Abstract
Understanding the mechanisms that regulate cell cycle progression in vascular smooth muscle cells (VSMCs) is key to understanding and modulating vascular lesion formation. Results of the present study provide the first evidence that phosphorylation of the helix-loop-helix factor Id3 in VSMCs occurs in vitro and in vivo and provides a regulatory switch controlling Id3-induced regulation of p21Cip1 and VSMC growth.
Keywords: muscle, vascular, smooth, cell cycle, helix-loop-helix motifs, phosphorylation
Targeting cell cycle progression has emerged as a promising strategy for limiting vascular smooth muscle cell (VSMC) proliferation in vivo.1,2 The cyclin-dependent kinase inhibitor p21Cip1 regulates VSMC cell cycle progression and proliferation both in vitro and in vivo.3,4 The promoter region of p21Cip1 contains 8 E-box elements, consensus sites for basic helix-loop-helix (bHLH) transcription factors, such as E47, and bHLH expression can promote p21Cip1 transcription.5
The Id family of HLH proteins function at least in part as dominant-negative inhibitors of bHLH-mediated transcription. Id3 has recently emerged as an important regulator of VSMC growth. Antisense inhibition of Id3 can block VSMC proliferation and S-phase entry while increasing p21Cip1 protein levels.6 In addition, Id3 expression is upregulated in VSMCs in vivo after vascular injury in mice and rats.7,8 Id3 contains an N-terminal cdk2 consensus site where it can be phosphorylated at a serine residue (Ser5) by cyclin-cdk2 complexes.9 Ser5 phosphorylation can alter the ability of Id3 to prevent bHLH dimers from binding DNA in vitro as well as the ability of Id3 to promote S-phase entry in cultured fibroblasts.9 However, the specific mechanism(s) whereby cdk2 phosphorylation of Id3 regulates cell cycle progression remain unknown.
Materials and Methods
Production of antisera specific for Ser5 phosphorylated Id3 is described in detail in the expanded Materials and Methods section of the online data supplement available at http://circres.ahajournals.org. Western blot analysis, transient transfections, luciferase and CAT assays, immunohistochemistry, and FACS analysis were performed as previously described8 and in the online data supplement.
Results
To study the effect of Id3 on VSMC proliferation, we cultured primary aortic VSMCs from wild-type (Id3+/+), homozygous Id3 knockout (Id3−/−) and heterozygous Id3 knockout (Id3+/−) mice. Id3−/− VSMCs proliferated at a significantly reduced rate relative to wild type controls, whereas Id3+/− cells proliferated at an intermediate rate (Figure 1a).
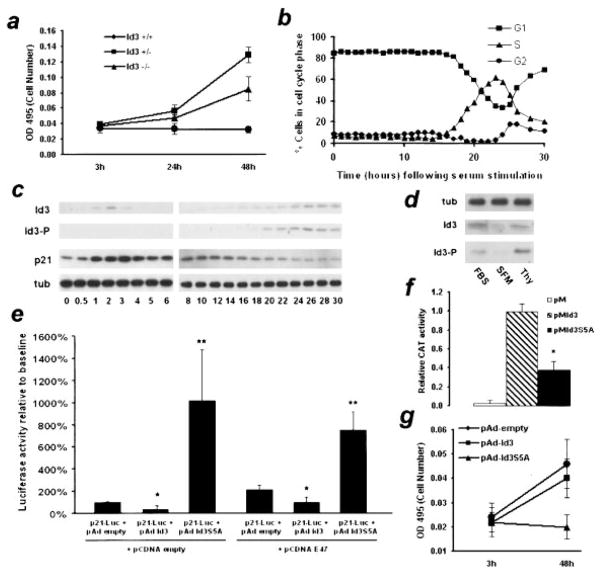
Figure 1.
a, VSMCs were cultured from Id3+/+, Id3+/−, and Id3−/− mice and assayed for cell number 3, 24, and 48 hours after plating. P< 0.05 (Id3+/+ vs Id3+/−) and <0.03 (Id3+/− vs Id3−/−). b, Quiescent VSMCs were serum-stimulated and analyzed by FACS for cell cycle content. c, Serum-stimulated cells were analyzed by Western blotting for total Id3, phosphorylated Id3, p21Cip1, and α-tubulin expression. Results were repeated in triplicate, and a representative blot is shown. d, VSMCs were grown asynchronously, growth-arrested via serum starvation, or treated with thymidine to arrest in S-phase and analyzed by Western blotting. Representative blot is shown from duplicate experiments. e, Full-length human p21Cip1 promoter-luciferase reporter construct (p21Luc) was cotransfected into VSMCs with a control vector (pcDNA) or a vector expressing E47 (pcDNAE47). Each group was also cotransfected with control vector (pAd empty) or wild-type Id3 (pAdId3) or Id3 with a serine to alanine mutation (pAdId3S5A). Luciferase activity was measured and normalized to control vector. *P< 0.05, **P< 0.01 relative to control vector. f, VSMCs were cotransfected with a CAT reporter construct and pVP16-Pan1 (rat homologue of E47) together with pM-empty vector, pM-Id3, or pM-Id3S5A. CAT activity was measured 48 hours after transfection using ELISA. *P< 0.001. g, VSMC were cotransfected with pAd-GFP and either pAd-empty vector, pAd-Id3, or pAd-Id3S5A. GFP-positive cells were sorted into 96-well plates and assayed for cell number at 3 and 48 hours after plating. P< 0.001 for Id3S5A vs control group.
To examine how the expression of Id3, phospho-Id3 and p21Cip1 correlates with cell cycle progression in VSMCs, quiescent cells were serum-stimulated and analyzed by FACS and Western blotting. Cells began to enter S-phase 16 hours after serum stimulation, with the number of cells in S-phase increasing until approximately 23 hours, when cells began to enter G2 (Figure 1b). Id3 protein was induced in a biphasic fashion. Not detectable in growth-arrested cells, Id3 was induced by 1 hour after serum stimulation, and declined by 4 hours (Figure 1c). This first peak in early G1 represents nonphosphorylated Id3, as no reactivity was observed using antisera specific for Ser5-phosphorylated Id3, and correlated with an increase in p21Cip1 protein levels. At the G1→ S transition, approximately 16 hours after stimulation, we observed induction of phosphorylated Id3, coincident with a decrease in p21Cip1 expression. Furthermore, VSMCs treated with thymidine to induce S-phase arrest displayed an enhanced level of phospho-Id3 compared with asynchronously proliferating cells (Figure 1d). FACS analysis confirmed that the number of cells in S-phase relative to proliferating controls increased approximately 4-fold in the thymidine-treated group (data not shown). PDGF-induced Id3 expression followed a similar time course, yet neither PDGF nor angiotensin II treatment resulted in Id3 phosphorylation at any time point (Figure 2, online data supplement, and data not shown).
Transient transfection studies demonstrated that wild-type Id3 induced a 50% reduction in p21Cip1 promoter activation, whereas Id3S5A increased p21Cip1 promoter activation approximately 1000% over baseline levels (Figure 1e). When E47 was coexpressed, baseline p21Cip1 promoter activation was increased approximately 2-fold. Wild-type Id3 inhibited E47-mediated p21Cip1 promoter activity by 50%, whereas Id3S5A again demonstrated an increase in p21Cip1 promoter activation, by approximately 400%. To determine whether Id3 phosphorylation affected its ability to dimerize with E47, a mammalian two-hybrid assay was performed. Relative to wild-type Id3, Id3S5A displayed a significantly reduced ability to dimerize with Pan-1, the rat homologue of E47 (Figure 1f). To examine the effects of Id3S5A on VSMC proliferation, VSMCs were transiently transfected with pAd-GFP and empty vector, wild-type Id3, or Id3S5A. Id3 and control transfected cells doubled in number after 48 hours. In contrast, VSMCs transfected with Id3S5A did not increase in number (Figure 1g).
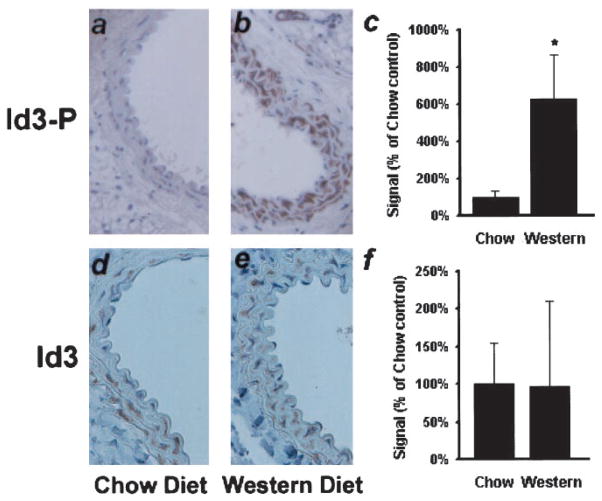
To determine whether Id3 phosphorylation is induced in vivo, we performed immunohistochemistry on carotid artery sections from ApoE−/− mice after a 5-week feeding of high-cholesterol Western or Chow control diets. Results indicate that expression of phospho-Id3 was enhanced approximately 6-fold in Western fed animals, whereas there was no significant change in total Id3 protein levels (Figure 2).
Figure 2.
ApoE−/− mice were fed either a chow (a and d) or high-cholesterol Western (b and e) diet for 28 days, at which time their carotid arteries were examined for expression of phospho-Id3 (a and b) or total Id3 (d and e) by immunohistochemistry. c and f, Sections were scored for immunoreactivity. *P< 0.04; (n=5 animals in each group).
Discussion
Regulation of gene expression is a common method of controlling cellular responses to external stimuli. This is often accomplished through signaling cascades that regulate the phosphorylation state of target transcription factors, and thus represents a major means by which signal transduction and gene expression are integrated. The present study provides the first evidence that phosphorylation provides a regulatory switch controlling the effects of Id3 on activation of the p21Cip1 promoter.
Traditionally, p21Cip1 has been regarded solely as an inhibitor of cell cycle progression, acting to inhibit cyclin-cdk activity required for G1→ S progression. However, recent evidence indicates that p21Cip1 can also act to stabilize cyclin D-cdk4 complexes, active in early G1, facilitating cell cycle entry.10 This may explain the seemingly paradoxical observation reported in the present study and elsewhere that p21Cip1 is transiently increased early in G1 following mitogen stimulation.11,12 Our observation that the increase in p21Cip1 expression occurs early in G1 as levels of nonphosphorylated Id3 peak, coupled with the attenuated ability of a phosphoablating Id3 mutant to dimerize with E47 and the enhanced ability of Id3S5A to promote p21Cip1 promoter activation, suggests that nonphosphorylated Id3 may dimerize with another bHLH factor that acts as a transcriptional repressor of p21Cip1. Indeed, Id3 serine 5 phosphorylation is known to regulate Id3 dimerization partner specificity in vitro11. These data suggest a novel mechanism for serum-induced p21Cip1 expression.
In contrast to Id3S5A, Id3 containing an intact Ser5 phosphorylation site acts to inhibit endogenous and E47-mediated promoter activation of p21Cip1 in proliferating VSMCs, where significant levels of Id3 phosphorylation are observed. Given that Id3 phosphorylation occurs at the G1→ S transition, our results suggest phosphorylation of Id3 contributes to S-phase entry by inhibiting expression of p21Cip1. Consistent with this paradigm, VSMCs arrested in S-phase displayed enhanced levels of phosphorylated Id3. Overexpression studies with wild-type Id3 and Id3S5A provide further support that serine 5 phosphorylation is important for VSMC growth. The growth inhibition observed with serine 5 to alanine mutation in Id3 could be attributable to S-phase arrest with resultant inhibition of cell cycle progression or induction of apoptosis. Interestingly, results demonstrate that although induction of Id3 protein expression appears to be a common mechanism mediating mitogen-induced VSMC proliferation, Id3 phosphorylation is not induced by PDGF-BB and angiotensin II.
Cyclin E-cdk2 is capable of phosphorylating multiple target proteins, including Rb and Id3. Phosphorylation of Id3 may thus act as a positive feedback mechanism; by inhibiting p21Cip1, itself an inhibitor of cyclin E-cdk2, Id3 may act to promote its own phosphorylation at the G1→ S transition, further reducing p21Cip1 levels. Given their critical role in regulating cell cycle progression, phosphorylation of target proteins by cyclin-cdk complexes represents a clinically important aspect of pathological VSMC growth. Indeed, antisense and small molecule inhibition of cyclin E-cdk2 activity have been shown to inhibit VSMC proliferation in vivo.2,13 Our in vivo studies demonstrate that external signals resulting from high cholesterol feeding regulate the phosphorylation of Id3 within a cdk2 consensus site. Thus, the functional consequences of Id3 phosphorylation observed in cultured VSMC may also be important in vivo during vascular lesion development.
Many important questions remain unanswered. What specific growth factors or external signals regulate Id3 phosphorylation? Does inhibition of Id3 phosphorylation modulate vascular lesion development? Studies to answers these and other important questions may provide new insights into the mechanisms whereby signal transduction and gene expression are integrated in the regulation of VSMC growth and in vascular lesion formation.
Supplementary Material
Acknowledgments
This work was supported by NIH grants T32HL007284 (S.T.F.), R01 HL062522, and P01 HL55798 (C.A.M.). We thank Dr Yuan Zhuang (Duke University) for the generous gift of Id3−/− mice and Melissa Bevard and John Sanders for excellent technical assistance with immunostaining.
References
- 1.Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, Orav EJ, Ehsan A, Dell’Acqua G, Dzau VJ. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki J, Isobe M, Morishita R, Aoki M, Horie S, Okubo Y, Kaneda Y, Sawa Y, Matsuda H, Ogihara T, Sekiguchi M. Prevention of graft coronary arteriosclerosis by antisense cdk2 kinase oligonucleotide. Nat Med. 1997;3:900–903. doi: 10.1038/nm0897-900. [DOI] [PubMed] [Google Scholar]
- 3.Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZY, Simari RD, Perkins ND, San H, Gordon D, Nabel GJ, Nabel EG. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci U S A. 1996;93:7905–7910. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller C, Baudler S, Welzel H, Bohm M, Nickenig G. Identification of a novel redox-sensitive gene, Id3, which mediates angiotensin II-induced cell growth. Circulation. 2002;105:2423–2428. doi: 10.1161/01.cir.0000016047.19488.91. [DOI] [PubMed] [Google Scholar]
- 7.Nickenig G, Baudler S, Muller C, Werner C, Werner N, Welzel H, Strehlow K, Bohm M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002;16:1077–1086. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura ME, Li F, Berthoux L, Wei B, Lobe DR, Jeon C, Hammarskjold ML, McNamara CA. Vascular injury induces posttranscriptional regulation of the Id3 gene: cloning of a novel Id3 isoform expressed during vascular lesion formation in rat and human atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:752–758. doi: 10.1161/01.atv.21.5.752. [DOI] [PubMed] [Google Scholar]
- 9.Deed RW, Hara E, Atherton GT, Peters G, Norton JD. Regulation of Id3 cell cycle function by Cdk-2-dependent phosphorylation. Mol Cell Biol. 1997;17:6815–6821. doi: 10.1128/mcb.17.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 11.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Hannon GJ, Beach D, Stillman B. Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Curr Biol. 1996;6:189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 13.Brooks EE, Gray NS, Joly A, Kerwar SS, Lum R, Mackman RL, Norman TC, Rosete J, Rowe M, Schow SR, Schultz PG, Wang X, Wick MM, Shiffman D. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J Biol Chem. 1997;272:29207–29211. doi: 10.1074/jbc.272.46.29207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.