioglitazone and only 2.4% by rosiglitazone. Finally, LDL cholesterol was increased 12.3% by pioglitazone and 21.3% by rosiglitazone, with overall particle concentration being reduced by pioglitazone secondary to a greater increase in particle size [40]. Therefore, it would appear that pioglitazone may be more beneficial in terms of lipid modification; however, it is unclear if the differential effects on lipids between agents have an impact on clinical events.
Several other clinical effects of these agents suggest that their overall effect on atherosclerosis progression will be favorable. Both rosiglitazone and pioglitazone decrease urinary microalbumin, which is known to be a marker of coronary artery disease progression [41,42]. Both agents increase brachial artery flow-mediated dilation in response to shear stress or acetylcholine infusion, suggesting an improvement in endothelial function, again a marker of coronary artery disease progression [10•]. Further, decreases in rates of restenosis following angioplasty and stent placement have been observed with both agents [14,34]. In addition, pioglitazone decreases carotid intimal-medial thickness as determined by ultrasound [43].
However, these beneficial effects come at the expense of weight gain and fluid retention. This has proven to be problematic in patients with underlying congestive heart failure, and in some cases has led to clinical decompensation in patients with controlled heart failure, particularly in concert with insulin therapy.
Nevertheless, the final verdict of the thiazolidinediones awaits the results of large clinical trials, of which many are ongoing with few completed. However, multi-center randomized trials have been encouraging. PIPOD (Pioglitazone In Prevention Of Diabetes) [44], one of the earliest completed trials with pioglitazone, studied Hispanic women who had a previous history of gestational diabetes. Treatment with pioglitazone resulted in improved insulin sensitivity and halted decline in β-cell function. The recently completed PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) study addressed the impact of pioglitazone therapy on patients with diabetes and macrovascular disease [45]. Although the primary composite end point (composite of all-cause mortality, nonfatal myocardial infarction, stroke, acute coronary syndrome, coronary artery bypass or percutaneous intervention, major leg amputation or leg revascularization) was not statistically significant, the secondary composite end point (composite of all-cause mortality, nonfatal myocardial infarction, and stroke) revealed a significant 16% reduction in comparison with placebo. One might argue, as many have, that the secondary end points of this study are more relevant end points. Further studies are needed to resolve this issue.
Several other trials are on the horizon. The STARR (Study of Atherosclerosis with Ramipril and Rosiglitazone) trial is addressing the effects of rosiglitazone on atherosclerotic progression as determined by B mode carotid ultrasound in patients with impaired glucose tolerance or impaired fasting glucose who have not yet developed diabetes. A subset of this trial, the DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) trial, will look specifically at the effects of rosiglitazone on mortality and progression to diabetes. The PPAR (Pioglitazone Protects DM Patients Against Re-Infarction) trial, currently ongoing in Japan, will investigate the effects of PPAR-γ agonism on mortality, hospitalization for cardiovascular events, progression to diabetes, and a host of secondary end points. VICTORY (Vein-Coronary Atherosclerosis and Rosiglitazone after Bypass Surgery) will provide information on the effects of rosiglitazone on vein graft disease progression in patients with diabetes. IRIS (Insulin Resistance in Stroke), an interesting ongoing trial, will determine the effects of thiazolidinediones on recurrent stroke in patients without diabetes. ADOPT (A Diabetes Outcome Progression Trial), RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia), BARI-2D (Bypass Angioplasty Revascularization Investigation–Type 2 Diabetes), PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation), and VADT (Glycemic Control and Complications in Diabetes Mellitus Type 2) are also ongoing and are investigating the effects of rosiglitazone on various outcomes in patients with diabetes.
Future Directions
The complete story of the effects of thiazolidinediones on the heart continues to be written. Whether or not full agonism of PPAR-γ will prove to be the most beneficial paradigm for prevention of atherosclerosis awaits further research because partial agonism, as well, appears to be beneficial with a reduction in the unwanted effects of weight gain due to increases in adiposity and fluid retention. Because the results from PROactive showed a significant increase in heart failure hospitalizations with pioglitazone treatment compared with placebo (6% vs 4%, P = 0.007), this presents a real clinical problem associated with use of these drugs [45].
Interestingly, mice partially deficient in PPAR-γ (PPAR-γ ±) had increased insulin sensitivity compared with wild-type mice and the decline in insulin sensitivity was less with increasing age [46]. Studies are conflicting as to whether a Western diet induces insulin resistance or atherosclerosis in this model. In addition, partial activation of PPAR-γ, appealingly, is weight neutral or even results in weight loss [46]. In even greater contrast, PPAR-γ pharmacologic antagonism in animal models has also been shown to increase insulin sensitivity [47].
This conflicting data leads to the provocative idea that partial PPAR-γ agonism may be more beneficial than full agonism. Partial pharmacologic agonism of PPAR-γ has been studied in animal models as well. Partial agonism results in PPAR association with different coactivators than those associated with during full agonism. This leads to similar glucose lowering and improvements in insulin sensitivity, as seen with full agonism with a significant decrease in weight gain [48]. Perhaps partial PPAR-γ agonism will prove to be more beneficial in multiple aspects involving the heart. Studies are ongoing in animal models with agents currently not approved for human use.
Conclusions
Given the current literature, it can be confidently stated that PPAR-γ agonism is beneficial in the treatment of insulin resistance and the majority of available evidence points to a beneficial effect on atherosclerosis progression. It must be kept in mind that this is dependent on careful patient selection in order to avoid increases in heart failure events.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bonow RO, Gheorghiade M. The diabetes epidemic: a national and global crisis. Am J Med. 2004;116(suppl):10S. doi: 10.1016/j.amjmed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–334. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Kramer D, Raji A, Plutzky J. Prediabetes mellitus and its links to atherosclerosis. Curr Diab Rep. 2003;3:11–8. doi: 10.1007/s11892-003-0047-4. [DOI] [PubMed] [Google Scholar]
- 4.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 5.Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 6.Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: rationale and evidence for correcting the lipid imbalance. Am Heart J. 2005;150:859–870. doi: 10.1016/j.ahj.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Verges B. New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab. 2005;31:429–439. doi: 10.1016/s1262-3636(07)70213-6. [DOI] [PubMed] [Google Scholar]
- 8.Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 9.Plutzky J. The vascular biology of atherosclerosis. Am J Med. 2003;115(suppl 8A):55S–61S. doi: 10.1016/j.amjmed.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10•.Staels B. PPARgamma and atherosclerosis. Curr Med Res Opin. 2005;21(suppl 1):S13–S20. doi: 10.1185/030079905X36440. This is an interesting and fairly comprehensive review of the many effects of PPAR-γ on the atherosclerotic process. [DOI] [PubMed] [Google Scholar]
- 11.Mather KJ, Lteif A, Steinberg HO, et al. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 12.Marx N, Froehlich J, Siam L, et al. Antidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:283–288. doi: 10.1161/01.atv.0000054195.35121.5e. [DOI] [PubMed] [Google Scholar]
- 13.Thogersen AM, Jansson JH, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 14.Bruemmer D, Law RE. Thiazolidinedione regulation of smooth muscle cell proliferation. Am J Med. 2003;115(suppl 8A):87S–92S. doi: 10.1016/j.amjmed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 16.Chinetti-Gbaguidi G, Fruchart JC, Staels B. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: new approaches to therapy. Curr Opin Pharmacol. 2005;5:177–183. doi: 10.1016/j.coph.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Barish GD, Evans RM. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocrinol Metab. 2004;15:158–165. doi: 10.1016/j.tem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 19.Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 20.Cock TA, Houten SM, Auwerx J. Peroxisome proliferator-activated receptor-gamma: too much of a good thing causes harm. EMBO Rep. 2004;5:142–147. doi: 10.1038/sj.embor.7400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy L, Tontonoz P, Alvarez JG, et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Chawla A. Role of PPARgamma in macrophage biology and atherosclerosis. Trends Endocrinol Metab. 2004;15:500–505. doi: 10.1016/j.tem.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Delerive P, Martin-Nizard F, Chinetti G, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 25.Calnek DS, Mazzella L, Roser S, et al. Peroxisome proliferator-activated receptor gamma ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–57. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- 26.Diep QN, El Mabrouk M, Cohn JS, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 27.Zirlik A, Leugers A, Lohrmann J, et al. Direct attenuation of plasminogen activator inhibitor type-1 expression in human adipose tissue by thiazolidinediones. Thromb Haemost. 2004;91:674–682. doi: 10.1160/TH03-06-0384. [DOI] [PubMed] [Google Scholar]
- 28.Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 29.Rangwala SM, Rhoades B, Shapiro JS, et al. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPAR-gamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 31.Corti R, Osende JI, Fallon JT, et al. The selective peroxisomal proliferator-activated receptor-gamma agonist has an additive effect on plaque regression in combination with simvastatin in experimental atherosclerosis: in vivo study by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;43:464–473. doi: 10.1016/j.jacc.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Li AC, Brown KK, Silvestre MJ, et al. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXRABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi M, Hirata A, Yamaguchi H, et al. Characterization of an inhibitory effect of pioglitazone on balloon-injured vascular smooth muscle cell growth. Metab Clin Exp. 2001;50:955–962. doi: 10.1053/meta.2001.24869. [DOI] [PubMed] [Google Scholar]
- 35.Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–781. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Carey DG, Cowin GJ, Galloway GJ, et al. Rosiglitazone increases insulin sensitivity and reduces factors associated with insulin resistance in type 2 diabetics. Diabetes Res Clin Pract. 2000;50(suppl 1):64–65. [Google Scholar]
- 37.Marx N, Imhof A, Froehlich J, et al. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107:1954–1957. doi: 10.1161/01.CIR.0000069272.06194.91. [DOI] [PubMed] [Google Scholar]
- 38.Peters Harmel AL, Kendall DM, Buse JB, et al. Impact of adjunctive thiazolidinedione therapy on blood lipid levels and glycemic control in patients with type 2 diabetes. Curr Med Res Opin. 2004;20:215–223. doi: 10.1185/030079903125002937. [DOI] [PubMed] [Google Scholar]
- 39.Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs. 2003;63:1373–1405. doi: 10.2165/00003495-200363130-00004. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 41.Bakris G, Viberti G, Weston WM, et al. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003;17:7–12. doi: 10.1038/sj.jhh.1001444. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Ushiyama C, Shimada N, et al. Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion in diabetes patients. J Diabetes Complications. 2000;14:250–254. doi: 10.1016/s1056-8727(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 43.Koshiyama H, Shimono D, Kuwamura N, et al. Rapid communication: inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metabol. 2001;86:3452–3426. doi: 10.1210/jcem.86.7.7810. [DOI] [PubMed] [Google Scholar]
- 44.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomized controlled trial. Proactive Investigators [no authors listed] Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 46.Miles PD, Barak Y, He W, et al. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieusset J, Touri F, Michalik L, et al. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endorinol. 2002;16:2628–2644. doi: 10.1210/me.2002-0036. [DOI] [PubMed] [Google Scholar]
- 48.Rocchi S, Picard F, Vamecq J, et al. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell. 2001;8:737–747. doi: 10.1016/s1097-2765(01)00353-7. [DOI] [PubMed] [Google Scholar]