Summary
DEET is the most widely used insect repellent worldwide. In Drosophila olfactory receptor neurons (ORNs), DEET is detected through a mechanism that employs the olfactory receptor, OR83b. However, it is controversial as to whether ORNs respond directly to DEET or whether DEET blocks the response to attractive odors. Here, we showed that DEET suppressed feeding behavior in Drosophila and this effect was mediated by gustatory receptor neurons (GRNs). DEET was potent in suppressing feeding as <0.1% DEET elicited aversive behavior. Inhibition of feeding by DEET required multiple gustatory receptors (GRs), which were expressed in inhibitory GRNs. DEET stimulated action potentials in GRNs that respond to aversive compounds, and this response was lost in Gr32a, Gr33a and Gr66a mutants. Since 0.02% DEET elicited action potentials, we conclude that DEET directly activates of GRNs. We suggest that the effectiveness of DEET in pest control owes to its dual action in inducing avoidance simultaneously via GRNs and ORNs.
Introduction
Insect pests are among the most serious causes of disease and starvation. Disease-transmitting insect vectors such as mosquitoes, biting flies, chiggers, fleas, and ticks spread infections to hundreds of millions of people each year. Malaria alone afflicts at least half a billion people annually, and this high incidence is due to the efficient transfer of the Plasmodium parasite by the mosquito, Anopheles gambiae (Snow et al., 2005). Insect pests also have devastating effects on crops and contribute to food shortages and famines. ~14% of all crops are lost to insect destruction, resulting in economic losses estimated at $200 billion/year in the United States and $2 trillion worldwide (Pimentel, 2009).
A common strategy to control insect pests is to repel them. Volatile insect repellents are detected through the olfactory sense, and can be applied directly to human skin and clothing to ward off mosquitoes and other disease vectors. Non-volatile repellents are typically sprayed on crops and are detected by insects through the sense of taste. Given that these latter repellents elicit a repulsive behavior, they inhibit the insect’s urge to consume the crops and are therefore referred to as antifeedants. Most plants produce antifeedants to reduce feeding (van der Goes van Naters and Carlson, 2006), and these compounds typically have insecticidal properties if they are ingested.
During the last 50 years, the most widely used insect volatile repellent worldwide is the synthetic compound, DEET (N,N-diethyl-m-toluamide) (Katz et al., 2008). Advantages of DEET are that it is associated with relatively low toxicity when it is not consumed, and that it can last for several hours when used at the highest concentrations. However, the potency of DEET is low as it is used most commonly at levels ranging from 25% to 100% (Katz et al., 2008). A controversial issue concerns the mode of action of DEET. There is evidence that DEET does not act directly as a volatile repellent, but inhibits the positive olfactory responses to attractive compounds such as lactic acid (Dogan et al., 1999; Ditzen et al., 2008). However, another study shows that ORNs in the mosquito, Culex quinquefasciatus, respond directly to DEET (Syed and Leal, 2008). Whether DEET is also capable of acting through the sense of taste is not clear, although DEET has been reported to reduce landing behavior (Syed and Leal, 2008).
Here, we demonstrate that fruit flies are exquisitely sensitive at detecting minute concentrations of DEET through the gustatory response. We found that levels of DEET as low as 0.05% suppressed feeding. This behavior was mediated by direct activation of avoidance GRNs, since application of DEET elicited action potentials in these sensory neurons. In further support of this conclusion, DEET-induced action potentials were nearly eliminated by mutations disrupting any of three GRs (GR33a, GR66a and GR32a), which are broadly expressed in avoidance GRNs.
Results
DEET is a highly effective antifeedant
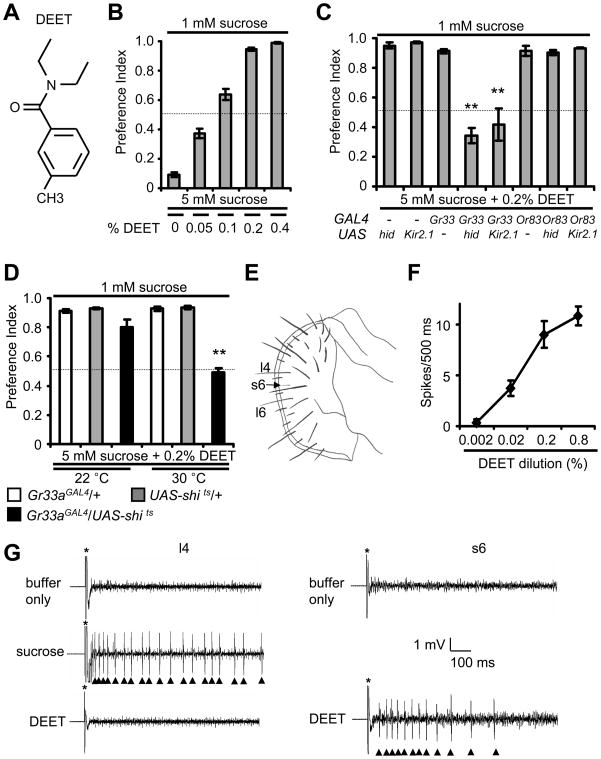
To determine whether DEET (Figure 1A) can inhibit feeding by the fruit fly, we used a binary choice assay (Montell, 2009). We placed flies in a 72 well microtiter dish containing either 1 mM sucrose or 5 mM sucrose and various concentrations of DEET. The wells were mixed with either red or blue food coloring so we could assess whether the animals consumed the lower or higher concentration of sugar by inspecting the colors of the abdomens. Complete preferences for 1 mM or 5 mM sucrose result in preference indexes (PIs) of 1.0 and 0 respectively, while a lack of discrimination between the two alternatives results in a PI of 0.5. In the absence of DEET, flies exhibit a strong preference for 5 mM sucrose (Figure 1B and Table S1A). Introduction of only 0.4% DEET to the 5 mM sucrose induced almost complete avoidance (Figure 1B and Table S1A). Nearly as effective avoidance occurred with 0.2% DEET, and even 0.05% DEET reduced consumption of 5 mM sucrose (Figure 1B and Table S1A).
Figure 1. Behavioral avoidance to DEET is mediated by gustatory receptor neurons.
(A) Structure of DEET (B–D) Binary food choice assays using Drosophila melanogaster. All assays were performed with 5 mM sucrose (either alone or mixture with each indicated concentration of DEET) and 1 mM sucrose. The dotted lines indicate a lack of bias between the two alternative food choices (P.I.=0.5). The experiments presented were conducted in a blind manner. (B) Dose response curve using the wild-type control flies (w1118) and the indicated concentrations of DEET. (C) Binary assays performed after the ORNs or GRNs were either ablated or inactivated. The cell death gene (UAS-hid) or the Kir2.1 channel (UAS-Kir2.1) were expressed in ORNs or GRNs under control of the Gr33a-GAL4 (Gr33aGAL4/+) or the Or83b-GAL4. The normal DEET avoidance in Or83b-GAL4/UAS-Kir2.1 flies was not due to ineffectiveness of these transgenes to effect olfaction since these flies did not avoid 0.1% benzaldehyde (Figure S1B). (D) Transient synaptic ablation of aversive GRNs using the Gr33a-GAL4 (Moon et al., 2009) and UAS-shits1. The assays were performed at the permissive (30°C) and non-permissive temperatures for the shits1. (E) Schematic illustration of gustatory sensilla on the fly labellum. We used the short sensillum (s6) for most tip recordings. (F) Dose response curve using DEET and s6 sensilla. The error bars indicate S.E.M.s. (G) Representative tip recordings obtained from an s6 sensillum and a long (l4) sensillum using buffer only, 0.2% DEET or 50 mM sucrose. The asterisks indicate the addition of the recording pipets to the sensilla. Arrowheads indicate tastant-induced action potentials.
Antifeedant action of DEET mediated by direct activation of GRNs
The highly sensitive repulsion to sucrose laced with DEET would appear to occur through avoidance GRNs. However, current evidence is that the repulsive action of DEET is mediated by olfactory avoidance through ORNs (Ditzen et al., 2008; Syed and Leal, 2008). There is no clear documentation that DEET is sensed through GRNs. The GRNs and ORNs are housed in hairlike projections, referred to sensilla (Vosshall and Stocker, 2007; Montell, 2009). The olfactory sensilla are distributed on the antenna and maxillary palps. The main gustatory organ is the labellum, which is situated on the proboscis. Additional taste sensilla are distributed on the wing margins, leg tarsi and female ovipositor.
To identify the class of receptor cells that were required for repulsion to DEET in the food choice assay, we took advantage of the GAL4/UAS system to selectively inactive or kill GRNs or ORNs. To ablate these cells, we used the hid gene, which induces apoptosis cell autonomously. We expressed UAS-hid (Zhou et al., 1997) using either the Or83b-GAL4 (Larsson et al., 2004), which directs expression in nearly all ORNs, or under the control of a GAL4 introduced into the Gr33a locus by homologous recombination (Gr33aGAL4) (Moon et al., 2009). The GAL4 in Gr33aGAL4/+ flies is expressed in virtually all GRNs that respond to aversive chemicals via contact chemosensation (Moon et al., 2009). We found that expression of UAS-hid in a Gr33aGAL4/+ background greatly decreased the aversion to 5 mM sucrose plus DEET (Figure 1C and Table S1B). In contrast, the UAS-hid or the UAS-hid/Or83b-GAL4 flies displayed the same repulsion to DEET as the wild-type control (+/Or83b-GAL4; Figure 1C). Thus, DEET is very effective at inhibiting feeding.
To confirm that DEET suppresses feeding via contact chemosensation, we performed the binary food choice assay after inactivating the GRNs and ORNs by overexpressing the potassium channel, Kir2.1 (Baines et al., 2001). Increased expression of Kir2.1 suppresses action potential firing by hyperpolarizing neurons. We found that induction of Kir2.1 in GRNs but not in ORNs caused the avoidance behavior to low concentrations of DEET (Figure 1C).
Because cell ablation or long-term distortion of electrical activity in GRNs could have unintended consequences on neighboring cells, we transiently inactivated the GRNs using a genetically encoded dominant temperature-sensitive blocker of synaptic transmission, shibirets1 (shits1) (Kitamoto, 2001). At elevated temperatures such as 30°C, synaptic transmission in shits1 expressing neurons is rapidly blocked. Therefore, we reared Gr33aGAL4/UAS-shits1 flies at the permissive temperature (22°C). Consistent with the effects resulting from expression of hid or Kir2.1, we found that introduction of shits1 in GRNs reduced the repulsion to sucrose plus DEET, but only after the temperature shift from the permissive (22°C) to the restrictive temperature (30°C) (Figure 1D and Table S1C). The combination of data using hid, Kir2.1 and shits1 all support the conclusion that the potent suppression of feeding by DEET is due to detection of DEET through the sense of taste rather than smell.
To address whether DEET can induce action-potentials in GRNs, we performed tip recordings. We introduced recording electrodes with DEET over the dendritic tips of two types of taste sensilla. These include small (s-type) and large (l-type) sensilla, which respond to aversive and attractive tastants respectively. We obtained spikes from s6 bristles upon application of 0.2% DEET (Figure 1E–G). Even 0.02% DEET produced action potentials in these sensilla (Figure 1F and Table S1D). Four out of five additional s-type sensilla also responded to DEET (Figure S1A). The highest frequencies of action potentials were produced in s5, s6, s7 and s10 sensilla (Figure S1A). The s8 sensilla were unresponsive to DEET, while the s9 sensilla produced a significantly lower frequency of action potentials relative s5, s6, s7 and s10 (Figure S1A). Neither of the two types of l (large)-type sensilla tested (l4 and l6) responded to DEET (Figures 1G and S1A). These latter results were not to a general deficit in responsiveness of the l4 sensilla, as we observed sucrose-induced potentials (Figure 1G). As has been observed with other aversive compounds (Meunier et al., 2003), DEET reduced the frequency of sucrose-induced action potentials in l-type sensilla (Figures S1C and S1D).
DEET response in GRNs requires gustatory receptors
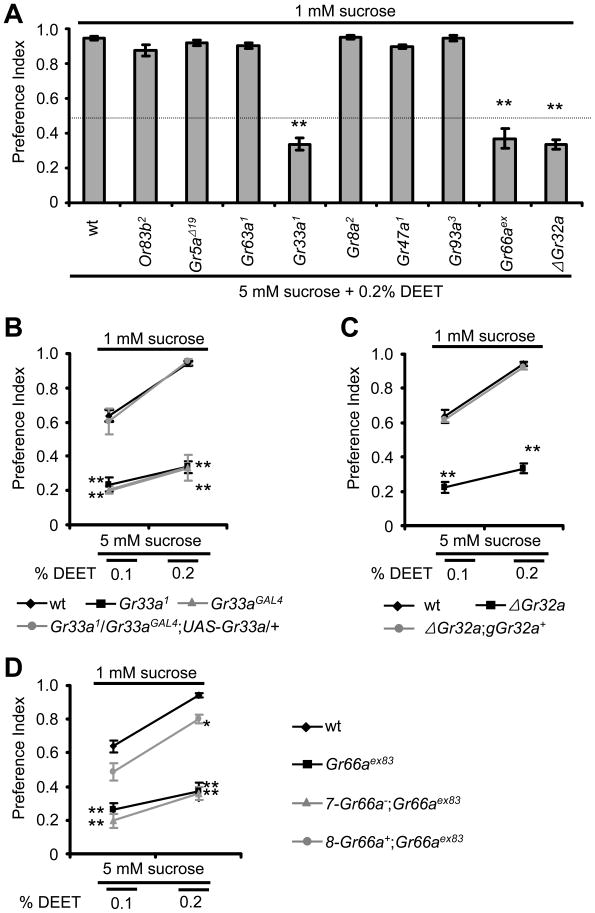
The ability to avoid DEET through the sense of smell requires the odorant co-receptor, OR83b (Ditzen et al., 2008). However, we found that in the absence of OR83b the flies effectively avoided eating the sucrose with DEET (Figure 2A and Table S1E). These results underscore that the ORNs and odorant receptors (ORs) are not the primary receptor cells or molecules involved in repulsion to DEET in the binary food assay.
Figure 2. Three gustatory receptors are required for DEET avoidance using the binary food choice assay.
(A) Survey of Gr mutants and the Or83b2 mutant for defects in the aversion to 0.2% DEET. Gr66aex represents Gr66aex83. (B–D) Rescue of the avoidance defects in response to 0.1% and 0.2% DEET using wild-type Gr transgenes. Most data were collected in a blind manner. However, similar results were obtained when the data were collected in non-blinded experiments (e.g. Figures S2A and S2B). (B) Two Gr33a alleles, Gr33a1 and Gr33aGAL4, displayed similar impairments in aversion to DEET. The behavior was rescued by expression of UAS-Gr33a+ under control of the Gr33aGAL4. See Figure S2A and S2B for additional information. (C) The deficit in ΔGr32a was rescued with a Gr32a+ genomic fragment, gGr32a+. See Figure S2D for additional rescue data using the GAL4/UAS system. (D) Rescue of the Gr66aex83 DEET avoidance defect. The genomic DNA included in the 8-Gr66a+ transgene encoded Gr66a+ and two flanking genes (CG7066 and CG7188) (Moon et al., 2006). The genomic DNA in 7-Gr66a− included the two flanking genes, but not Gr66a. The error bars indicated S.E.M.s.
To determine which molecules are involved in the detection of DEET, we tested candidate gustatory receptors (GRs). GRs are predicted to include seven transmembrane domains and most are expressed either in GRNs that respond to attractive or aversive compounds (Montell, 2009). Fly GRs are unrelated to mammalian gustatory receptors and their sequence similarities to Drosophila ORs are minimal (Robertson et al., 2003). We found that mutation of the gene encoding GR5a, which is expressed in GRNs and is required for the response to several sugars but not sucrose (Dahanukar et al., 2007), had no effect on DEET avoidance in the two-way choice tests (Figure 2A and Table S1E). Furthermore, mutation of Gr63a1, which encodes a CO2 receptor expressed in ORNs (Jones et al., 2007; Kwon et al., 2007), did not impair the suppressive effects of DEET (Figure 2A and Table S1E).
The prime candidate GRs for functioning in the detection of DEET are those that are required for responding to aversive compounds. Recently, we provided evidence that GR33a functions in collaboration with other GRs for detecting all compounds through contact chemosensation (Moon et al., 2009). Indeed, we found that mutation of Gr33a1 impaired the repulsion to DEET (Figure 2A), and introduction of a wild-type Gr33a+ transgene rescued the phenotype (Figure 2B and Table S1F). These data indicate that the ability to avoid DEET through contact chemosensation requires at least one GR.
Because GR33a appears to be a broadly required co-receptor (Moon et al., 2009), we wondered whether other GRs are required in concert with GR33a to avoid DEET. Mutations in two genes, Gr8a2 and Gr47a1 (Lee, Moon and Montell, unpublished), which are expressed in aversive GRNs did not disrupt DEET avoidance (Figure 2A). Disruption of Gr93a3 also had no impact on the repulsion to DEET (Figure 2A), consistent with our previous observation that it affects caffeine sensing only (Lee et al., 2009). Surprisingly, Gr66aex83 mutant animals, which are also compromised in caffeine avoidance (Moon et al., 2006), were impaired in DEET repulsion, similar to Gr33a1 animals (Figure 2A). Even more surprising, the ΔGr32a mutant, which displays increased male-to-male courtship behavior (Miyamoto and Amrein, 2008), was also required for avoiding DEET (Figures 2A and 2C; Tables S1E and S1G).
Given the expression of Gr32a in aversive taste GRNs situated on leg tarsi (Miyamoto and Amrein, 2008), we tested whether the gustatory sensilla on the forelegs were responsive to DEET using an alternative assay, the proboscis extension response (PER). Application of sucrose only to wild-type forelegs resulted in extension of the proboscis in most animals tested (Figure S2E). Addition of DEET to the sucrose resulted in a significant reduction in the PER (Figure S2E). In contrast, application of sucrose plus DEET to the leg tarsi of ΔGr32a flies did not reduce the PER produced by presentation of sucrose alone (Figure S2E). These results indicate that the leg tarsi and Gr32a contribute to DEET avoidance.
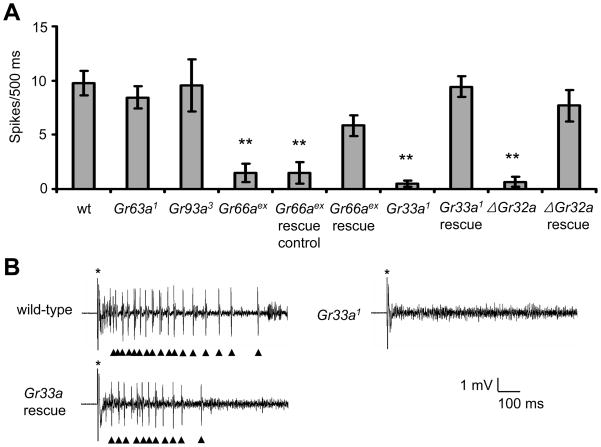
To provide additional evidence that Gr66a, Gr33a and Gr32a functioned in GRNs for the detection of DEET, we performed tip recordings. Consistent with the behavioral assays, DEET-induced action potentials were profoundly reduced in Gr66aex83, Gr33a1, and ΔGr32a mutant animals (Figure 3A and Table S1I), and, these defects were significantly reversed by introduction of the wild-type transgenes (Figures 3A and 3B). The mutations affecting other GRs, such as Gr63a1 or Gr93a3 did not impair DEET-induced action potentials (Figure 3A).
Figure 3. DEET-induced action potentials required Gr66a, Gr33a and Gr32a.
(A) Tip recordings showing the mean responses in s6 sensilla to 0.2% DEET. The deficits in the Gr66aex83, Gr33a1 and ΔGr32a mutants were rescued significantly by the wild-type transgenes. The rescue control for Gr66a was 7-Gr66a− and the rescue transgene was 8-Gr66a+. Gr66aex stands for Gr66aex83. The Gr33a rescue construct was UAS-Gr33a+ and the Gr32a rescue was performed using the genomic transgene, gGr32a+. The error bars indicate S.E.M.s. (B) Representative traces of DEET-induced action potentials in the wild-type control, Gr33a1 and Gr33a expressing the wild-type transgene: Gr33a1/Gr33aGAL4;UAS-Gr33a/+. The times when the recording pipets were applied to the s6 sensilla are indicated by the asterisks. Arrowheads indicate DEET-induced action potentials.
Since Gr66a, Gr33a and Gr32a are all required for the DEET response, they would be expected to be co-expressed in the same GRNs. We have shown previously that Gr66a and Gr33a are co-expressed in all of the same GRNs in the adult labellum (Moon et al., 2009). Using a Gr32-GAL4 reporter, Gr32a has been reported to be expressed in 10 out of 12 s-type sensilla that express Gr66a (Hiroi et al., 2002). To explore further the co-expression of Gr32a with the other two Grs (Gr33a and Gr66a) that participate in the DEET response, we ablated Gr33a-expressing cells using the UAS-hid and Gr33a-GAL4, and performed RT-PCR using primers specific for Gr32a. We found the product of the expected size, which was observed in wild-type, was absent from the labellum of the flies expressing UAS-hid under control of the Gr33a-GAL4 (Figure S2F). However, expression of UAS-hid using the Gr32-GAL4 did not eliminate Gr32a RNA production in the labellum (Figure S2F). The combination of these data indicate that Gr32a RNA was expressed in more GRNs than the Gr32a-GAL4 and suggests that it may be co-expressed in all of the same s-type sensilla that express Gr66a.
Our data indicate that at least three GRs are required for sensing minute concentrations of DEET in GRNs. However, the minimum number may exceed three since misexpression of UAS-Gr66a, UAS-Gr33a, and UAS-Gr32a in either water- or sugar-activated GRNs, using the NP1017-GAL4 or the Gr5a-GAL4 respectively did not produce DEET-induced action potentials in these cells (data not shown).
Gr32a and Gr66a required for detecting multiple naturally occurring antifeedants
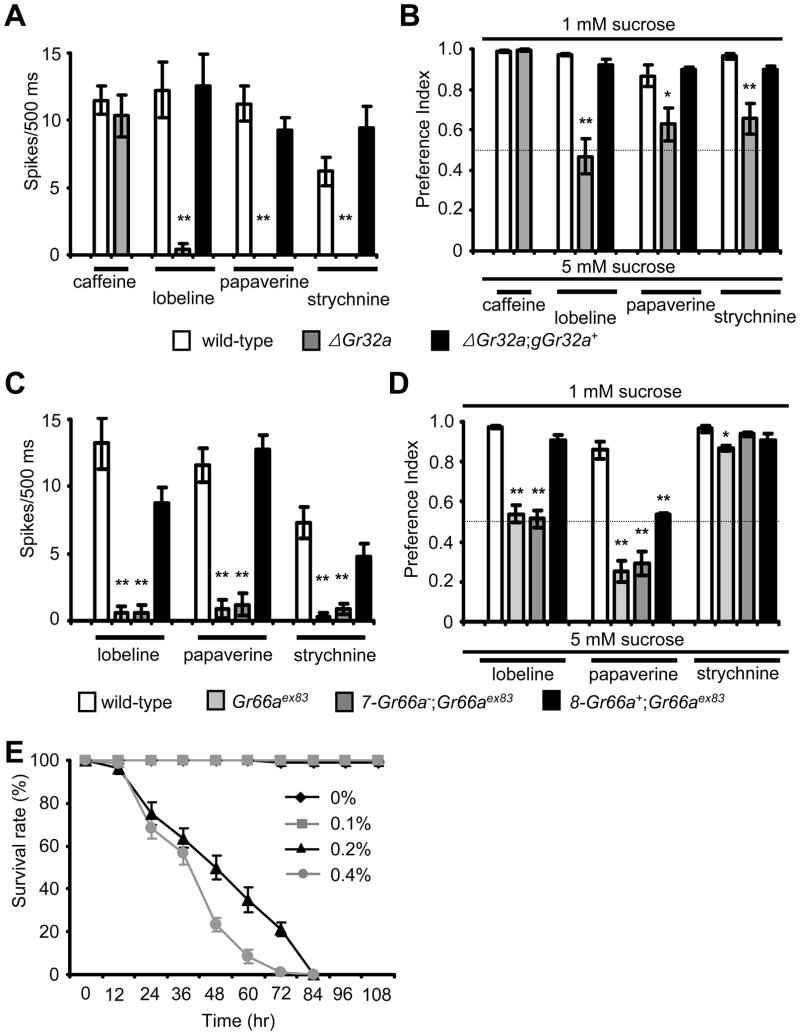
The results that Gr32a is required for DEET avoidance behavior and for DEET-induced action potentials in the labellum, raise the question that Gr32a might also be required for sensing other aversive compounds through the sense of taste. To address this question, we performed electrophysiological as well as two-way choice assays. We found that ΔGr32a mutant animals showed impaired electrophysiological and behavioral response to all aversive chemicals tested, with the exception of caffeine, and these defects were rescued fully by the wild-type transgene (Figures 4A, 4B and S3; Tables S1J and S1K).
Figure 4. Durability and toxicity of DEET and requirements for Gr32a and Gr66a for the responses to multiple naturally occurring repellent compounds.
(A) and (C) Tip recordings. (B) and (D) Two-way choice tests. (A) ΔGr32a flies showed reduced frequencies of action potentials in response to several repellent compounds (also refer to Figure S3A), but a normal caffeine response. (B) ΔGr32a flies were impaired in behavioral avoidance to multiple repellent compounds (also refer to Figure S3B). (C) The frequencies of action potentials induced by lobeline, papaverine and strychnine were nearly eliminated in Gr66aex83. These defects were rescued significantly by 8-Gr66a+, but not by 7-Gr66a−. (D) The behavioral avoidances to lobeline and papaverine were reduced in Gr66aex83. These defects were reversed by 8-Gr66a+, but not by 7-Gr66a−. The slight reduction in the behavioral avoidance to strychnine was statistically significant. (E) Time-dependent effects on the survival of wild-type control flies resulting from consuming 1% sucrose combined with the indicated concentrations of DEET. The error bars indicated S.E.M.s.
We also examined further the Gr66a gustatory phenotype, since Gr66a is required for sensing DEET and therefore does not function specifically for sensing caffeine. Using aversive compounds that we did not test earlier (Moon et al., 2006), we found Gr66aex83 flies were impaired in the behavioral and electrophysiological responses to lobeline and papaverine (Figures 4C and 4D; Tables S1L and S1M). As was the case for the Gr33a1 mutant flies (Moon et al., 2009), the Gr66aex83 mutant elicited very few strychnine-induced action potential; however, the behavioral response to strychnine was not impaired (Figures 4C and 4D). Since GR33a is required for producing action potentials induced by a wide array of aversive compounds (Moon et al., 2009), these results demonstrate that all three GRs that are necessary for responding to DEET, function broadly in the detection of noxious compounds.
Toxicity of DEET
The observation that flies and mosquitoes avoid consuming DEET suggests that this antifeedant is toxic to the flies. To test this proposal, we compared the survival of flies maintained on 1% sucrose, or after lacing the sucrose with small concentrations of DEET (0.1 – 0.4%) (Figure 4E and Table S1N). 0.1% DEET did not cause lethality over the time course examined. However, 0.2% DEET caused lethality among 50% of the animals after 48 hrs (LT50). The LT50 decreased to 38.4 hrs in the presence of 0.4% DEET. These results suggest that DEET is not only an antifeedent, but is also an insecticide.
Discussion
Despite the extensive worldwide application of DEET for pest control, its mode of action remains controversial. While it is clear that the DEET is detected at least in part through ORNs and requires the olfactory co-receptor, OR83b, there is dispute as to whether this volatile chemical directly activates ORNs or masks the responses to attractive odorants, such as those that are food-derived (Ditzen et al., 2008; Syed and Leal, 2008). However, of particular relevance here, it was not clear if DEET was detected through the sense of taste. Multiple lines of evidence support the conclusions that DEET is a highly potent at preventing feeding, and this behavioral aversion is mediated by direct activation of GRNs by DEET. These include the observations that levels of DEET as low as 0.05% discourage feeding, and minute concentrations (0.02%) induce action potentials in GRNs that are known to mediate avoidance behavior. Moreover, elimination or inactivation of GRNs that mediate avoidance responses greatly reduced the aversion to DEET. The detection of DEET through contact chemosensation may be increased further due to the distribution of DEET responsive gustatory sensilla on several body parts such as the legs and labellum.
We uncovered the identities of three Grs (Gr32a, Gr33a and Gr66a) that were critical for the detection of DEET. Expression of these Grs correlated well with the types of sensilla that responded to DEET, and the magnitudes of the responses. First, the l (large)-type sensilla do not express any of these Grs (Hiroi et al., 2002; Thorne et al., 2004; Wang et al., 2004; Moon et al., 2006; Moon et al., 2009) and do not respond to DEET. Second, the three Grs may be co-expressed in all s-type sensilla. Expression of Gr33a and Gr66a appear to overlap completely in s-type sensilla (Moon et al., 2009). Although the Gr32a-GAL4 has been reported to be expressed in 10 out of 12 s-type sensilla (Hiroi et al., 2002), we have presented evidence that it is produced in additional s-type sensilla, and limited to Gr33a-expressing GRNs. Third, the four s-type sensilla that elicit the highest DEET-induced spike frequencies (s5, s6, s7 and s10) express relatively high levels of Gr66a (Hiroi et al., 2002). Conversely, the two s-type sensilla that produced either no detectable (s8) or relatively low levels (s9) of DEET-induced action potentials express comparably low levels of Gr66a (Hiroi et al., 2002).
We suggest that the repertoire of GRs that are minimally required for the DEET response may be four or more since misexpression of Gr32a, Gr33a and Gr66a in GRNs that normally do not respond to DEET was insufficient to confer a DEET response to these neurons. These results are reminiscent of the findings that three GRs are required but not sufficient for the responses to bitter compounds such as caffeine (Moon et al., 2009). Alternatively, we cannot exclude that an additional non-GR subunit is required in concert with GRs for function. Nevertheless, as we have discussed earlier, the complexities of Drosophila GRs exceed that of the Drosophila heterodimeric CO2 receptor and mammalian taste receptors (Lee et al., 2009).
The potent ability of DEET to prevent feeding, was strictly dependent on GRNs and did not involve ORNs, since inactivation of ORNs had no effect. Furthermore, elimination of the broadly required olfactory co-receptor, OR83b, had little if any impact on the fly’s gustatory aversion for DEET. Nevertheless, DEET is a volatile compound, and is also detected through non-contact chemosensation through ORNs. Thus, the effectiveness of DEET in pest control may result from its dual action in deterring insects simultaneously through contact and non-contact chemosensation, rather than exclusively through the olfactory response.
Experimental Procedures
Fly stocks
Or83b-GAL4, Or83b2, Gr63a1, UAS-Kir2.1 and UAS-hid were from the Bloomington Stock Center. We described Gr93a3 (Lee et al., 2009), Gr66aex83 (Moon et al., 2006), Gr33a1, Gr33aGAL4 and UAS-Gr33a (Moon et al., 2009) in previous studies. The 8-Gr66a+ genomic transgene included Gr66a+ and the two flanking genes (CG7066 and CG7188) and the 7-Gr66a− transgene included CG7066 and CG7188 only (Moon et al., 2006). The Gr66a-GAL4 (Thorne et al., 2004), ΔGr32a, UAS-Gr32a and the Gr32a+ genomic rescue transgene, gGr32a+, were provided by H. Amrein (Miyamoto and Amrein, 2008). The NP1017-GAL4 (Inoshita and Tanimura, 2006), UAS-shits1 (Kitamoto, 2001) were described previously. We used w1118 as the wild-type control.
Behavioral assays
The binary food-choice assays (Meunier et al., 2003) were performed as described previously (Moon et al., 2006) using 0.3 mM strychnine, 0.3 mM lobeline, 0.2 mM denatonium, 0.05 mM berberine, 1 mM papaverine, 10 mM caffeine, and 0.5 mM quinine. The chemicals were obtained from Sigma-Aldrich and Wako pure Chemical Industries. The PIs were determined using the numbers of the flies or mosquitoes that were blue (NB), red (NR) or purple (NP): PI=(NB+0.5NP)/(NR+NB+NP) or (NR+0.5NP)/(NR+NB+NP). Every experiment with DEET was carried out ≥6 times. The behavioral assays with other aversive compound were conducted ≥4 times. We used w1118 as the wild-type control. A detailed protocol is included with the Supplemental Materials.
Survival assays
20 wild-type control flies, (w1118) were starved for 12 hr on 1% agarose. We transferred the flies to 1% sucrose/1% agarose and counted the number of live flies every 12 hr for 108 hr. Each experiment was carried out ≥4 times.
Electrophysiology
To determine the frequencies of chemical-induced action potentials in the GRNs, we carried out tip recordings as described previously (Moon et al., 2006), using 1 mM strychnine, 1 mM lobeline, 1 mM denatonium, 0.1 mM berberine, 1 mM papaverine, 10 mM caffeine, and 1 mM quinine. Every experiment was conducted ≥7 times. The wild-type control was w1118. A more detailed protocol is provided with the online Supplemental Materials.
Statistical analyses
We used single factor ANOVA wth Scheffé’s analysis as a post hoc test to compare two sets of data. p value<0.05 or p value<0.01 were indicated with single or double asterisks, respectively.
Highlights.
Fruit flies are exquisitely sensitive to avoiding DEET through the sense of taste.
Gustatory receptors are required for detecting DEET in gustatory receptor neurons.
Minute quantities of DEET directly activate gustatory receptor neurons.
Supplementary Material
Acknowledgments
We thank Drs. H. Amrein and T. Tanimura for fly stocks, S.J. Moon for the unpublished Gr33a-GAL4 line, and M. Jin for the drawing in Figure 1E. SHK was supported in part by a Samsung Fellowship. This work was supported by a grant to CM from the NIDCD (DC007864).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- Dogan EB, Ayres JW, Rossignol PA. Behavioural mode of action of deet: inhibition of lactic acid attraction. Med Vet Entomol. 1999;13:97–100. doi: 10.1046/j.1365-2915.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci USA. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Katz TM, Miller JH, Hebert AA. Insect repellents: historical perspectives and new developments. J Am Acad Dermatol. 2008;58:865–871. doi: 10.1016/j.jaad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D. Pesticides and pest control. In: Peshin R, Dhawan AK, editors. Integrated Pest Management: Innovation-Development Process. Springer; 2009. pp. 83–87. [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci U S A. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.