Abstract
The locus coeruleus (LC) lies in the dorsal pons and supplies noradrenergic (NA) input to many regions of the brain, including respiratory control areas. The LC may provide tonic input for basal respiratory drive and is involved in central chemosensitivity since focal acidosis of the region stimulates ventilation and ablation reduces CO2-induced increased ventilation. The output of LC is modulated by both serotonergic and glutamatergic inputs. A large percentage of LC neurons are intrinsically activated by hypercapnia. This percentage and the magnitude of their response are highest in young neonates and decrease dramatically after postnatal day P10. The cellular bases for intrinsic chemosensitivity of LC neurons are comprised of multiple factors, primary among them being reduced extracellular and intracellular pH, which inhibit inwardly rectifying and voltage-gated K+ channels, and activate L-type Ca2+ channels. Activation of KCa channels in LC neurons may limit their ultimate response to hypercapnia. Finally, the LC mediates central chemosensitivity and contains pH-sensitive neurons in amphibians, suggesting that the LC has a long-standing phylogenetic role in respiratory control.
Keywords: rodent, amphibian, respiration, chemosensitive signaling, serotonin, glutamate, K channel, hypercapnia, development
1. Introduction
Locus coeruleus (LC) is a well-delineated cluster of noradrenergic neurons located bilaterally adjacent to the fourth ventricle in the pontine region of the brainstem (Dahlström and Fuxe, 1964). It is estimated that ~50% of all the noradrenergic projections in the central nervous system originate in the LC which are directed toward the forebrain, cerebellum, brainstem and spinal cord (Aston-Jones et al. 1995; Berridge and Waterhouse, 2003). The LC exhibits arousal-state-dependent activity and it is considered a major wakefulness promoting nucleus with activation of the LC resulting in an increase in EEG and signs of alertness (Samuels and Szabadi, 2008)
The LC is implicated in the control of many homeostatic functions including maintenance of attention, motivation, arousal states (Svensson and Thorén, 1979; Bhaskaran and Freed, 1988), sleep (Aston-Jones and Bloom, 1981), circadian regulation of arousal and performance (Aston-Jones et al., 2001), cognitive behaviors (for review see Sara, 2009), fever response (Almeida et al., 2004), control of breathing (Oyamada et al. 1998; Fabris et al., 1999; Hilaire et al. 2004; Putnam et al., 2004; Viemari et al. 2004, Biancardi et al., 2008; de Souza-Moreno et al., 2010), and cardiovascular function (Sved and Felsten, 1987). This nucleus is involved in some pathophysiological states as well, such as panic disorder (Charney et al., 1990; Kaplan, 1992; Sullivan et al., 1999; Bailey et al., 2003; Griez and Schruers, 2003) and Rett syndrome (RTT) (Taneja et al., 2009).
Regarding the regulation of breathing, LC neurons have been shown to be involved in the central respiratory network (Coates et al., 1993; Oyamada et al., 1998; Biancardi et al., 2008). Further, CO2/H+ sensitive neurons have been identified in the LC (Elam et al., 1981; Pineda and Aghajanian, 1997; Filosa et al., 2002). In fact, LC neurons have been extensively studied with respect to the bases for and mechanisms of chemosensitive signaling (Putnam et al., 2004).
In this review, we will discuss the evidence for involvement of LC in basal respiratory drive as well as central chemosensitivity. We will further consider studies of the basis for cellular CO2/H+ sensitivity in chemosensitive LC neurons. Finally, we will examine evidence that shows LC neurons play a role in the control of breathing in amphibians as well as in mammals.
2. LC neurons and basal respiratory drive
In mammals there are some studies demonstrating that LC neurons display a respiratory-related activity, i.e., they have direct access to information about the timing of the respiratory output from the medullary respiratory centers (Oyamada et al., 1998, 1999; Andrzejewski et al., 2001). Electrical and chemical stimulation applied to the LC attenuates the inspiratory inhibition caused by electrical stimulation at the Bötzinger Complex, suggesting that the LC also plays a role in the modulation of the inspiratory inhibition of Bötzinger Complex stimulation (Wang et al., 2004). LC neurons also exert a tonic inhibitory effect on IX respiratory activity in neonatal rat brainstem-spinal cord preparations via alpha2 adrenergic receptor indicating that LC regulates upper-airway expiratory activity as well (Yamanishi et al., 2008).
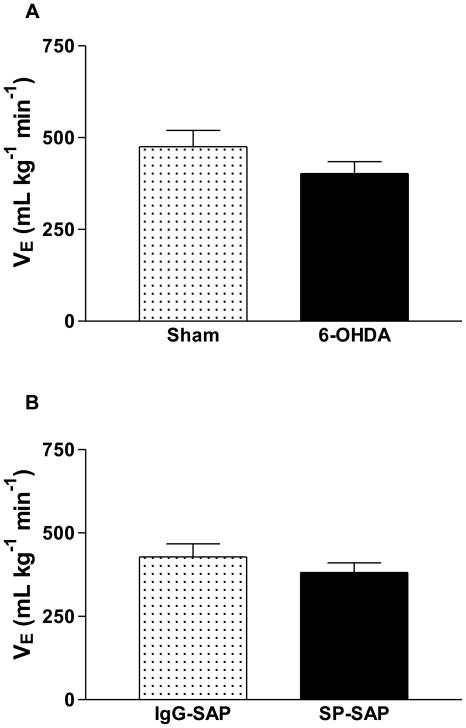
The Phox2a gene is responsible for differentiation of catecholaminergic neurons in restricted areas such as LC (Viemari et al., 2004). The inactivation of Phox2a leads to the agenesis of the LC proper, but leaves intact all the other noradrenergic centers: the locus subcoeruleus and groups A7, A5, A2, and A1 (Morin et al., 1997; Pattyn et al., 2000). Complete Phox2a inactivation produces depression of the central respiratory generator and elimination of LC and sensory afferent neurons (Wrobel et al., 2007). Elimination of the LC in Phox2a−/− mutants is linked to a severe decrease in the breathing frequency (Viemari et al. 2004). LC contributes to the adaptation of breathing to physiological needs and according to some studies it provides a tonic excitatory drive that contributes to a normal breathing rate in rats (Guyenet et al., 1993; Jodkowski et al., 1997; Oyamada et al., 1998; Dawid-Milner et al., 2001; Li and Nattie 2006) and mice (Shirasawa et al., 2000; Hilaire et al., 2004). In this context, Hilaire et al. (2004) demonstrated that LC noradrenergic neurons provide a tonic excitatory stimulus that maintains breathing frequency and are necessary for the development of a normal respiratory rhythm. Recently, Li and Nattie (2006) showed that substantial lesions of brainstem catecholaminergic neurons (including LC) slow breathing frequency during air breathing and that this effect is present in both wakefulness and in NREM sleep. Thus, taken together these data suggest that LC noradrenergic neurons provide a tonic drive to breathe. However, Biancardi et al. (2008) demonstrated that selective lesion of the LC in adult rats using 6-OHDA (a toxin that selectively eliminates catecholaminergic neurons) did not change basal ventilation and breathing frequency (Fig. 1A), suggesting that noradrenergic neurons located in the LC play no role in respiratory control under resting conditions in adults. Further, injection of a potent toxin conjugate, SP-SAP, in the LC of adult rats for killing neurons expressing the neurokinin-1 (NK-1) receptor did not alter adult breathing under basal conditions (Fig. 1B, Carvalho et al., in press). In agreement with this notion, LC unilateral cooling in neonatal sheep did not affect breathing in normoxic normocarbic conditions (Moore et al., 1996). The differences between these results and Li and Nattie’s study may be due to the fact the elimination of noradrenergic neurons also results in the loss of neurons not located in the LC, since DBH-SAP was injected via the 4th ventricle promoting elimination of other catecholaminergic groups such as A5, A7, C1 and C2. Another possible explanation could be that most of the previous cited studies were performed using anesthetized rats (Guyenet et al., 1993; Jodkowski et al., 1997; Dawid-Milner et al., 2001) or neonatal preparations (Oyamada et al., 1998; Shirasawa et al., 2000; Hilaire et al., 2004) whereas we used unanesthetized and adult animals. Whatever the explanation, in all of our lesion or microinjection studies using animals with manipulations specifically performed in the LC, no difference in basal ventilation has been observed (Biancardi et al., 2008; Biancardi et al., 2010; de Souza-Moreno, 2010; Carvalho et al., in press).
Figure 1.
A. Minute ventilation (VE) of the sham (n=8) and 6-OHDA rats (n=10) groups during normocapnia (adapted with permission from Biancardi et al., 2008). B. Minute ventilation (VE) of the IgG-SAP rats (control, n=9) and SP-SAP (n=8) groups during normocapnia (adapted with permission from Carvalho et al., in press). Values are means ± SEM. There is no difference between groups in both A and B.
3. LC and central chemosensitivity
3.1. Studies in intact animals
3.1.1. Lesion and stimulation studies
Some studies have demonstrated that the c-fos technique can be used to identify neurons involved in the responses elicited by hypercapnia (Haxhiu et al., 1996; Teppema et al., 1997; Berquin et al., 2000). Although neuronal function cannot be inferred from Fos expression, these studies brought new insight into the anatomical distribution of putative intrinsically chemosensitive neurons within chemoreflex pathways (Berquin et al., 2000). In mammals, studies under in vivo conditions showed that CO2 stimulation increases the expression of the c-Fos gene in LC neurons (Haxhiu et al., 1996; Teppema et al., 1997). In addition, extracellular recordings from LC neurons in both neonatal and adult rats showed that they respond to systemic hypercapnia with an increase in spike frequency under in vivo conditions (Elam et al., 1981). Elam et al. (1981) demonstrated a dose-dependent increase in firing frequency of LC neurons in response to hypercapnia (3%–20% CO2) under in vivo conditions, such that the response at ~7% CO2 would correspond to a ~25% increase in firing frequency. Additionally, using a brain slice preparation, Stunden et al. (2001) reported a ~44% increase in firing frequency of LC neurons when solution CO2 was increased from 5 to 10% and Filosa et al. (2002) saw a 93% increase in LC firing rate when solution CO2 was increased from 5 to 15% CO2. Thus, the firing response of LC neurons to hypercapnia appears to be dose dependent both in in vivo and in vitro preparations, although this response also appears to saturate at high levels (between 10–20%) of CO2 (Pineda and Aghajanian, 1997; Ritucci et al., 2005).
One of the first pieces of evidence that demonstrated that LC neurons may function directly as respiratory CO2/pH chemosensors was reported by Coates et al. (1993). In this study, the authors injected acetazolamide, which produces a small and focal acidosis, into various brainstem sites (Coates et al., 1993). Focal acidification of LC noradrenergic neurons promotes a large increase in phrenic nerve discharge (37%) in cats. The LC neurons are of particular interest in CO2 challenge since >80% of these neurons are found to be chemosensitive, responding to hypercapnia with an increased firing rate (Pineda and Aghajanian, 1997; Oyamada et al., 1998; Filosa et al., 2002). Recently, Johnson et al. (2008) using Prp57 transgenic mice, which express green fluorescent protein (GFP) in the LC, reported that LC neurons exhibited chemosensitivity in culture after pharmacological block of fast excitatory and inhibitory synaptic transmission. According to the authors, more than 85% of GFP-positive LC neurons are stimulated by elevated CO2/H+ in culture after pharmacological block of fast excitatory and inhibitory synaptic transmission.
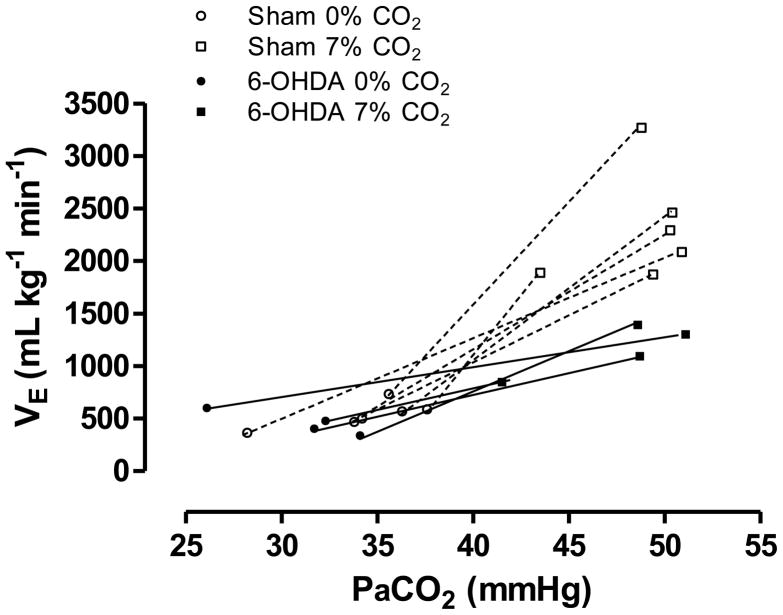
Lesions of catecholaminergic (CA) neurons of the rat brainstem, resulting in approximately 84% of LC-CA neurons being eliminated, significantly decreased the ventilatory response to 7% CO2, by 28% during sleep and wakefulness, suggesting that brainstem CA neurons participate in central chemoreception in vivo both in NREM sleep and wakefulness (Li and Nattie, 2006). These findings suggest that the LC is an important component of the hypercapnic ventilatory response. Recently, Biancardi et al. (2008) have investigated the participation of LC noradrenergic neurons in the CO2-induced drive to breathing. The data indicate that LC noradrenergic neurons modulate the hypercapnic ventilatory response since chemical lesion of this structure reduced the ventilatory response to 7% CO2, due to a decreased VT (Biancardi et al., 2008). A reduction of approximately 80% of the noradrenergic neurons of LC was associated with an approximately 64% decrease in the ventilatory effect on response to CO2, indicating once again that the LC exerts a profound the CO2-induced drive to breathe (Fig. 2). The 64% reduction in the ventilatory response after lesioning only the A6 region compared with the 28% reduction after lesions of noradrenergic (A5, A6, A7) and adrenergic (C1 and C3) neurons (Li and Nattie, 2006) could be due to: 1) differences in the percentage and the magnitude of the response of chemosensitive neurons from various brainstem areas (Putnam et al., 2004). It is well known that catecholaminergic cell groups have different brain projections that differentially modulate breathing. Some sites are excitatory to hypercapnic ventilatory response, while others are inhibitory. For instance, C1 adrenergic neurons provide a direct inhibitory input to the great majority of LC noradrenergic neurons (Aston-Jones et al., 1992); and 2) LC contains the highest percentage (>80%) of CO2-activated neurons of any brainstem region (cf. Putnam et al., 2004), thus it is expected that a lesion of this site should have a significant effect on the ventilatory response to inspired CO2, as observed by Biancardi et al. (2008).
Figure 2.
The relationship between pulmonary ventilation (VE) and CO2 arterial partial pressure (PaCO2) of the sham and 6-OHDA-lesioned groups exposed to normocapnia (0% CO2) and hypercapnia (7% CO2).
In summary, these data suggest that catecholaminergic neurons within the LC are involved in the central chemoreceptive response that mediates hyperventilation in response to inspired CO2 but may not play a significant role in the drive to breathe under normocapnic conditions (see Section 2), at least in adults.
3.1.2. Serotonergic modulation
A number of studies have suggested that 5-HT modulates the firing rate of LC noradrenergic neurons (Pickel et al., 1977; Cedarbaum and Aghajanian, 1978; Leger and Descarries, 1978; Haddjeri et al., 1997; Kaehler et al., 1999; Pudovkina et al., 2002; Kim et al., 2004) and that LC receives dense serotonergic projections coming mainly from dorsal raphé and pericoerulear 5-HT neurons (Aston-Jones et al., 1991; Kaehler et al., 1999; Kim et al., 2004). Indeed, the dorsal raphé (DR) nucleus exerts effects on LC activity through activation of 5-HT1A and 5-HT2A receptors (Svensson et al., 1975; Haddjeri et al., 1997).
In a recent study it was reported that inhibition of caudal medullary serotonergic neurons with 8-OH-DPAT had no significant effect on the inhibition of the raphé obscurus ventilatory response to CO2, but simultaneous and the retrotrapezoid nucleus (RTN) produced an enhanced hypoventilation and a greater reduction of the CO2 response (Li et al., 2006) suggesting that 5-HT neurons modulate breathing by interaction with RTN.
It is possible that 5-HT neurons modify LC output just as they do with RTN neurons. In fact, hypercapnia induced an increase in 5-hydroxyindole-3-acetic acid (5-HIAA) levels and serotonergic turnover (5-HTTIA/5-HT ratio) in the LC (de Souza Moreno et al., 2010). In this study, WAY-100635 (a 5-HT1A receptor antagonist), 8-OHDPAT (5-HT1A/7 agonist), Ketanserin (5-HT2A antagonist), or DOI (5-HT2A agonist) were microinjected into the LC of conscious, freely behaving rats to determine what 5-HT receptors were involved in the ventilatory response to CO2. Microinjection of WAY-100635 into the LC decreased the ventilatory response to CO2, probably through inhibition of NA neurons, while microinjection of Ketanserin increased the ventilatory response to hypercapnia due to an increase in VT, suggesting that 5-HT acting on 5-HT2 receptors in the LC exerts an inhibitory modulation on the ventilatory response to hypercapnia. Reinforcing this idea, it was shown that the 5-HT2A receptor agonist DOI microinjected in the LC attenuates the CO2 drive to breathing.
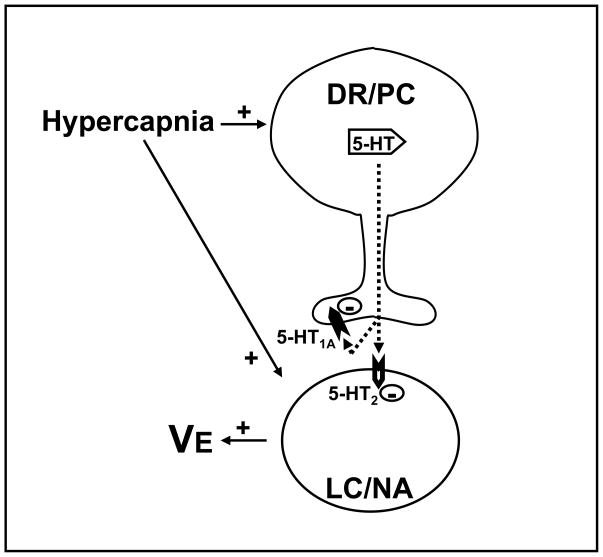
A possible mechanism through which serotonin (5-HT) modulates the role of the LC in the hypercapnia-induced hyperpnea is shown in Figure 3. According to this model, 5-HT release increases in the LC during hypercapnia and this 5-HT inhibits the LC stimulatory role in the hypercapnic ventilatory response, acting through postsynaptic 5-HT2A receptors in this nucleus. Furthermore, this 5-HT release seems also to be regulated by presynaptic 5-HT1A receptors, a mechanism that may play a role in preventing too great of an inhibition by serotonin of LC neurons during hypercapnia.
Figure 3.
Schematic diagram showing the proposed mechanism through which serotonin (5-HT) from dorsal raphe (DR) or pericoeruleus region (PC) modulates the LC role in the hypercapnia-induced hyperpnea. Hypercapnia induces an increase in the release of 5-HT in the LC, which acts on postsynaptic 5-HT2A receptors to inhibit noradrenergic (NA) neurons and may act on presynaptic 5-HT1A receptors to regulate its own release. Adapted from de Souza Moreno et al. (2010).
3.2. In vitro studies
Considerable progress has been made over the last 10–15 years in understanding the cellular pathways of CO2/H+ sensing in LC neurons. We now have a much clearer picture of the development of intrinsic chemosensitivity, the signaling pathways that activate LC neurons in response to elevated CO2/H+, and the ion channels that respond to these signals. These studies have been done using in vitro approaches with reduced preparations, almost exclusively involving pontine slices, although a recently described preparation with cultured LC neurons (Johnson et al., 2008) and a slice study using voltage-sensitive dyes (Erlichman et al., 2009), should prove to be quite useful for studying chemosensitive signaling. Below we will highlight the understanding that has been gained from the use of in vitro preparations.
3.2.1. Intrinsic chemosensitivity
In order to study the signaling of an LC neuron in response to increased CO2/H+ we need to assure that we are studying the intrinsic response of that neuron, and not the response of some other neuron within the slice that excites the neuron that we are studying through synaptic transmission, either chemical or electrical (gap junctions). There are several approaches to eliminating chemical and electrical synaptic activity and it is virtually impossible to assure, in all but isolated cultured cells, that there is no input from another neuron. However, one technique that has been commonly used (Dean et al., 1990; Richerson, 1995; Oyamada et al., 1998; Wellner-Kienitz and Shams, 1998) is to employ solutions with elevated Mg2+ and in some cases reduced Ca2+ as well. This blocks the entry of Ca2+ pre-synaptically and prevents neurotransmitter release as is shown by the disappearance of post synaptic potentials in the presence of this synaptic block medium (Nichols et al., 2009). This approach has been used in conjunction with carbenoxolone, a blocker of gap junctions to study intrinsic chemosensitivity in LC neurons as well as neurons from the nucleus of the solitary tract (NTS), another chemosensitive area (Dean et al., 2001; Conrad et al. 2009; Nichols et al., 2009). The use of blockers of both chemical and electrical synapses to study intrinsic chemosensitivity in neurons from the solitary tract (Conrad et al., 2009) has been described as “…one of the two most thorough analyses of intrinsic neuronal chemosensitivity that I know…” (Leiter, 2009), the other study being conducted on LC neurons (Hartzler et al., 2007). The results of the latter study will be discussed in the next section.
3.2.2. Development of intrinsic chemosensitivity
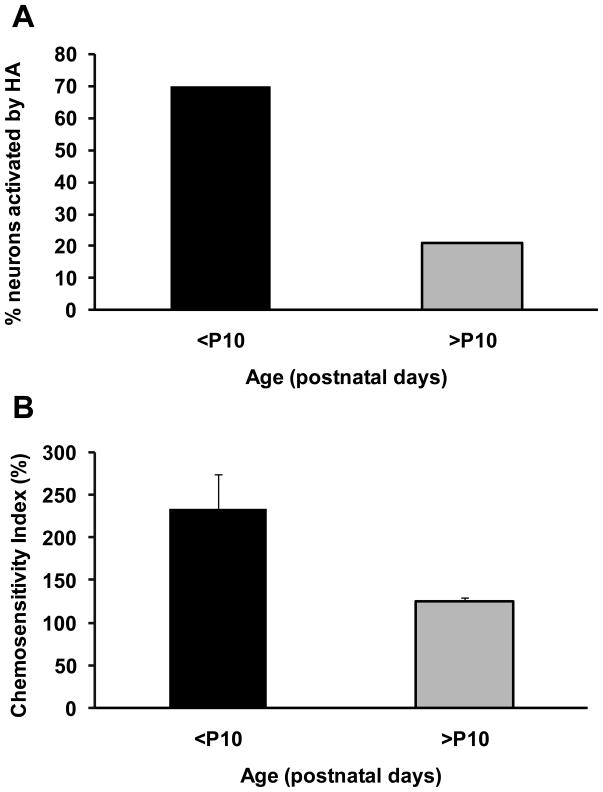
In an earlier study it was reported that the firing rate response of LC neurons to hypercapnia was essentially constant from the first postnatal day (P1) until near weaning (P16) (Stunden et al., 2001). These measurements were made without the use of either chemical synaptic blockade or gap junction blocker. Thus, in more recent studies these measurements have been repeated in the presence of synaptic block medium (elevated Mg2+, 11.4 mM, and reduced Ca2+, 0.2 mM) and carbenoxolone (100 μm) (Hartzler et al., 2007; Nichols et al., 2008). Interestingly, under these conditions there is a marked developmental change in the intrinsic chemosensitivity of LC neurons. In LC neurons from neonates younger than postnatal day P10, a high percentage (70–80%) of neurons were chemosensitive (Fig. 4A) and the magnitude of the response, as measured by the chemosensitivity index (CI; Wang and Richerson, 1999), was very high (about 235%) (Fig. 4B), similar to some of the most chemosensitive neurons measured in vitro from the medullary raphé (Wang et al., 1998). Neither the percentage of neurons nor the chemosensitivity index were affected by synaptic blockade medium or carbenoxolone in LC neurons from young neonates (<P10) indicating that these neuronal responses to hypercapnia were intrinsic (Hartzler et al., 2007; Nichols et al., 2008). In contrast, in LC neurons from older neonates (>P10) a high percentage of neurons were also activated by hypercapnia but the magnitude of the response was markedly lower (CI of 125%; CI of 100% indicates a nonchemosensitive neuron). As in younger neonates, synaptic blockade medium had very little effect on these values but, remarkably, carbenoxolone dramatically reduced the percentage of intrinsically chemosensitive neurons (around 20%) (Fig. 4A) without affecting the CI (Fig. 4B) (Hartzler et al., 2007; Nichols et al., 2008). These studies indicate that around 10 days after birth, LC neurons undergo a major reduction in chemosensitivity with the percentage of intrinsically chemosensitive neurons and the magnitude of their response both decreasing dramatically. In LC neurons from rats older than P10, gap junctions appear to play a significant role in the response to hypercapnia, with relatively few intrinsic chemosensitive neurons coupled to adjacent nonchemosensitive neurons, resulting in hypercapnia activating a larger proportion of LC neurons than are intrinsically sensitive.
Figure 4.
Intrinsic chemosensitive response to hypercapnic acidosis in LC neurons from the neonatal rat brainstem. Both high Mg2+/low Ca2+ and carbenoxolone were used to prevent synaptic communication between neurons. CS responses divided into two age classes: post-natal days P0-9 and P10-19, referenced as <P10 and >P10, respectively. N=23 for <P10, N=24 for >P10. A. Percent of neurons activated (increased firing rate) by HA (15% CO2). B. Chemosensitive index (relative magnitude of the chemosensitive response) for those neurons activated by HA. N=16 for <P10, N=5 for >P10. The height of the bars represents the means and the error bars represent one SEM.
This dramatic reduction in the CO2 sensitivity of catecholaminergic LC neurons during early development is reminiscent of a similar change in peripheral catecholaminergic cells, rat adrenal chromaffin cells (Muñoz-Cabello et al., 2005). In these cells, hypercapnia induced a marked release of catecholamines in over 60% of the cells from rats younger than P10. However, in adult rats (>P30) only 35% of the cells responded and those that responded had a substantially reduced release of catecholamines (Muñoz-Cabello et al., 2005). These data, together with those from LC neurons, suggest that in very young rats, hypercapnia will induce a substantial systemic catecholamine release as well as major activation of the main noradrenergic center in the brain. We suggest that this may serve as a simple form of ventilatory control in young neonates, increasing ventilation in response to hypercapnia due to catecholamine release both systemically and centrally. We would further suggest that during early development, this system weakens and is replaced by a more nuanced adult form of ventilatory control.
The possibility of a neonatal form of ventilatory control that is distinct from mature, fully developed respiratory control has been suggested previously (Stunden et al., 2001; Putnam et al., 2005) but is controversial (Davis et al., 2006). Stunden et al. (2001) reported a marked ventilatory increase to inspired CO2 that was most clear in very young rats (P0-3) but then waned during the first week of life, with an increase in the ventilatory response to inspired CO2 re-appearing after age P10. The authors suggested the ventilatory response in young neonates was due to “neonatal” central chemosensitivity while the response in older rats was due to an “adult” form of central chemosensitivity. During the transition period (around P7 to P10) there was a very low ventilatory response to inspired CO2. A similar response, although with a much smaller “neonatal” hypercapnic ventilatory increase in ventilation, was seen by Serra et al. (2001). Further suggesting a distinct neonatal response to hypercapnia, Wickström et al. (2002) showed in very young neonates (P2-4) that inspired CO2 resulted in a marked increase in respiratory frequency while in older neonates (>P8) this response was reduced and ventilation largely increased due to increased tidal volume. It was once again suggested that central chemosensitivity appears to show marked developmental changes during the first 10 days of life in rats. Finally, the presence of a neonatal form of chemosensitivity early after birth is suggested by the findings of embryonic (~E20) respiratory activity in rats whose frequency is reduced by decreased CO2 and whose activity is modulated by pontine adrenergic input, although this modulation is inhibitory (Di Pasquale et al., 1992). This activity is similar to activity in young neonates but these authors state that “…the fetal respiratory network might be affected by cell migration, differentiation and death; so the fetal network could be replaced during development by a different one…” (Di Pasquale et al., 1992). In fact, a shift in the respiratory network has been observed in rats between ages P0-1 and P2-4 (Oku et al., 2007). The idea of a shift in the respiratory network early in postnatal development is in essence what we are proposing here with respect to central chemosensitivity, with LC neurons playing a part in the early network (“neonatal” or “fetal”) and having a reduced role in the later network (“adult”).
There is contradicting evidence suggesting that there is very little hypercapnic ventilatory response in neonates before about P15 (Davis et al., 2006). It is not clear why the results of this study were so different from the early study of Stunden et al. (2001) with respect to the hypercapnic ventilatory response in young neonates. Davis et al. (2006) suggested the difference is due to the normalization of ventilation for body weight (see for example Fig. 9 in Stunden et al., 2001) since they found no hypercapnic ventilatory response in young neonates when increased ventilation in hypercapnia was expressed as a percentage of resting ventilation. This cannot be the explanation of the differences between the two studies, however, since Stunden et al. (2001) also expressed CO2-induced increased ventilation as a percentage of resting ventilation (see Fig. 10 in Stunden et al., 2001) and still saw a marked hypercapnic ventilatory response in young neonates (P1-P3). Whatever the explanation, the findings of Davis et al. (2006) indicate that it is possible that there is not a neonatal form of ventilatory control. If this is the case the high degree of CO2 responsiveness of LC neurons from young neonates (Fig. 4) might indicate that LC neurons play some other role in these young rats, such as stimulating arousal or serving as a suffocation alarm during periods of hypercapnia. These issues seem worth addressing more fully in future studies.
3.2.3. Chemosensitive signals
Progress has also been made understanding the cellular signals that allow a neuron to respond to hypercapnia. In theory, when a cell is exposed to hypercapnic acidosis (increased CO2 at constant HCO3 with an associated decrease in extracellular pH, pHo), there are many signals that could affect neuronal excitability and thus firing rate. These signals include the increase of CO2, the reduction of pHo, or decreased pHi. A first approach to differentiating the role of these various signals was studying the firing rate response of LC neurons to various acid challenges, some involving increased CO2 and others not and some with a decreased pHo and others not (Filosa et al., 2002). Using such an approach, it was shown that the parameter that best correlated with the increased firing rate was the change in pHi (Filosa et al., 2002) indicating that a change of intracellular pH plays a major role in chemosensitive signaling in LC neurons.
A problem with this approach is that all acid challenge solutions result in a fall of pHi so it is difficult to assess the necessity for a change of pHi in chemosensitive signaling. To address this issue, a technique was developed to study the firing rate response of LC neurons to hypercapnia while clamping pHi at a constant value (Hartzler et al., 2008). Using this technique, it was shown that hypercapnic acidosis still resulted in an increased firing rate even though pHi was clamped constant at its initial value (Hartzler et al., 2008). These results clearly indicate that while a change of pHi can lead to increased firing rate in response to hypercapnia in LC neurons, a change of pHi is not a necessary signal. However, when pHi was clamped at its initial value and LC neurons were exposed to isohydric hypercapnic conditions (increased solution CO2 and HCO3 such that pHo is maintained constant at its initial value) their firing rate did not increase (Hartzler and Putnam, unpublished observations). Thus, a decrease in either pHi or pHo, but not an increase of CO2, is capable of increasing the firing rate of LC neurons.
Another response of LC neurons to hypercapnia has been reported. LC neurons exhibit subthreshold rhythmic oscillations which are small oscillating changes in membrane potential believed to be mediated by opening of Ca2+ channels (Williams et al., 1984; Williams and Marshall, 1987; Oyamada et al., 1999). The interesting finding was made that the frequency of these oscillations are increased by hypercapnia (Filosa and Putnam, 2003), suggesting that acidosis stimulates these Ca2+ channels. This is contrary to the usual observation that Ca2+ channels are inhibited by acidosis (Tombaugh and Somjen, 1997; Shah et al., 2001). However, hypercapnia-induced activation of Ca2+ channels has been reported in peripheral chemosensitive cells from the carotid body (Summers et al., 2002). This activation of Ca2+ channels in carotid body cells appears to be due to activation of soluble adenylyl cyclase (sAC) during hypercapnia by increased intracellular HCO3− (Summers et al., 2002), a known potent activator of sAC (Zippin et al., 2001). Thus, hypercapnia not only appears to inhibit K+ channels in LC neurons but also to activate Ca2+ channels, in agreement with the proposed multiple factors model of chemosensitive signaling in LC neurons (Putnam et al., 2004).
The activation of Ca2+ channels by hypercapnia in LC neurons raises an intriguing possibility. The increased activity of Ca2+ channels in response to hypercapnia should increase intracellular Ca2+ and activate Ca-activated K+ (KCa) channels. These channels are known to be present in LC neurons (Williams et al., 1984). If these channels are activated during hypercapnic exposure, their activity could serve as a brake on the chemosensitive response of LC neurons. Such an effect has previously been suggested. Aghajanian et al. (1983) concluded that “…the influx of calcium into locus coeruleus neurons appears to serve a negative feedback function in the regulation of both spontaneous activity and reactivity to orthodromic stimulation”. This could in part explain why the firing rate response to hypercapnia plateaus at higher levels of CO2 (Pineda and Aghajanian, 1997; Ritucci et al., 2005). If KCa channels serve such a braking role on the chemosensitive response, inhibiting these channels should increase the firing rate in response to hypercapnia and could offer a novel approach to increasing the gain of central chemosensitivity.
3.2.4. Ion channel targets of chemosensitive signals
Just as the response of LC neurons to hypercapnia involves several signals (including changes of pHo, pHi, Ca2+ channel activity and Cai), emerging evidence suggests that there are several ion channel targets of these signals in chemosensitive LC neurons (for recent review see Putnam, 2010). Numerous channels have been suggested to be sensitive to changes of pH, especially K+ channels (Putnam et al., 2004). In fact, it has been proposed that multiple pH-sensitive channels with differing pH sensitivities could explain why neurons can be sensitive to pH changes over a broad range (Su et al., 2007).
The first channel to be shown to be involved in the response of LC neurons to hypercapnia was the inwardly rectifying K+ channel (Kir) (Pineda and Aghajanian, 1997). Although changes of pHi are known to directly affect Kir channel activity (Zhu et al., 1999; Xu et al., 2000), Pineda and Aghajanian (1997) reported an indirect effect in LC neurons. Decreased pHi is known to change the charge state of intracellular polyamines, and polyamines are known to inhibit Kir channel activity. Thus, hypercapnia-induced acidification results in Kir channel inhibition in LC neurons (Pineda and Aghajanian, 1997). Several Kir channel subunits are expressed in LC neurons, including Kir1.1, Kir 2.3, Kir4.1 and Kir5.1, the latter two being expressed most strongly (Wu et al., 2004). This is of interest since Kir4.1-5.1 make a heteromeric channel whose pK is in the physiological range (Xu et al., 2000), making these channels highly susceptible to changes of pH.
In accord with the multiple factors model, other K+ channels in LC neurons have recently also been shown to be inhibited by hypercapnia. An A current (rapidly activating and inactivating voltage-sensitive K+ channel) is inhibited by a decrease in pHo (and possible in part by decreased pHi) in LC neurons (Li and Putnam, 2009; Putnam, 2009; Putnam and Li, 2009). These findings are in agreement with studies showing that 4 aminopyridine, an inhibitor of the A current, significantly reduces, but does not eliminate, the firing rate response of LC neurons to hypercapnia (Martino and Putnam, 2007). It has further been shown that a sustained current that is slowly activated, very slowly inactivated and inhibited by tetraethyl ammonium (probably a delayed-rectifying K+ channel, Kdr) is also inhibited by hypercapnia in LC neurons (Li and Putnam, 2009; Putnam, 2009; Putnam and Li, 2009). Combined with the data discussed above on hypercapnia-activation of L-type Ca2+ channels, there is thus evidence for the activity of 4 different channels (Kir, A current, Kdr, and L-type Ca2+ channels) being affected by hypercapnia in LC neurons. There is likely a relationship between the activation of several signaling pathways and the alteration of the activity of several types of ion channels by hypercapnia in LC neurons.
3.2.5. Somatic vs. dendritic responses to hypercapnia
Neurons are not homogeneous cells. They contain several specialized domains including the axon, dendrites and soma. As we have gained increasing knowledge about the cellular signals and the ion channel targets involved in neuronal responses to hypercapnia, it becomes a significant question to know whether chemosensitivity is mediated largely by events on the dendrites, on the axons or both. This is especially imperative since pH responses to acid challenges differ in dendrites and soma (Schwiening and Willoughby, 2002; Willoughby and Schwiening, 2002) and the distribution of ion channels is often not symmetric in neurons (Korngreen and Sakmann, 2000; Riazanski et al., 2001). Based largely on anatomic data, a number of studies have suggested that chemosensitive signaling might be based on the dendrites in neurons from various chemosensitive regions. Chemosensitive neurons from the ventral medulla send dendritic projections to the ventral surface, suggesting that these dendrites are sampling the solution at the ventral surface (Kawai et al., 1996). A more dramatic example of this is shown by chemosensitive neurons from the retrotrapezoid nucleus, that send dendrites to the marginal layer at the ventral surface which run for several hundred microns along the ventral surface (Mulkey et al., 2004). A variation of these anatomical arguments has been offered for medullary raphé neurons. Bradley et al. (2002) have shown that chemosensitive serotonergic neurons have dendritic projections that closely approach large medullary arteries and have suggested that these neurons may be sensing blood CO2 (Severson et al., 2003).
We are aware of but a single study that directly addresses whether the response to hypercapnia in chemosensitive neurons resides on the soma, on the dendrites, or on both. Ritucci et al. (2005) studied the firing rate response of LC neurons in brainstem slices to hypercapnia applied locally by a superfusion pipette. When a large dendrite was exposed to the hypercapnic solution, there was no increase in neuronal firing rate. In contrast, when the soma (and proximal dendrites) were exposed to hypercapnic solution, LC neuron firing rate increased nearly as much as if the entire slice had been exposed to solution equilibrated with 15% CO2 (Ritucci et al., 2005). These findings suggest that in LC neurons, at least, the chemosensitive machinery resides within the soma and/or the most proximal portion of the dendrites. This appears to be at odds with the suggested distal chemosensitive signaling suggested by the anatomical studies discussed above. It may well be that chemosensitive neurons from different regions have a different cellular locus for the chemosensitive signaling machinery. Alternatively, as pointed out by Mulkey et al. (2004), “…Proximity to the ventral surface does not necessarily in itself confer CO2 sensitivity…” and it may be that chemosensitive neurons from all regions respond to hypercapnia predominantly based on signaling pathways and ion channels residing close to the soma.
4. LC and chemosensitivity in anuran amphibians
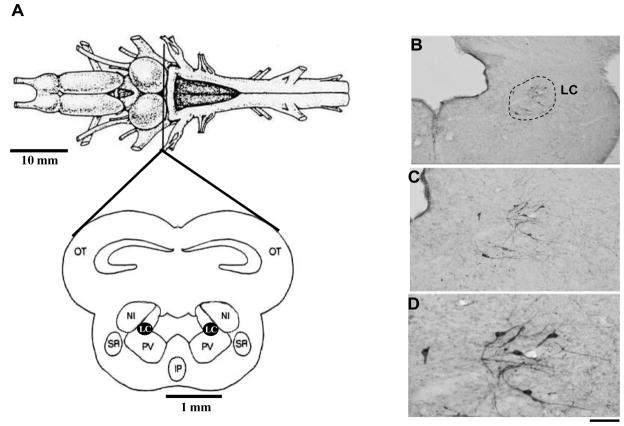
In anurans, González and Smeets (1991) distinguished a large tyrosine-hydroxylase (TH) immunoreactive, but dopamine negative, group of cells at the isthmus region (Fig. 5), which lies at the rostral end of the hindbrain. This isthmic cell group contains noradrenaline (González and Smeets, 1991, 1993) and innervates the spinal cord, cerebellum and telencephalon (Parent, 1975; González and Smeets, 1991; 1993). This area is considered to be homologous to the LC of mammals. This homology is based on its position, noradrenergic content, and projections to both the telencephalon and spinal cord (Marin et al., 1996). Since LC is important for the control of breathing and is considered to be a chemosensitive site in mammals, Noronha-de-Souza et al. (2006) investigated the participation of this nucleus in the control of breathing and central chemoreception of unanesthetized toads (Rhinella schneideri, formerly Bufo parcnemis). Initially, morphologic evidence was provided, i.e., the expression of c-fos in neurons of the LC after hypercarbic challenge. In this study, an increased inspired CO2 concentration (5% CO2) induced Fos-like immunoreactivity in the LC of toads, reinforcing the idea that the LC of amphibians is homologous to the LC of mammals.
Figure 5.
A. Dorsal view of the anuran brain, indicating the level of midbrain section illustrated below. Adapted from Gargaglioni and Branco (2009). B, C and D. Photomicrographs of the isthmus region showing the catecholaminergic cell bodies identified by tyrosine hydroxylase immunohistochemical staining of the brains. Detailed (higher magnification) morphology of these neurons is shown in B and C. Scale bars: 200 μm in A, 100 μm in B, and 50 μm in C. Adapted from Noronha-de-Souza et al. (2004). Abbreviations: Aq, Aqueduct of Sylvius; LC, Locus coeruleus; NI, nucleus isthmi; Otec, optic tectum. LC, locus coeruleus; IV, fourth ventricle.
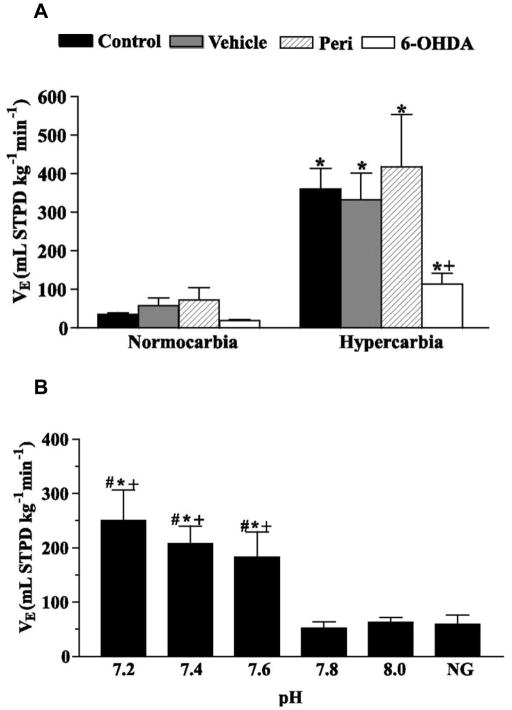
In addition, selective chemical lesion of LC catecholaminergic neurons was used to verify the possible involvement of this nucleus in the respiratory responses to CO2. Similar to mammals, LC chemical lesion with 6-OHDA decreased the hypercarbia-induced hyperventilation (Fig. 6A), largely due to a decreased tidal volume response, but did not affect ventilation under resting conditions (Noronha-de-Souza et al., 2006). This finding associated with the fact that the isthmic catecholaminergic cell group of amphibians (where LC is placed) does not contain dopaminergic or adrenergic cell bodies (González and Smeets, 1993), strongly suggests that noradrenergic LC neurons are involved in processing or modulating central chemoreceptor information in amphibians.
Figure 6.
A. Ventilation (VE) of the control, vehicle, peri, and 6-OHDA groups exposed to normocarbia and hypercarbia (5% CO2). * Significant effect of hypercarbia compared with the normocarbic value (paired t-test), + Significant differences between 6-OHDA and all other groups during hypercarbia (P < 0.05, one-way ANOVA). B. VE after microinjection of mock CSF of pH 7.2, 7.4, 7.6, 7.8 (control pH value), and 8.0. NG means VE before the injection (no injection group). * Significant difference from pH 7.8 group, # Significant difference from pH 8.0 group; + Significant difference from NG group (one-way ANOVA). Adapted from Noronha-de-Souza et al. (2004).
To test whether LC neurons are intrinsically pH-sensitive, focal acidification was performed by microinjecting mock CSF with different pH values (7.2, 7.4, 7.6, 7.8 and 8.0) into the area of the LC. Mock CSF perfusion is a well established method for studying the central chemoreceptor drive to breathe (Hitzig and Jackson, 1978; Branco et al., 1992). Interestingly, pulmonary ventilation increased after local reduction of the pH (mock CSF of 7.2, 7.4 and 7.6), which suggests that LC may be a chemosensitive site in the CNS of amphibians (Fig. 6B). Future research will be necessary to specify the stimulus or stimuli responsible for LC activation (intracellular pH, extracellular recordings. It will also pH, and/or molecular CO2) by using electrophysiological be interesting to investigate the role of LC in chemosensitivity during the development of amphibians. Given the functional homology of the LC between amphibians and rodents, we are currently examining the cellular level chemosensitive properties of LC neurons to determine whether this homology in function is derived from the same neuronal cellular signaling pathways.
5. Conclusions
We have discussed evidence that LC neurons are involved in central chemosensitivity in both mammals and amphibians and that this response is most likely mediated by CO2/H+-sensitive neurons within the LC. The development of chemosensitivity within the LC appears to be unusual, with a strong cellular response to CO2 during the first week of life which subsides after day P10. This pattern suggests an especially important role in central chemosensitivity for the LC in very early periods of development. LC continues to play a role in central chemosensitivity throughout development and into adulthood, but it may also play other roles, such as stimulating arousal, as part of the suffocation alarm system or as an agent in the initiation of panic disorders. A detailed study of the developmental role of LC neurons in central chemosensitivity appears warranted.
We have also reviewed evidence that the output of the LC can be modulated by various neurotransmitters. The role of the neurotransmitters serotonin and glutamate in the regulation of LC neuronal responses to CO2/H+ needs to be delineated in detail, especially with regard to the role such modulation may play in state-dependent changes of LC output.
A great deal has been learned about the multiple cellular signaling pathways and ion channel targets involved in the chemosensitive response of LC neurons to CO2/H+ but more needs to be learned. It is especially important to understand which ion channel(s) are involved in the response of LC neurons to various chemosensitive signals, how these pathways are modulated by different states of the organism and how the signals and ion channel targets change during development. Recent suggestions for an important role for Ca2+ channels and intracellular Ca2+ need to be studied fully, especially given the possibility that this pathway may lead to activation of KCa channels which could serve as a brake on the magnitude of the chemosensitive response of LC neurons. If this is the case, certain respiratory pathologies involving disordered breathing may arise from loss of this braking pathway.
Finally, given the similarities between LC responses in anurans and mammals, it is likely that the chemosensitive function of LC neurons is a basal trait of terrestrial vertebrates. These similarities in LC responses also further support the proposed functional homology of this nucleus in both groups. Detailed studies of the role played by the LC in the control of breathing and the cellular basis of CO2/H+-sensing in LC neurons from other vertebrates (especially amphibians) are needed to gain insight into whether or not chemosensitivity is highly conserved in the LC and other putative chemosensitive regions in the brainstem.
Acknowledgments
This work was supported in part by National Heart Lung and Blood Institute Grant (R01 HL56683) to RWP, a Ruth L. Kirschstein National Research Service Award (F32 HL080877) to LKH and by FAPESP and Instituto Nacional de Ciência e Tecnologia em Fisiologia Comparada (INCT-Fisiologia Comparada) to LHG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. doi: 10.1152/japplphysiol.00977.2004. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Vendermaelen CP, Andrade R. Intracellular studies on the role of calcium in regulating the activity and reactivity of locus coeruleus neurons in vivo. Brain Res. 1983;273:237–243. doi: 10.1016/0006-8993(83)90848-x. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Coimbra NC, Branco LGS. Thermoeffector neuronal pathways in fever: role of the locus coeruleus. J Physiol. 2004;558(1):283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andrzejewski M, Mückenhoff K, Scheid P, Ballantyne D. Synchronized rhythms in chemosensitive neurons of the locus coeruleus in the absence of chemical synaptic transmission. Respir Physiol. 2001;129(1–2):123–140. doi: 10.1016/s0034-5687(01)00300-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Astier B, Ennis M. Inhibition of noradrenergic locus coeruleus neurons by C1 adrenergic cells in the rostral ventral medulla. Neuroscience. 1992;48(2):371–381. doi: 10.1016/0306-4522(92)90497-p. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;8:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience. 1985;15:765–777. doi: 10.1016/0306-4522(85)90077-6. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego, CA: 1995. pp. 183–213. [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol. 2003;17(3):252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857(1–2):30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bhaskaran D, Freed CR. Changes in neurotransmitter turnover in locus coeruleus produced by changes in arterial blood pressure. Brain Res Bull. 1988;21(2):191–199. doi: 10.1016/0361-9230(88)90231-6. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurones and CO2 drive to breathing. Eur J Physiol. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Silva LT, Bicego KC, Gargaglioni LH. Role of Locus coeruleus noradrenergic neurons in cardiorespiratory and thermal control during hypoxia. Respir Physiol Neurobiol. 2010;170(2):150–156. doi: 10.1016/j.resp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nature Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Branco LGS, Glass ML, Hoffmann A. Central chemoreceptor drive to breathing in unanesthetized toads, Bufo paracmenis. Respir Physiol. 1992;87:195–204. doi: 10.1016/0034-5687(92)90059-6. [DOI] [PubMed] [Google Scholar]
- Brown CM, Mackinnon AC, Mcgrath JC, Spedding M, Kilpatrick AT. α2-Adrenoceptor subtypes and imidazoline-like binding sites in the rat brain. Br J Pharmacol. 1990;99:803–809. doi: 10.1111/j.1476-5381.1990.tb13010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho D, Bícego KC, Silva GF, Castro OW, Garcia-Cairasco N, Gargaglioni LH. Role of Neurokinin-1 expressing neurons in the Locus coeruleus on ventilatory and cardiovascular responses to hypercapnia. Respir Physiol Neurobiol. doi: 10.1016/j.resp.2010.04.016. In press. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178(1):1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Nagy LM, Southwick SM, Krystal JH, Heninger GR. Noradrenergic function in panic disorder. J Clin Psychiatry. 1990;51(Suppl A):5–11. [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. 5-Hydroxytryptamine2 agonist augments gamma-aminobutyric acid and excitatory amino acid inputs to noradrenergic locus coeruleus neurons. Neuroscience. 1993;54(2):409–420. doi: 10.1016/0306-4522(93)90262-e. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Resp Physiol Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curet O, de Montigny C. Electrophysiological characterization of adrenoceptors in the rat dorsal hippocampus. III. Evidence for the physiological role of terminal alpha 2-adrenergic autoreceptors. Brain Res. 1989;499:18–26. doi: 10.1016/0006-8993(89)91131-1. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Dawid-Milner M, Lara J, Gonzales-Baron S, Spyer K. Respiratory effects of stimulation of cell bodies of the A5 region in anesthetized rats. Pflügers Arch. 2001;441:434–443. doi: 10.1007/s004240000450. [DOI] [PubMed] [Google Scholar]
- de Souza Moreno V, Bícego KC, Szawka RE, Anselmo-Franci JA, Gargaglioni LH. Serotonergic mechanisms on breathing modulation in the rat locus coeruleus. Pflügers Arch. 2010;459(3):357–368. doi: 10.1007/s00424-009-0741-4. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Milhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:201–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol. 2001;129(1–2):83–100. doi: 10.1016/s0034-5687(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurones and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brainstem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol. 2009;102:1577–1590. doi: 10.1152/jn.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris G, Anselmo-Franci JA, Branco LG. Role of nitric oxide in hypoxia-induced hyperventilation and hypothermia: participation of the locus coeruleus. Braz J Med Biol Res. 1999;32(11):1389–1398. doi: 10.1590/s0100-879x1999001100009. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Amer J Physiol Cell Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin- releasing hormone and norepinephrine potentiation of inspiratory- related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJ. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol. 1991;303(3):457–477. doi: 10.1002/cne.903030311. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJ. Noradrenaline in the brain of the South African clawed frog Xenopus laevis: a study with antibodies against noradrenaline and dopamine-beta-hydroxylase. J Comp Neurol. 1993;331:363–374. doi: 10.1002/cne.903310306. [DOI] [PubMed] [Google Scholar]
- Griez E, Schruers K. Mechanisms of CO2 challenges. J, Psychopharmacol. 2003;17(3):267–268. doi: 10.1177/02698811030173003. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol Regul Integr Comp Physiol. 1993;264:1035–1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, de Montigny C, Blier P. Modulation of the firing activity of noradrenergic neurones in the rat locus coeruleus by the 5-hydroxtryptamine system. Br J Pharmacol. 1997;120:865–875. doi: 10.1038/sj.bjp.0700968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. Developmental changes in the chemosensitive response in locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr. 2007;33 Prog. No. 297.8. [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Biol Med. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol. 1996;105:35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generation by the pontine A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hitzig BM, Jackson DC. Central chemical control of ventilation in the unanesthetized turtle. Am J Physiol. 1978;235(5):R257–264. doi: 10.1152/ajpregu.1978.235.5.R257. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82:377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing Locus Coeruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol. 2008;105:1301–1311. doi: 10.1152/japplphysiol.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler ST, Singewald N, Philippu A. Dependence of serotonin release in the locus coeruleus on dorsal raphe neuronal activity. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(5):386–393. doi: 10.1007/pl00005365. [DOI] [PubMed] [Google Scholar]
- Kaplan GB. Neurobiological aspects of panic disorder. R I Med. 1992;75(5):247–251. [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026(1):56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;525:621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger L, Descarries L. Serotonin nerve terminals in the locus coeruleus of adult rat: a radioautographic study. Brain Res. 1978;145:1–13. doi: 10.1016/0006-8993(78)90791-6. [DOI] [PubMed] [Google Scholar]
- Leiter JC. Intrinsic chemosensitivity: How is it measured; what does it mean; and how does it help us understand the ventilatory response to CO2? Resp Physiol Neurobiol. 2009;166:13–15. doi: 10.1016/j.resp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie EE. Catecholamine neurons in rats modulate sleep, breathing, central chemoreception, and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K-Y, Putnam RW. Hypercapnia inhibits both transient and sustained potassium currents in chemosensitive neurons from neonatal rat locus coeruleus (LC) FASEB J. 2009;23 Program No. 621.5. [Google Scholar]
- Marin O, Smeets WJ, Gonzalez A. Do amphibians have a true locus coeruleus? Neuroreport. 1996;7:1447–1451. doi: 10.1097/00001756-199605310-00025. [DOI] [PubMed] [Google Scholar]
- Martino PF, Putnam RW. The effect of 4 aminopyridine (4AP) on the hypercapnic response of locus coeruleus (LC) neurons. Soc Neurosci Abstr. 2007;33 Program No. 297.7. [Google Scholar]
- Moore PJ, Ackland GL, Hanson MA. Unilateral cooling in the region of Locus coeruleus blocks the fall in respiratory output during hypoxia in anaesthetized neonatal sheep. Experimental Physiology. 1996;81:983–994. doi: 10.1113/expphysiol.1996.sp003998. [DOI] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Burnet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–423. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7(12):1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cabello AM, Toledo-Aral JJ, López-Barneo J, Echevarria M. Rat adrenal chromaffin cells are neonatal CO2 sensors. J Neurosci. 2005;25:6631–6640. doi: 10.1523/JNEUROSCI.1139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Biol Med. 2008;605:348–352. doi: 10.1007/978-0-387-73693-8_61. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex (SC) neurons from adult rats. Amer J Physiol Regul Integr Comp Physiol. 2009;296:R763–R773. doi: 10.1152/ajpregu.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-de-Souza CR, Bicego KC, Michel G, Glass ML, Branco LG, Gargaglioni LH. Locus coeruleus is a central chemoreceptive site in toads. Am J Physiol. 2006;291(4):997–1006. doi: 10.1152/ajpregu.00090.2006. [DOI] [PubMed] [Google Scholar]
- Oku Y, Massumiya H, Okada Y. Postnatal developmental changes in activation profiles of the respiratory neuronal network in the rat ventral medulla. J Physiol. 2007;585:175–186. doi: 10.1113/jphysiol.2007.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada Y, Andrzejewski M, Mückenhoff K, Scheid P, Ballantyne D. Locus coeruleus neurones in vitro: pH-sensitive oscillations of membrane potential in an electrically coupled network. Respir Physiol. 1999;118(2–3):131–147. doi: 10.1016/s0034-5687(99)00088-2. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A. The monoaminergic innervation of the telencephalon of the frog, Rana pipiens. Brain Res. 1975;99(1):35–47. doi: 10.1016/0006-8993(75)90606-x. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000;15(3):235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Reis DJ. A serotonergic innervation of noradrenergic neurons in nucleus locus coeruleus: demonstration by immunocytochemical localization of the transmitter specific enzymes tyrosine and tryptophan hydroxylase. Brain Res. 1977;131(2):197–121. doi: 10.1016/0006-8993(77)90515-7. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Cremers TI, Westerink BH. The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. Eur J Pharmacol. 2002;445(1–2):37–42. doi: 10.1016/s0014-2999(02)01663-1. [DOI] [PubMed] [Google Scholar]
- Putnam RW. Role of pH in cellular CO2 chemosensitivity. J Appl Physiol. 2010 In press. [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287(6):C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Li K-Y. Transient and sustained potassium currents are inhibited by hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr. 2009;35 Program No. 89.16. [Google Scholar]
- Riazanski V, Becker A, Chen J, Sochivko D, Lie A, Wiestler AD, Elger CE, Beck H. Functional and molecular analysis of transient voltage-dependent K+ currents in rat hippocampal granule cells. J Physiol. 2001;537:391–406. doi: 10.1111/j.1469-7793.2001.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol. 2005;289:C1094–C1104. doi: 10.1152/ajpcell.00329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization-included pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538.2:371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol. 2001;91:1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shah MJ, Meis S, Munsch T, Pape HC. Modulation by extracellular pH of low- and high voltage-activated calcium currents of rat thalamic relay neurons. J Neurophysiol. 2001;85:1051–1058. doi: 10.1152/jn.2001.85.3.1051. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth KA, Brown GA, Horning S, Arata S, Okumura K, Sasazuki T, Korsmeyer SJ. Rnx deficiency results in congenital central hypoventilation. Nat Genet. 2000;24(3):287–290. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Stupfel M. Carbon dioxide and temperature regulation of homeothermic mammals. In: Nahas G, Schaefer KE, editors. Carbon dioxide and metabolic regulations. Springer; New York: 1974. pp. 163–186. [Google Scholar]
- Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurones. J Physiol. 2007;578:831–841. doi: 10.1113/jphysiol.2006.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46(9):1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar N. CO2 and pH independently modulate L-type Ca2+ currents in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Sved AF, Felsten G. Stimulation of the locus coeruleus decreases arterial pressure. Brain Res. 1987;414(1):19–32. doi: 10.1016/0006-8993(87)91332-1. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Bunney BS, Aghajanian GK. Inhibition of both NA and 5-HT neurons in brain by the α-adrenergic agonist clonidine. Brain Res. 1975;92:291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Thorén P. Brain noradrenergic neurons in the locus coeruleus: inhibition by blood volume load through vagal afferents. Brain Res. 1979;172(1):174–178. doi: 10.1016/0006-8993(79)90908-9. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci. 2001;13(11):2077–2087. doi: 10.1046/j.0953-816x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effects of serotonin (5-hydroxytryptamine, 5-HT) reuptake inhibition plus 5-HT(2A) receptor antagonism on the firing activity of norepinephrine neurons. J Pharmacol Exp Ther. 2002;302(3):983–991. doi: 10.1124/jpet.102.033282. [DOI] [PubMed] [Google Scholar]
- Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of locus coeruleus neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29(39):12187–12195. doi: 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high and low-threshold Ca2+ currents in isolated rat CA1 neurons. J Neurophysiol. 1997;77:639–653. doi: 10.1152/jn.1997.77.2.639. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Bévengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Yu S, Zhang F, Li Y, Cao Y, Li Q, Song G, Zhang H. Modulation of inspiratory inhibition of the Bötzinger complex by raphe pallidus and locus coeruleus in rabbits. Adv Exp Med Biol. 2004;551:127–133. doi: 10.1007/0-387-27023-x_20. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JJ, Richerson GB. Chemosensitivity of rat medullary raphé neurones in primary tissue cultures. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Wang T, Chen L, Li Q. Noradrenergic lesion of the locus coeruleus increases the firing activity of the medial prefrontal cortex pyramidal neurons and the role of alpha2-adrenoceptors in normal and medial forebrain bundle lesioned rats. Brain Res. 2010;1324:64–74. doi: 10.1016/j.brainres.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Shams H. CO2-sensitive neurons in organotypic cultures of the fetal rat medulla. Respir Physiol. 1998;111:137–151. doi: 10.1016/s0034-5687(97)00124-2. [DOI] [PubMed] [Google Scholar]
- Wickström R, Hökfelt T, Lagercrantz H. Development of CO2-response in the early newborn period in rat. Respir Physiol Neurobiol. 2002;132:145–158. doi: 10.1016/s1569-9048(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Williams JT, North KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol. 2002;544.2:487–499. doi: 10.1113/jphysiol.2002.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel LJ, Ogier M, Chatonnet F, Autran S, Mézières V, Thoby-Brisson M, McLean H, Taeron C, Champagnat J. Abnormal inspiratory depth in Phox2a haploinsufficient mice. Neuroscience. 2007;145(1):384–392. doi: 10.1016/j.neuroscience.2006.11.055. [DOI] [PubMed] [Google Scholar]
- Wu J, Shen W, Jiang C. Expression and coexpression of CO2- sensitive Kir channels in brainstem neurons of rats. J Memb Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi T, Koizumi H, Komaki M, Ishihama K, Adachi T, Enomoto A, Takao K, Iida S, Kogo M. Possible involvement of neurons in locus coeruleus in inhibitory effect on glossopharyngeal expiratory activity in a neonatal rat brainstem-spinal cord preparation in vitro. Neurosci Res. 2008;60(1):2–9. doi: 10.1016/j.neures.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra and extracellular acidification on single channel Kir2.3 currents. J Physiol. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Levin LR, Buck J. CO2/HCO3-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab. 2001;12:366–370. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]