Abstract
Interleukin-6 is a definitive pleiotropic cytokine with varied systemic function. Implicated in a variety of disease states, interleukin-6 plays a major role in inflammatory processes. It modulates the transcription of several liver-specific genes during acute inflammatory states, particularly C-reactive protein (CRP), and controls the proliferation of normal plasmablastic cells. In addition, interleukin-6 has been implicated in hematopoiesis as a cofactor in stem cell amplification and differentiation, prompting the hypothesis that immunomodulatory therapy given postoperatively may be beneficial in cancer patients. This article is the first all-inclusive review of the decade-spanning clinical investigations of anti–interleukin-6 mAb in the treatment of cancer and related lymphoproliferative disorders.
The preliminary clinical evidence from six structured clinical trials of monoclonal antibodies to anti–interleukin-6 including BE8 and CNTO 328 in the treatment of cancer including multiple myeloma, renal cell carcinoma, and B-lymphoproliferative disorders have produced a number of interesting observations: in all cases, anti–interleukin-6 mAb treatment had a substantial impact on CRP levels, and in most instances levels were reduced to below detectable limits. Patients exhibited good tolerance and no toxic side effects were observed in the vast majority of studies. The therapeutic impact of anti–interleukin-6 mAb on cancer-related anorexia and cachexia may also be of clinical significance in a vast number of cancer patients.
Keywords: Antibodies, Monoclonal; chemistry; C-Reactive Protein; biosynthesis; Carcinoma, Renal Cell; therapy; Humans; Immunotherapy; methods; Interleukin-6; chemistry; physiology; Kidney Neoplasms; therapy; Lymphoma, B-Cell; therapy; Multiple Myeloma; therapy; Neoplasms; therapy
Keywords: Interleukin-6, monoclonal antibody, therapy for cancer
Introduction
Interleukin-6 (IL-6) is a definitive pleiotropic cytokine, a member of a family of proteins with varied systemic function.{Barton 1997 32109/id} Secreted by a number of cell types, IL-6 is present in elevated levels in association with active disease and synthesis of other cytokines stimulated by infection, trauma, and immunologic challenge.{Jones, Horiuchi, et al. 2001 36225/id} {Kishimoto, Tanaka, et al. 1995 36243/id} As testimony to the pleiotropic nature of IL-6, the cytokine was initially assigned a variety of names based on function, including interferon beta-2 (IFN-β2), IL-1 inducible 26-kD protein, hepatocyte-stimulating factor, cytotoxic T-cell differentiation factor, and B-cell stimulatory factor.{Kishimoto, Taga, et al. 1989 36241/id} As the various attendant physiologic effects of the molecule became associated with a common gene, the name interleukin-6 was proposed.{Poupart, Vandenabeele, et al. 1987 36238/id}
The physiologic activity of IL-6 is complex, producing both pro-inflammatory and anti-inflammatory effects in the immune system (Figure 1). Interleukin-6 promotes inflammation by contributing to the activation and proliferation of T cells, stimulating the differentiation of B cells, and inducing the acute-phase reactants of the hepatocyte population.{Jones, Horiuchi, et al. 2001 36225/id} In contrast, IL-6 also inhibits aspects of the inflammatory cascade. Both of the two primary inflammatory cytokines, tumor necrosis factor alpha (TNF-α) and IL-1, stimulate the production of prostaglandins, nitric oxide, and matrix metalloproteinases. Interleukin-6, on the other hand, does not promote the production of these inflammatory mediators, and it is hypothesized that IL-6 may play a role in regulating or turning off the in vivo synthesis of TNF-α and IL-1.{Barton 1997 32109/id} Despite these functions, IL-6 modulates the transcription of several liver-specific genes during acute inflammatory states, particularly C-reactive protein (CRP), and controls the proliferation of normal plasmablastic cells, as demonstrated in reactive plasmacytosis by using monoclonal antibody (mAb) directed against IL-6 {Gavarotti, Boccadoro, et al. 1985 38022/id}In addition, IL-6 has been shown to be an activator or an inhibitor of T-cell responses, depending on the target and the system used in vitro. This intricate interaction of pro-inflammatory and anti-inflammatory activities hints at the critical role IL-6 potentially plays in regulating the physiologic response to disease.
Figure 1.
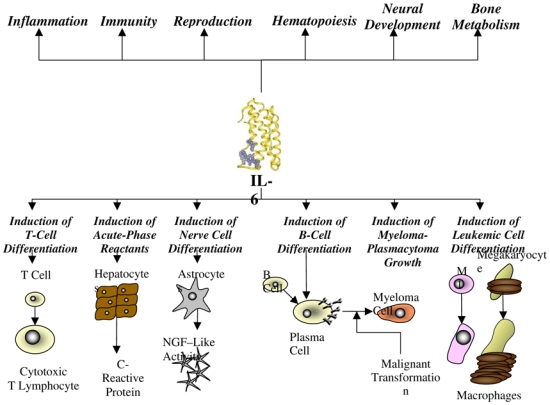
Physiologic activity of interleukin-6 (NGF, nerve growth factor).
Increased production of IL-6 has been implicated in a variety of disease processes, including neoplasia, Alzheimer’s disease, autoimmunity (e.g., rheumatoid arthritis), inflammation, myocardial infarction, aging, Paget’s disease, osteoporosis, neoplasia (renal cell carcinoma [RCC], prostatic and bladder cancers, certain neurologic cancers), B-cell malignancies (e.g., Castleman’s disease), some lymphoma subtypes, and, particularly, multiple myeloma (MM) {Keller, Wanagat, et al. 1996 36226/id}{Simpson, Hammacher, et al. 1997 35743/id}{Tupitsyn, Kadagidze, et al. 1998 36232/id}. In addition, IL-6 is implicated in proliferation pathways as a central proliferation factor or acting in cooperation with other factors, such as heparin-binding epithelial growth factor and hepatocyte growth factor (Oncogene 2002, 21:460; Cancer Res 2001, 61: 383; {Wang, De Vos, et al. 2002 38024/id}This reinforces the hypothesis that blocking IL-6 may have significant benefit in a large variety of pathologic situations. In the following discussion we review the role of IL-6 in the etiology and pathogenesis of cancer, as well as a comprehensive review of clinical trials of targeted cancer therapy using mAb to IL-6.
Interleukin-6/Interleukin-6 Receptor Interaction
Interleukin-6 is a multifunctional cytokine that binds to a specific IL-6 receptor (ɑ chain, IL-6R, or CD126) on target cells. This IL-6/IL6R complex associates with two molecules of the ubiquitously expressed gp130 (β chain, CD130), the second chain of the receptor, resulting in the formation of high-avidity IL-6 binding receptors {Kishimoto, Akira, et al. 1992 38003/id}; {Ward, Howlett, et al. 1994 38023/id}The gp130 functions as an affinity converter, since the resulting affinity of IL-6 for the ternary complex is around 10−11 M, instead of 10−9 M for IL-6R. Whereas gp80 binds specifically to IL-6, gp130 is a common signal-transducing receptor for a subfamily of cytokines, including IL-6, IL-11, leukemia-inhibiting factor (LIF), ciliary neurotrophic factor (CNTF), oncostatin M (OM), and cardiotropin 1 (CT-1), named the gp130 cytokine family. After binding to their specific receptors, all these cytokines induce homodimerization of gp130 or its heterodimerization with the LIF receptor (LIFR), which initiates cell signaling {Kishimoto, Akira, et al. 1992 38003/id}. In contrast with the wide distribution of gp130, gp80 is limited to hepatocytes and specialized subsets of leukocytes, including monocytes, neutrophils, T cells, and B cells (Jones et al 2001). Stimulation of gp130 is essential for hematopoiesis in vivo.
The system is complicated by the presence of soluble forms of both gp80 and gp130. These circulating compounds are cleaved from the cell membrane molecule or translated from an alternative spliced mRNA, yielding a protein that differs at its COOH-terminus by 14 amino-acid residues {Mullberg, Schooltink, et al. 1993 38008/id}{Horiuchi, Koyanagi, et al. 1994 38030/id}. Cleavage of transmembrane proteins can be done by a transmembrane metalloproteinase, distinct from matrix-type metalloproteases, that belongs to the family domains containing metalloproteinases (ADAM) {Wolfsberg & White 1996 37992/id}. Soluble (s) IL-6R or gp55 retains its capability to bind IL-6, and the complexes formed are able to activate the gp130 transducer receptor. Therefore, in contrast with other soluble cytokine receptors, which are generally antagonists, sIL-6R is an agonist molecule, promoting IL-6 activity. This capability may explain a possible activation of gp130 despite the lack of gp80, if sIL-6R molecules circulate in great quantity, as demonstrated in certain pathologic situations. Cells that do not express specific receptors for IL-6, IL-11, or CNTF are not able to respond to these cytokines. The presence of sIL-6R leads to responsiveness of these cells, and this process has been named transsignaling. Sera from healthy individuals contain sIL-6R (mean value, 89 ng/mL; range, 17–300 ng/mL). Serum sIL-6 was increased by 116% in overt MM (mean value, 193 ng/mL) {Gaillard, Bataille, et al. 1993 38029/id}. Soluble gp130 (sgp130) has been observed in human plasma and may bind soluble and membrane-anchored IL-6/IL-6R complexes, thus appearing as an endogenous IL-6 antagonist. In MM, the serum level of sgp130 is above 800 ng/mL.
Interleukin-6, A Major Cytokine Implicated in Self-Renewal and Differentiation of Early Progenitor Cells
Interleukin-6 plays a major role in inflammatory processes. In addition, IL-6 has been implicated in hematopoiesis as a cofactor in the amplification and differentiation of stem cells. Early hematopoietic stem cells express low levels of FLT-3 and c-kit receptors as well as gp130 receptor, but do not express IL-6R (Kollet O et al., Blood 1999, 94: 923). Therefore, IL-6/IL-6R complexes are efficient in amplifying and maintaining early progenitor cells as well as other cytokines, including SCF and FLT-3 or, to a lesser extent, IL-1. Primitive CD34-positive progenitors provide a soluble positive-feedback signal that induces cytokine production by stromal cells, including IL-6 (Gupta P, and al., Blood 1998, 91: 3724). Serum sIL-6R levels reflect proliferative kinetics of the stem cells after mobilization (Omura H, et al., Leuk Lymphoma 2002, 43: 623). The differentiation of various cells, including mast cells and cardiomyocytes, is also under the control of IL-6 and the gp130-family, in addition to other cytokines (Tsuruda T et al., Circ Res 2002, 90: 128).
Recently, it was shown that self-renewal of embryonic stem cells required sustained signaling by gp130 cytokines, particularly LIF and also IL-6, in a concentration-dependent manner, with thresholds in ligand-receptor signaling that modulate control of stem cell differentiation (Viswanathan S, et al., Stem Cells 2002, 20: 119).
On the other hand, IL-6/IL-6R complexes and the gp130 cytokine family were shown to be implicated in neural cell differentiation (Edoff K, Jerregard H J Neurosci Res 2002, 67: 255) and in osteoclast differentiation. The gp130 family and, particularly, LIF regulate osteoprogenitor differentiation in different models (Malaval L et al., J Bone Miner Res 1998, 13: 175).
Interleukin-6, Possible Role in Cancer
Comparable to the role of IL-6 in inflammation, inhibition and stimulation of cancer cell proliferation are also functions of this cytokine, depending on the cell type and the presence or absence of IL-6R.{Keller, Wanagat, et al. 1996 36226/id} In addition to modulating the antitumor activity of macrophages, IL-6 takes part in the production of lymphokine-activated killer (LAK) cells and protects neutrophils from apoptosis, increasing their cytotoxic effect on tumor cells. Also, through the stimulation of increased synthesis of CRP, IL-6 indirectly influences the binding of this protein to phospholipids on tumor cells, activating C1q of the complement system, which leads to tumor cell lysis in certain cases.
During the later stages of tumor growth, tumoral expression is associated with an increase in the levels of IL-1, IL-6, and acute-phase proteins. Table 1 lists investigations that define the role of IL-6 in a number of neoplastic diseases. In the majority of studies, active disease is associated with elevated serum levels of IL-6, which are related to disease severity and outcome. In many of these cancers, increased IL-6R expression was also detected,{Jones, Horiuchi, et al. 2001 36225/id} and a proliferative mechanism has been suggested.
Table 1.
Cancers Associated with Abnormal Interleukin-6 Production
| Cancer Type | Serum IL 6 Levels | Study Findings | Reference(s) |
|---|---|---|---|
| Breast cancer | ↑ | Median serum IL-6 level ~ 10 times higher in patients with metastatic disease than in those with localized disease | Benoy et al., 2002 |
| ↑ | Significantly higher serum IL-6 levels in patients with more than 1 metastatic site | Zhang and Adachi, 1999 | |
| ↑ | IL-6 and IL-8 levels significantly higher in patients with progressive disease | Yokoe et al., 1997 | |
| Cancer-related cachexia | … | Decrease in IL-6 serum levels associated with subjective improvement following therapy | Yamashita and Ogawa, 2000 |
| ↑ | High serum levels of IL-1, IL-6, and TNF-in advanced stage cancer patients, particularly those with cachexia | Mantovani et al., 1998 | |
| Gastrointes tinal cancer | ↑ | Serum IL-6 levels were elevated in advanced gastrointestinal cancer patients and correlated with overall survival | De Vita et al., 2001 |
| ↑ | Serum IL-6 levels indicative of tumor proliferative activity in colorectal cancer patients | Kinoshita et al., 1999 | |
| ↑ | High serum levels of IL-6 mark patients with cholangiocarcinoma and correlate with tumor burden | Goydos et al., 1998 | |
| ↑ | Serum levels of IL-1, IL-6, and TNF-elevated in patients with squamous cell carcinoma of the oral cavity | Jablonska et al., 1997 | |
| ↑ | Mean serum levels of IL-6 significantly higher in patients with gastric cancer | Wu et al., 1996 | |
| Leukemia | … | Serum levels of IL-6 in chronic lymphocytic leukemia patients not significantly different from that of control subjects, IL-6 levels in patients treated with cladribine significantly lower, epecially in patients who achieved remission | Robak et al., 1999 |
| Lymphoma | ↑ | Elevated serum IL-6 levels seen in 25% of indolent non-Hodgkin’s lymphomas, predictive of poor outcome | Fayad et al., 1998 |
| ↑ | IL-6 serum levels frequently elevated in patients with Hodgkin’s disease; these normalize with remission | Seymour et al., 1997 | |
| ↑ | Serum IL-6 levels elevated in patients with diffuse large cell lymphoma | Preti et al., 1997 | |
| Lung cancer | … | IL-6, IL-8, and TNF- found in higher concentrations in malignant pleural effusion than in serum | Alexandrakis et al., 2000 |
| ↑ | Serum IL-6 levels higher in patients with extensive small cell lung cancer than in patients with limited-stage disease | Dowlati et al., 1999 | |
| ↑ | Increased IL-6 level related to extensive disease, impaired performance status, and enhanced acute-phase response | Martin et al., 1999 | |
| ↑ | Mean IL-6 concentrations significantly higher in non–small cell lung cancer patients than in control subjects | De Vita et al., 1998 | |
| ↑ | Serum concentration of IL-6 significantly higher in mesothelioma than in lung adenocarcinoma | Nakano et al., 1998 | |
| Melanoma | ↑ | Significantly higher serum IL-6 and IL-12 levels observed in patients with localized and metastatic melanoma | Moretti et al., 2001 |
| ↑ | Baseline serum IL-6 level significantly higher in patients with metastatic malignant melanoma | Mouawad et al., 1996 | |
| Multiple myeloma | ↑ | Increased proportion of T cells producing IL-6 in MM patients with active disease | Frassanito et al., 2001 |
| ↑ | Significantly increased serum concentration of IL-6 in MM patients | Urbanska-Rys et al., 2000 | |
| ↑ | Serum levels of IL-6 significantly higher in MM patients, highest levels seen in patients with progressive disease | Wierzbowska et al., 1999 | |
| ↑ | Serum levels of IL-6 and of IL-6R significantly higher in patients with MM who died within 3 years than in those who survived | Pulkki et al., 1996 | |
| Ovarian cancer | ↑ | Median serum levels of IL-6 significantly elevated in ovarian cancer patients | Tempfer et al., 1997 |
| Pancreatic cancer | ↑ | Increased serum levels of IL-6 detected in 54.5% of pancreatic cancer patients; significantly among the patients with weight loss | Okada et al., 1998 |
| Prostate cancer | … | Serum IL-6 levels significantly correlated with clinical stage of prostate cancer | Nakashima et al., 2000 |
| ↑ | Serum levels of IL-4, IL-6, and IL-10 significantly elevated in hormone- refractory prostate cancer | Wise et al., 2000 | |
| ↑ | Levels of IL-6 and transforming growth factor (TGF) correlate with tumor burden and clinically evident metastases | Adler et al., 1999 | |
| ↑ | Serum IL-6 level significantly elevated in hormone-refractory prostate cancer | Drachenberg et al., 1999 | |
| ↑ | Serum levels of IL-6 related to the metastatic burden to osseous tissue in patients with prostate cancer | Akimoto et al., 1998 | |
| Renal cell cancer | ↑ | Serum IL-6 levels prior to surgery were significantly higher in renal cell cancer patients with short survival | Kallio et al., 2001 |
| ↑ | Levels of serum IL-6 and basic fibroblast growth factor were significantly higher in renal cell cancer patients with malignant cysts | Hayakawa et al., 1998 | |
| … | 56% of patients with metastatic renal cell carcinoma had detectable serum levels of IL-6 | Walther et al., 1998 | |
| ↑ | Serum IL-6 levels were significantly higher in renal cell cancer patients with paraneoplastic fever and weight loss | Blay et al., 1997 | |
| … | There was a significant difference in survival among renal cell carcinoma patients with detectable levels of IL-6 | Costes et al., 1997 | |
| ↑ | Survival time was significantly shorter for renal cell carcinoma patients with serum IL-6 levels above the median level for all patients studied | Ljungberg et al., 1997 | |
Interleukin-6 Is Implicated in Proliferation Pathways for B-Cell Malignancies, Particularly MM
The function of IL-6 in the pathogenesis of MM is well documented (Klein B Blood 1995, 85:863). Multiple myeloma is a plasma cell dyscrasia characterized by the malignant proliferation of bone marrow plasma cells. Interleukin-6 is the central factor of proliferation, even though few of the plasma cells may secrete IL-6, particularly when cells are cultured in vitro under specific conditions. The major potential mechanism of the proliferative expression of IL-6 in MM is attributed to a paracrine direct cell-to-cell interaction between MM cells and the bone marrow stromal cells, including cell-cell contact and also the production of diverse molecules by tumoral cells, such as IL-1 (Costes V et al Br J Haematol 1998, 103: 1152). A certain number of IL-6–dependent plasma cell lines have been generated, with some of them sensitive to other gp130 cytokine family members, depending on the presence of other receptors in this family and the action of IL-10 (Klein B et al., Leuk Lymphoma 1999, 34: 63, Gu ZJ et al., Blood 1996, 88: 3972). Serum IL-6 levels are correlated with prognosis in different B-cell malignancies, including chronic B-lymphocytic leukemia (Fayad L et al., Blood 2001, 97: 256) and lymphoma (Legouffe E et al., Leuk Lymphoma 1998, 31:351).
In addition, sIL-6 plays a role in the pathogenesis of the disease, via the formation of complexes with IL-6 producing a 10-fold increase in the sensitivity of human IL-6–dependent cell lines (Gaillard JP et al., Eur J Immunol 1993, 23: 820). The presence of high levels of sIL-6R in the serum of patients with MM, independent of tumor cell mass and status of the disease, suggests an important functional role of this circulating protein in the pathogenesis of monoclonal gammopathies (Gaillard JP 1993). Clinical investigations have shown that serum IL-6 and CRP levels are correlated, and are indicative of disease severity and progression (Bataille R Bocadoro Blood).
Interleukin-6 Is Also Implicated in Proliferation Pathways for Other Solid Cancers
The increased levels of IL-6 seen in progressive disease are also associated with other malignant conditions, including breast cancer and RCC (Table 1). Interleukin-6 is produced by some RCC cell lines and tumor cells in vivo (Takenawa J, et al., J Natl Cancer Inst 1991, 83: 1668–72; Walther McM J Urol 1998). Proliferative expression of IL-6 in some RCC cell lines was attributed to an autocrine mechanism (Miki S et al., FEBS Lett 1989, 250: 607–10), with stat3 activation and p53 modulation (Horiguchi A et al., Kidney Int 2002, Angelo LS et al., Cancer Res 2002). We demonstrate that IL-6R is present on tumor cells, in correlation with circulating IL-6 and disease aggressiveness (Costes V, et al., J Clin Pathol 1997). We and others have demonstrated that circulating IL-6 level correlated with serum CRP level and that both are prognostic factors, with a serum CRP level of 50 mg/L statistically discriminant (Blay JY Cancer Res 1992, Walther MM J Urol 1998; Ljungberg B et al., Eur J Cancer 1997).
Interleukin-6 Is a Cooperation Factor for Alternative Tumour Promoting Mechanisms
Recently, IL-6 was shown to be linked to drug-resistance mechanisms, including glutathione S-transferase (GST) through demonstration of the sensitization of human RCC cell lines to cisplatin by blocking IL-6 (Mizutani Y et al., Cancer Res 1995, 55: 590) or multidrug resistance (MDR) in breast cancer. In different conditions, including breast cancer, prostatic cancer, and MM, IL-6 amplifies the proliferation linked to epithelial growth factor (EGF)/ErbB molecules or hepatocyte growth factor (HGF) (Oncogene 2002, 21:460; Cancer Res 2001, 61:383; Wang YD et al., Oncogene 2002, 21: 2584).
Interleukin-6 Is Implicated in Paraneoplastic Syndrome
Cancer-related anorexia and cachexia are serious complications associated with these malignant conditions and affect as many as 87% of patients.{Loprinzi, Ellison, et al. 1990 36290/id} Although the etiology of cachexia is complex and multifactorial, IL-6 has been implicated in the progressive wasting, with most of the evidence that points to IL-6 as a cachectic agent having been obtained from animal studies.{Tisdale 1997 36231/id} In RCC as well as in other cancers, IL-6 and CRP serum levels are correlated with Stauffer’s syndrome, particularly neoplastic fever, body-weight loss, performance status (Blay JY Rossi JF et al. Int J Cancer 1997), depression (Musselmann Am J Psychiatr 2001, 158: 1252), anemia, leukocytosis, thrombocytosis, hypoalbuminemia, hypercalcemia, and other biologic symptoms.
The Role of Interleukin-6 in Renal Cell Carcinoma
Interleukin-6 is expressed by the majority of RCCs and is known to have an essential role in the proliferation of RCC cell lines.{Miki, Iwano, et al. 1989 36237/id}{Takenawa, Kaneko, et al. 1991 36289/id} The exact mechanism of enhanced production of IL-6 in renal cell tumors is unknown, but p53 mutations have been detected in up to 30% of primary kidney tumors and in the majority of metastatic tumors (70% to 80%).{Haitel, Wiener, et al. 2000 36278/id}{Oda, Nakatsuru, et al. 1995 36242/id}{Angelo, Talpaz, et al. 2002 36218/id}{Uhlman, Nguyen, et al. 1994 36390/id}{Reiter, Anglard, et al. 1993 36389/id} Findings were presented that confirmed that p53 mutations can result in overexpression of IL-6, and that wild-type (wt) p53 represses IL-6 expression by inhibiting transcription factor binding to the IL-6 promoter.
Analyzing sera and tissue samples from 38 patients with primary RCC, Costes and associates (1997){Costes, Liautard, et al. 1997 35780/id} found significant correlations between level of IL-6 and disease state. Serum IL-6 levels correlated with tumor size and stage. Tissue samples stained positive for IL-6R expression in 10 instances. The presence of IL-6R in tumors was significantly associated with tumor stage, nuclear grade, proliferation index, and serum level of IL-6. Study of this subgroup of patients with the most aggressive disease allowed the authors to identify patients in whom IL-6 and IL-6R played a critical role in tumor progression or proliferation and, thus, a group who may benefit from anti–IL-6 strategies.{Costes, Liautard, et al. 1997 35780/id} In a larger study that enrolled 122 patients with RCC, Yoshida and associates (2002){Yoshida, Ikemoto, et al. 2002 35741/id} confirmed serum levels of IL-6 in stage IV patients that were significantly higher than those in patients with disease of less severe grades or in the control group and concluded that serum IL-6 level and TNF-α may be a useful measure in the early diagnosis of RCC.
Metastatic RCC is also associated with a high incidence of paraneoplastic syndrome, a condition characterized by fever and elevated levels of acute-phase markers (e.g., CRP), a decrease in serum albumin, thrombocytosis, and anemia,{Papac & Poo-Hwu 1999 35742/id} which appears to be the consequence of abnormal cytokine production or immunogenic mechanisms. Blay and associates (1997){Blay, Rossi, et al. 1997 35779/id} measured serum levels of IL-6 in RCC patients with paraneoplastic fever and weight loss that were significantly higher than those in patients with RCC who did not have paraneoplastic symptoms, thus confirming the role of IL-6 in the syndrome. Three patients with paraneoplastic syndrome were enrolled in a 21-day, phase II trial of mAb to IL-6 therapy (murine B-E8, IgG1). All three patients who received mAb to IL-6 showed a reduction in levels of CRP, haptoglobin, and serum alkaline phosphatases during the 21-day test period. After therapy was stopped, the serum levels of these factors increased to pre-treatment levels or beyond,{Blay, Rossi, et al. 1997 35779/id} demonstrating the significance of IL-6 in paraneoplastic syndrome as well as the potential of targeted anti–IL-6 therapy.
Humoral hypercalcemia is a complication of malignancy related to paraneoplastic syndrome that is linked to the tumor’s production of substances that stimulate osteoclastic activity. Data from a study by Weissglas and colleagues (1995){Weissglas, Schamhart, et al. 1995 36234/id} indicate the possible role of IL-6 overexpression by RCC cells in hypercalcemia, most probably through its action on parathyroid hormone–related peptide, which highlights a potential supportive therapeutic role for anti–IL-6 treatment.
Another possible use of targeted anti–IL-6 therapy in RCC is as an adjuvant to cis-diamminedichloroplatinum (CDDP). Renal cell tumors are recalcitrant to CDDP therapy, and they could be a prime target of combination therapy. Mizutani and colleagues (1995){Mizutani, Bonavida, et al. 1995 33152/id} reported the effects of mAb to IL-6 and anti-IL-6R mAb on the sensitivity of human RCC cells to CDDP. In vitro anti–IL-6 or anti–IL-6R mAb enhanced the susceptibility of RCC cells to CDDP, implying clinical benefit with combined therapy.
Experimental and clinical findings supporting the role of IL-6 in active cancers provide a rationale for targeted therapeutic investigations. A variety of therapeutic agents have a mode of action that has an impact on IL-6 production. Known inhibitors of IL-6 include corticosteroids, nonsteroidal anti-inflammatory agents, estrogens, and cytokines, such as IL-4.{Lauta 2001 33087/id} Dexamethasone has also been shown to inhibit both IL-6 and IL-6R gene expression in myeloma cell lines. Targeted biologic therapies include toxic IL-6 and MAbs directed against IL-6 and IL-6R.{Brochier, Gaillard, et al. 1998 36221/id} {Lauta 2001 33087/id} Toxic IL-6 therapy is based on fusing the IL-6 gene to Pseudomonas exotoxin or diphtheria toxin genes, although the potential viability of this approach is questionable, as many normal cells express IL-6R, most notably hepatocytes.{Lauta 2001 33087/id} The remainder of this review will focus on the clinical experience with mAb therapy for cancer.
Clinical Experience with mAb to IL-6
The chronology of clinical investigations of mAb to IL-6 in the treatment of cancer and related lymphoproliferative disorders spans roughly a decade, beginning with the first report of a single patient with plasma cell leukemia (PCL) who was treated with mAb to IL-6 in 1991.{Klein, Wijdenes, et al. 1991 33100/id} The most recent effort was detailed in an article describing a phase I-II clinical trial that evaluated mAb therapy for B-lymphoproliferative disorder in recipients of organ transplants.{Haddad, Paczesny, et al. 2001 36223/id} The general characteristics of and clinical results from each of the investigations, in chronologic order, are summarized in Table 2. Initial investigations discussed below were conducted with mouse mAb to IL-6 (murine mAbs B-E4 and B-E8) and more recently, a humanized mouse mAb to Il-6 (human-mouse chimeric mAb to IL-6) with the investigational name CNTO 328 is current undergoing extensive clinical investigation for the treatment of MM, renal cell carcinoma and solid tumors. CNTO 328 contains the variable antigen-binding region of the murine anti-IL-6 antibody and the constant region of the human IgG1 kappa immunoglobulin {van Zaanen, Lokhorst, et al. 1998 32875/id}.
Table 2.
Chronology of Anti–interleukin-6 Monoclonal Therapy for Cancer
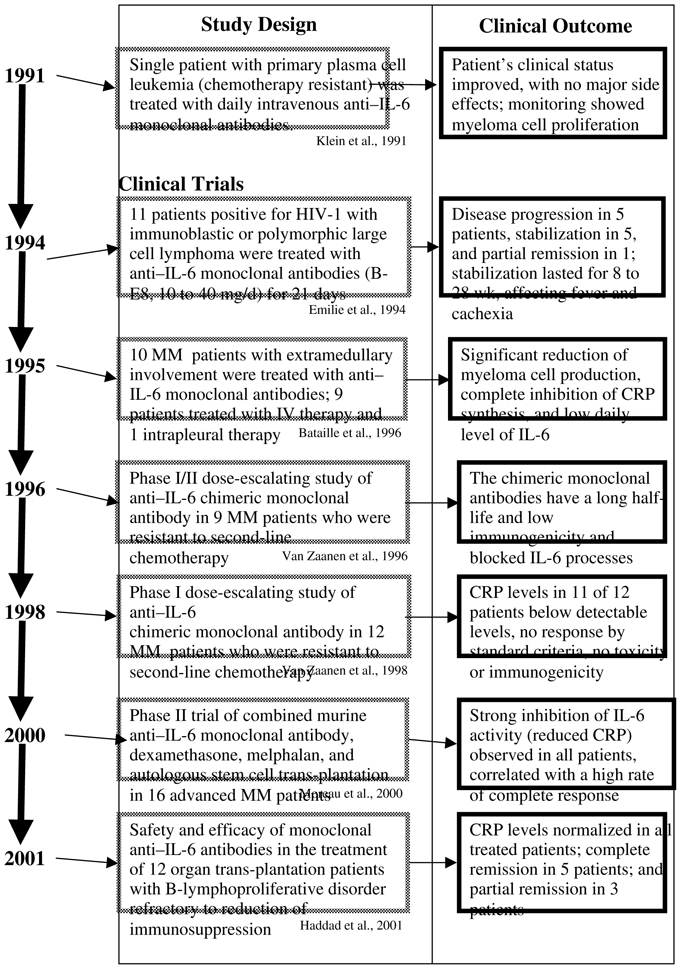 |
A patient with primary PCL that was recalcitrant to chemotherapy was the first reported recipient of mAb to IL-6 therapy.{Klein, Wijdenes, et al. 1991 33100/id} The patient was a 61-year-old male with primary PCL, bone lesions, hypercalcemia, renal deficiency, anemia, leukocytosis, bone marrow invasion by malignant plasma cells, and 25% myeloma cells in the peripheral blood. After informed consent was obtained, the patient received daily intravenous infusions of mAb to IL-6 (murine B-E4, and murine B-E8) in the following dosing regimen: days 0–5, 40 mg B-E4; day 6, 120 mg B-E4; days 7–10, 8 mg B-E8; days 11–14, 4 mg B-E8; days 15–16, 20 mg B-E4; days 17–23, no injection; days 24–59, 8 mg B-E8; days 60–63, no injection; days 64–67, 16 mg B-E8; and after day 68, no injection.
Clinical observations regarding treatment noted the blocking of myeloma cell proliferation in the bone marrow, along with a reduction in serum levels of calcium, monoclonal IgG, and CRP. Levels of CRP were reduced to undetectable, and overall no major side effects were noted. This study demonstrated the feasibility and potential of mAb to IL-6 therapy, resulting in a transient tumor cytostasis and reduction in toxicities from IL-6.{Klein, Wijdenes, et al. 1991 33100/id} This same patient developed a case of Escherichia coli sepsis during therapy, and serum levels of IL-6 in the form of monomeric complexes of IL-6/mAb to IL-6 remained very high for 20 days after sepsis, indicating the persistence of increased production of IL-6.{Lu, Brailly, et al. 1993 36228/id} This technique for measuring the IL-6/anti–IL-6 complex provides a means of estimating overall IL-6 production as well as a method for estimating the levels of mAb to IL-6 necessary for effective neutralization of IL-6.{Lu, Brailly, et al. 1993 36228/id}{Lu, Brailly, et al. 1995 33151/id}
Emilie (1994){Emilie, Wijdenes, et al. 1994 32969/id} also reported the results of an open, multicenter clinical trial of mAb to IL-6 (murine B-E8) for the treatment of patients positive for human immunodeficiency virus type 1 (HIV-1) who had immunoblastic or polymorphic large cell lymphoma.{Emilie, Wijdenes, et al. 1994 32969/id} Anti–IL-6 mAb (10–40 mg/day) was administered intravenously for a study period of 21 days. Clinical suppression of IL-6 activity was assessed by measurement of serum levels of CRP.
At total of 11 HIV-1–positive patients with lymphoma entered the study and were evaluated. Anti–IL-6 mAb therapy was observed to suppress the spontaneous growth of the lymphoma in 6 of the 9 patients, with detectable neutralization of endogenous IL-6. Five of these patients were classified as clinically stabilized, and one achieved partial remission.{Emilie, Wijdenes, et al. 1994 32969/id} For the patients with disease stabilization, follow-up assessments indicated that stabilization was sustained for 8 to 28 weeks. Overall, the antitumor activity was limited and inconsistent.{Emilie, Wijdenes, et al. 1994 32969/id}. Further, all patients developed an immune response to the murine mAb BE8 {Legouffe, Liautard, et al. 1994 36227/id} Side effects seen during therapy included consistent thrombocytopenia and, occasionally, decreases in neutrophil counts. The authors note that the most clear-cut effect associated with the BE8 therapy was its alleviation of the systemic symptoms, including fever, sweats, and cachexia. The authors conclude that in some cases IL-6–dependent growth of malignant lymphomas occurs, and that effective neutralization of endogenous IL-6 with targeted mAb therapy alleviates clinical symptoms.{Emilie, Wijdenes, et al. 1994 32969/id}
A subsequent study by Bataille and colleagues (1995){Bataille, Barlogie, et al. 1995 33014/id} reported the results of mAb to IL-6 therapy in the treatment of MM. A total of 9 of the 10 patients who had advanced and progressive MM with mainly primary or secondary PCL received intravenous mAb to IL-6 (B-E8) therapy (20 mg/day) for at least 4 days and for as long as 68 days. One patient with pleural effusion received local intrapleural administration of mAb-8, 20 mg/day for 3 days.
Three of the treated patients succumbed to the disease after less than 1 week of therapy, including the patient who received intrapleural treatment. Two of these patients with evaluable data showed marked inhibition of plasmablastic proliferation. The seven remaining patients received intravenous therapy for at least 1 week, with three exhibiting an objective antiproliferative effect as measured by myeloma cell labeling in the bone marrow, and one of these three patients showed a 30% regression of tumor mass. However, it is important to note that none of the patients in the study achieved remission or improvement as assessed by standard clinical criteria. Although the data were not reported, the authors noted that a beneficial effect of mAb to IL-6 therapy was the resolution of fever and hypercalcemia. The data suggest that mAb to IL-6 therapy could prove beneficial in patients with early-stage disease.{Bataille, Barlogie, et al. 1995 33014/id}
A phase I study of mAb to IL-6 therapy for MM has been presented by van Zaanen: reporting a dose-escalation analysis in patients with MM resistant to second-line chemotherapy.{van Zaanen, Koopmans, et al. 1996 36233/id}{van Zaanen, Lokhorst, et al. 1998 32875/id} The first report concerns the treatment of patients with end-stage, progressive MM, diagnosed according to the criteria of Durie and Salmon (1975).{Durie & Salmon 1975 36339/id} Nine patients entered the study and received human-mouse chimeric mAb to IL-6 (murine-human chimeric mAb [cMAb]) in two cycles of 14 daily intravenous infusions, starting on day 1 and day 28 of therapy. The dosing regimen was as follows: 5 mg/day in patients 1 to 3 (total dose, 140 mg); 10 mg/day in patients 4 to 6 (total dose, 280 mg); and 20 mg/day in patients 7 to 9 (total dose, 560 mg). This antibody is now known as CNTO 328.
Based on a one-compartment open pharmacokinetic model, the investigators were able to calculate the day-to-day endogenous production of IL-6 using the measured values of free IL-6 and the binding characteristics of the anti–IL-6 cMAb. The median half-life of the CNTO 328 was 17.6 days, and no human anti- CNTO 328 antibodies were produced in the treated patients.
Clinically, 8 of 9 patients had stabilization of their disease; however, clinical response, indicated by a decrease in levels of M protein of more than 50%, was not achieved by any of the patients treated. The significant clinical results from the study included the development of a methodology for calculating endogenous IL-6 production, and the finding that CNTO 328 therapy normalizes endogenous IL-6 production but does not have an impact on the IL-6 production associated with infection. These investigations suggest that CNTO 328 was able to block IL-6–dependent processes in vivo.{van Zaanen, Koopmans, et al. 1996 36233/id}
The second paper based on this dose-escalation study by van Zaanen and colleagues, was published in 1998 {van Zaanen, Lokhorst, et al. 1998 32875/id}. Twelve patients entered the study and received chimeric mAb to IL-6 (murine-human chimeric mAb) in two cycles of 14 daily intravenous infusions starting on day 1 and day 28 of therapy. The dosing regimen was as follows: 5 mg/day in patients 1 to 3 (total dose, 140 mg); 10 mg/day in patients 4 to 6 (total dose, 280 mg); 20 mg/day in patients 7 to 9 (total dose, 560 mg); and 40 mg/day in patients 10 to 12 (total dose, 1120 mg).
A total of 11 of 12 patients exhibited clinical stabilization of disease, and the twelfth patient with progressive disease responded to the second course of treatment. There were no toxic or allergic reactions to CNTO 328 therapy, although there was transient thrombocytopenia (2 patients) and granulocytopenia (6 patients). Although stabilization of disease was evident, no clinically significant response was seen, as none of the patients achieved a reduction in the level of M protein greater than 50%. As noted in the previous investigation, no immune response to anti–IL-6 cMAb occurred. Levels of CRP were also reduced to undetectable in 11 of 12 patients.{van Zaanen, Lokhorst, et al. 1998 32875/id}
The study concluded that there were no life-threatening side effects associated with anti–IL-6 cMAb therapy, and pharmacokinetic measurements indicated that the cMAb had a long half-life (17.8 days). Although none of the patients achieved remission, the authors hypothesized that the absence of a reduction in the level of M protein of more than 50% in treated patients who exhibited a profound reduction in CRP levels may be related to the presence of immature and mature myeloma cells. The immature myeloma cells are highly proliferative, whereas the mature cells have low levels of proliferative activity. The mature cells are also responsible for synthesis of M protein, implying that there is a population of IL-6–independent myeloma cells in end-stage MM{van Zaanen, Lokhorst, et al. 1998 32875/id} not targeted by mAb to IL-6.
More recently, Moreau and associates (2000){Moreau, Harousseau, et al. 2000 32876/id} investigated the potential of combination therapy including murine mAb to IL-6 (B-E8), dexamethasone (DXM), and high-dose melphalan (HDM220) 220 mg/m2, followed by autologous stem cell transplantation (ASCT), in the treatment of advanced MM. A total of 16 patients received treatment. Of these, at the time of enrollment, 2 were resistant to all chemotherapy and 14 patients had relapsed. A dose of 250 mg of B-E8 was infused over 4 days in combination with DXM 49 mg/day on days 1 to 4, followed by HDM220 infused over 30 minutes on day 5, and ASCT on day 7.{Moreau, Harousseau, et al. 2000 32876/id}
In general, IL-6 activity was strongly inhibited, as indicated by reduced CRP levels. Overall, 13 of 16 patients (81.2%) exhibited a response, with a complete response seen in 6 (37.5%). There were no toxic or allergic reactions reported, but the incidence of thrombocytopenia and neutropenia was increased.
This study, however, did not precisely determine the biologic parameters of the IL-6 blocking, particularly in terms of level and duration, a situation that could be associated with a possible and rapid regrowth of the disease. The treatment period associated with the major risk of triggering the proliferation by IL-6 is the period just after the autograft, because high levels of IL-6 could be produced during hematopoietic recovery.
In addition, tumoral cells are induced to apoptosis. If IL-6 is not controlled, apoptosis could be reversed with clonal selection in a period with immune deficiency. This period is also an optimal period for this strategy because of the synergism between melphalan and anti–IL-6. In a control group, we observed that CRP/IL-6 was enhanced after HDM administration, particularly during hematologic recovery. In other words, this strategy is a very promising if the blocking of IL-6 is total and lasting.
For all of these reasons, in a recent trial involving 34 patients with MM treated with melphalan 140 mg/m2 plus B-E8, a murine mAb, we carefully analyzed the biologic data by applying our mathematical model for calculating the daily IL-6 production and the efficacy of blocking, and we are waiting to provide longer follow-up data on this study (personal data, submitted to publication). As previously shown, the injection of mAb to IL-6 in a patient with MM induced the circulation of high amounts of IL-6 in the form of IL-6/anti–IL-6 monomeric complexes. Estimations of the daily production of IL-6 were given for some patients, including one who developed Eschericha coli sepsis during mAb to IL-6 treatment. The range of production may exceed 7 mg/day in this particular situation (Lu ZY et al., Cytokine 1993, 5:578).
In addition, we demonstrated that the use of a unique mAb to IL-6 in vivo was unable to efficiently block daily production of IL-6 at a level greater than 18μg/day (Lu ZY Blood 1995, 86: 3123). In this particular study, we observed that there was an inverse correlation between clinical response and daily production of IL-6 evaluated during the treatment if production exceeded this cut-off value. This confirms the importance of calculating this particular parameter for optimising anti–IL-6 dosing strategy. In addition, a certain number of patients presented delayed CRP/IL-6 production that was also associated with a lack of clinical efficacy. This means that efficient blocking of in vivo IL-6 production has to be complete and lasting. In addition, mAb to IL-6 had an effect on patient quality of life, as demonstrated by a reduction in mucositis episodes and in disease aggressiveness, a decrease in the number of red blood cell transfusions, with no difference in hematologic recovery and no increase in the infectious risk (personal data). However, in all the studies using mouse BE8, several problems were observed, including the necessity of having low daily IL-6 production, below 18 μg/day, and a shorter half-life when compared to the chimeric antibody CNTO 328. Therefore a chimeric antibody may permit chronic administration of mAb.
Alternative methods have been developed by using humanized anti–IL-6R mAb. One method is PM1, currently tested in Phase I-II trials in MM and in rheumatoid arthritis (Nishimoto N et al., Blood 2000, 95: 56; Yoshizaki K et al., Springer Sem Immunopathol 1998, 20: 247–259). Other methods include combinations of three anti–IL-6 or anti–IL-6R MAbs that shorten the half-life of the IL-6/IL-6R complexes (from 4 days to less than 20 minutes) in vivo, in addition to the formation of polymeric complexes instead of monomeric complexes, a situation compatible with increased clearance of these IL-6/IL-6R complexes (Klein B, Brailly H Immunol Today 1995, 16:216; Brochier J Current trends in Immunol 1998, 1:105; Brochier J et al., Eur J Immunol 2001, 31:259).
The most recent Phase I-II clinical trial that evaluated anti–IL-6 therapy investigated the treatment of B-lymphoproliferative disorder (BLPD).{Haddad, Paczesny, et al. 2001 36223/id} The open, multicenter trial examined the effect of murine mAb to IL-6 (B-E8) in 12 transplant recipients whose conditions were refractory to reduction of immunosuppression, in whom BLPD subsequently developed. A total of 5 of the 12 patients received a dose of 0.4 mg/kg per day, and the remaining 7 patients received 0.8 mg/kg per day for a scheduled treatment period of 15 days. Treatment was completed in 10 patients; therapy was discontinued in 2 patients, owing to disease progression. The patients tolerated treatment with no major side effects.
The study observed mAb to IL-6 therapy to be effective in 8 of the treated patients, with complete remission achieved by 5 patients 4 months after treatment was initiated. A partial remission was seen in 3 patients. At the time of the report, 7 patients were alive and well.{Haddad, Paczesny, et al. 2001 36223/id} This preliminary investigation suggests that mAb to IL-6 therapy is a potential option in the treatment of BLPD and should be explored further.
Anti-Interleukin-6 Monoclonal Antibodies in Metastatic Renal Cell Carcinoma
To our knowledge, there is only one study that used such a strategy in metastatic RCC, conducted by one of these authors and partly published (personal data and Blay et al. {Blay, Rossi, et al. 1997 35779/id}). Eighteen patients were included in this trial. Fourteen patients had progressive disease under IFN-α and/or IL-2 therapy, and 4 additional patients were not previously treated for their metastatic disease but had contraindications for immune therapy. The mAb was delivered at 20 mg/day for 21 consecutive days by the intravenous route. No toxicity was observed. All of the 18 patients had an increase in their performance status associated with analgesic effect, including 3 of 4 patients who stopped morphinic intake. In five patients who presented with specific fever, temperature normalization was correlated with the inhibition of CRP production. Conversely, in 1 patient with fever, temperature did not normalize, and this was associated with partial CRP inhibition. One patient who presented with hypercalcemia had a transient reduction in the serum calcium level. Two patients were inevaluable for response because the period of treatment was too short, being 4 and 6 days, respectively. A total of 3 of 16 patients presented a minor response (<50% reduction). All the patients were immunized, but this immunization did not affect the ability of the BE8 to block CRP production. All three of these patients had not been previously treated, and their mean CRP serum level was 24±11 mg/L, as opposed to the 5 patients with stable disease (mean CRP serum level, 42±47 mg/L), and the 8 patients with progressive disease (mean CRP serum level, 134±53 mg/L). Two additional patients presented a dissociated response, on the liver and lung. Maximal reductions of serum levels of acute-phase proteins were observed at day 7 for CRP (96±6% decrease), and at days 16, 17, and 19 for fibrin (55±8% decrease), haptoglobin (66±17% decrease), and orosomucoid (46±12% decrease), respectively. An increase in albumin level (+17%) was noted at day 20. White blood cells decreased by 34%, transiently at day 3. Four patients had an increase in hemoglobin level (+1.1±0.2 g/dayL). All the patients had a decrease in the platelet count by 45% at day 17, with normal bone marrow aspirates done in 3 patients. No changes in the levels of bleeding factors were observed, or in serum immunoglobulin level, and creatinine and liver enzymes. Slight decreases in IL-1 and TNF-α were observed in the serum or plasma from the 6 patients analyzed. No change in CD3, CD4, CD8, CD19, CD19DR+, CD56, CD3DR+, and CD14 positive cells were observed in peripheral blood using flow cytometry technique. Such therapeutic strategy may constitute a new way of treating RCC. It may be combined with standard immune therapy, as tested in 7 patients with combinations of IFN- and 1 patient with IL-2 and IFN-, demonstrating the reduction in toxicity and the maintenance of response for 1 previously untreated patient (JFR, personal data).
Daily Whole-Body Production of Interleukin-6
Recently, there have been developed cytokine-binding proteins (CBPs)—for example, monoclonal antibodies (MAbs) to cytokines or soluble cytokine receptors—in order to neutralize cytokine activity in vivo. A major difficulty in the evaluation of the efficacy of cytokine antagonists in vivo is the lack of data on whole-body production of a cytokine in normal and pathologic conditions. Indeed, such information is necessary for predicting the amount of CBP needed to neutralize the target cytokine in vivo. Two elements are of importance in such targeted therapy. First, in some pathologic circumstances, the quantity of the cytokine present in vivo may be largely beyond the blocking ability of the CBPs. Second, the half-life of IL-6, in the form of IL-6/anti–IL-6 complexes, was increased 200 fold {Lu, Brochier, et al. 1992 38028/id}. In order to predict the efficacy of anti–IL-6 treatments, whole-body IL-6 production has been estimated by our group (Lu ZY and al. Blood 1995, 86: 3123). The daily whole-body production of IL-6 was first estimated in 13 patients with MM or metastatic RCC who were treated with mAb to IL-6. Recently, this mathematical formula was applied to 34 additional patients with MM. As CRP production by hepatocytes is induced by the different cytokines that activate the gp130, the measurement of serum levels of CRP may serve as a pharmacodynamic marker of efficacy. Different parameters were also measured, including the diverse fractions of IL-6, free IL-6, and antibody bound IL-6 as well as the serum IL-6–like bioactivity, by using B9 hybridoma assay (Lu ZY et al. Blood 1995, 86: 3123). The calculation of daily IL-6 production was determined with a mathematical procedure developed and previously described in detail (Lu ZY Blood 1995). In brief, there are two forms of circulating mAb (free mAb and IL-6/mAb immune complexes) and one form of circulating IL-6 (IL-6/mAb immune complexes) in patients receiving mAb to IL-6 treatment. The half-life of IL-6/mAb immune complexes was similar to that of free mAb. Hence, IL-6 production on day “n,” which can be calculated as shown below:
In the formula above, M, [IL-6n], [IL-6n], [Mn], and [Mn-1] represent the daily amount of injected mAb, the IL-6 concentration on day n, the IL-6 concentration a day before day n, the mAb concentration on day n, and the mAb concentration one day before day n, respectively. The “b” was a coefficient concerning the half-life of mAb
In addition, a numerical model of mAb to IL-6’s ability to neutralize IL-6 binding to its high-affinity receptor was described and predicts the fraction of gp130 transducer activated by the IL-6/IL-6R or IL-6/sIL-6R complexes as a function of IL-6 and mAb to IL-6 concentrations. The affinities of the interactions of the different components were known and were integrated into the model and can be used to predict the efficacy of a treatment using mAb to IL-6 [(IL-6.IL-6R ~ IL-6 + IL-6R (kD = 500 pmol/L) and IL-6/IL-6R/gp130 ~ IL-6/IL-6R = gp130 (kD = 5 pmol/L; IL-6/sIL-6R ~ IL-6 + IL-6R (kD = 1 nmol/L) and IL-6/sIL-6R/sgp130 ~ IL-6/sIL-6R + sgp130 (kD = 10 nmol/L); IL-6/sIL-6R/gp130 ~ IL-6/sIL-6R + gp130 (kD =10 pmol/L)]. This mathematical model was tested in a group of patients with MM and metastatic RCC, thus confirming the validity of this mathematical modeling that predicted the range of efficacy of anti–IL-6 MAbs.
Conclusions
The experimental evidence linking IL-6 to the pathogenesis of a variety of cancers emphasizes the potential role of this cytokine in targeted biologic therapies. The evidence presented in this review indicates a complex role for IL-6 in a variety of cancer disease states. Interleukin-6 is implicated in proliferation pathways of B-cell malignancies especially MM, as well as a cooperation factor for tumor promotion, and in proliferation pathways for solid tumors. Evidence also supports a significant role for IL-6 in the etiology of renal cell carcinoma. The mAbs B-E8 and CNTO 328 have shown promising results in preliminary clinical trials for the treatment of B-lymphoproliferative disorders, plasma cell leukemia, lymphoma, multiple myeloma, and renal cell carcinoma, and warrant further investigation in these disease states.
The preliminary evidence from six structured clinical trials of mAb to IL-6 in the treatment of produced a number of interesting observations: in all cases, mAb to IL-6 therapy had a substantial impact on CRP levels, and in most instances levels were reduced to below detectable limits. Patients exhibited good tolerance, with no toxic side effects, and immunogenic responses toward anti–IL-6 were not observed in the vast majority of studies. The advanced cancer stages examined in the trials reviewed and the substantial impact of mAb to IL-6 on IL-6–mediated activity indicate that there may be significant potential for mAb to IL-6 in the treatment of cancers at an earlier stage. The therapeutic impact of mAb to IL-6 on paraneoplastic syndromes including cancer-related anorexia and cachexia may also be of clinical significance in a vast number of cancer patients with malignant disease.


