Abstract
Bacteria such as Staphylococcus aureus are successful as commensal organisms or pathogens in part because they adapt rapidly to selective pressures imparted by the human host. Mobile genetic elements (MGEs) play a central role in this adaptation process and are a means to transfer genetic information (DNA) among and within bacterial species. Importantly, MGEs encode putative virulence factors and molecules that confer resistance to antibiotics, including the gene that confers resistance to beta-lactam antibiotics in methicillin-resistant S. aureus (MRSA). Inasmuch as MRSA infections are a significant problem worldwide and continue to emerge in epidemic waves, there has been significant effort to improve diagnostic assays and to develop new antimicrobial agents for treatment of disease. Our understanding of S. aureus MGEs and the molecules they encode has played an important role toward these ends and has provided detailed insight into the evolution of antimicrobial resistance mechanisms and virulence.
Keywords: Mobile genetic elements, Staphylococcus aureus, Virulence, Antibiotic resistance, Horizontal gene transfer
Introduction
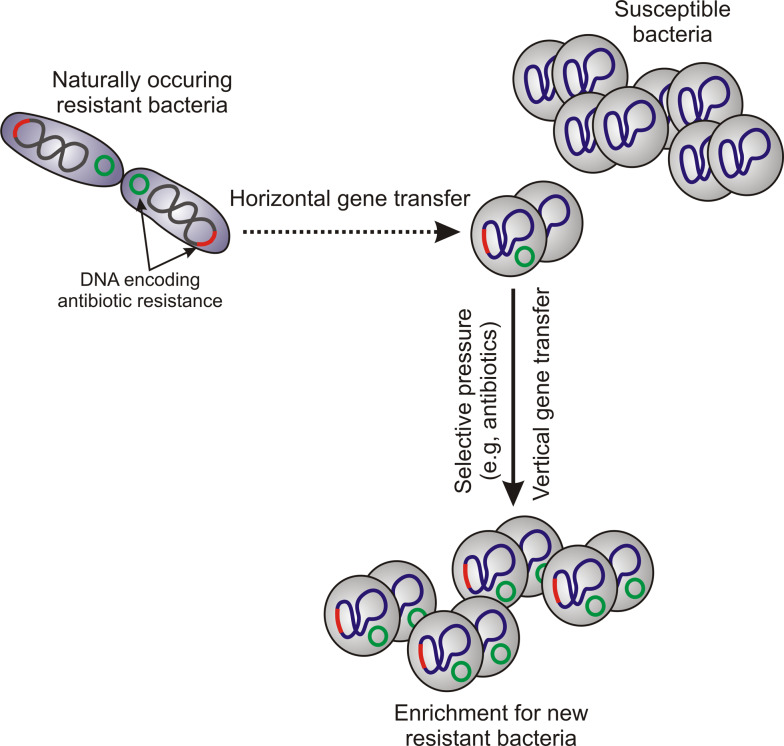
Mobile genetic elements (MGEs) were first described in the maize genome in the late 1940s [1, 2] and are an important means for transfer of genetic information among prokaryotes and eukaryotes. MGEs are typically identified as fragments of DNA that encode a variety of virulence and resistance determinants as well as the enzymes that mediate their own transfer and integration into new host DNA [3]. MGEs demonstrate intracellular and intercellular mobility, and those within one particular cell are called a “mobilome” [4]. Transfer of MGEs between cells is known as lateral or horizontal gene transfer (HGT). HGT occurs as prokaryote-to-prokaryote, prokaryote-to-eukaryote, and eukaryote-to-eukaryote transfer of DNA [5, 6] (Fig. 1). MGEs may consist of insertion sequences, transposons, phages, plasmids, pathogenicity islands, and chromosome cassettes. These segments of DNA are largely propagated by vertical gene transfer, which is transmission of genetic information from parent to progeny cell (Fig. 1).
Fig. 1.
Horizontal and vertical gene transfer
The bacterial genome consists of core and accessory genomes. The core genome contains all genes vital to cell survival, such as genes encoding molecules involved in metabolism, DNA and RNA synthesis, and replication. The accessory gene pool represents the diversity within bacterial species by encoding proteins required for adaptation of bacteria in different ecological niches (resistance, virulence factors, etc.). Accessory genes typically have a different G + C content than those in the core genome, often because they are obtained from other species of bacteria [7, 8]. Bacteria obtain genetic information from other cells or the surrounding environment in three ways: (1) uptake of free DNA from the environment (transformation), (2) bacteriophage transduction, and (3) direct contact between bacterial cells (conjugation).
In prokaryotes, transfer of genetic information between cells and among different species or genera is one of the main forces that generate “step change” or quantum leap evolution [7]. Extrachromosomal DNA elements such as MGEs play a crucial role in the plasticity of the genome, allowing bacteria to adjust readily to new environments. Selective pressure from the environment drives enrichment for specific genes that promote fitness and survival. An example of selective pressure is that imparted by use of antibiotics, which promotes development or acquisition of antibiotic resistance in bacteria. Inasmuch as S. aureus is notorious for acquiring resistance to antibiotics, some of which is encoded by MGEs, and also contains many putative virulence molecules on MGEs, it is an ideal model bacterium for the purpose of this review.
S. aureus MGEs
The genus Staphylococcus consists of Gram-positive bacteria that colonize human or animal skin and mucosal membranes. Although staphylococci are a part of normal human flora and thus commensal microorganisms, they are also opportunistic pathogens and cause a wide range of diseases. Among staphylococci, S. aureus is the most invasive species and an etiological agent of diverse human and animal maladies, including skin infections, abscesses, food poisoning, toxic shock syndrome, septicemia, endocarditis, and pneumonia [9–11]. S. aureus is one of the most prominent causes of nosocomial- and community-acquired bacterial infections worldwide [12]. Although the basis for this cadre of diseases is multifactorial and largely dependent on host susceptibility, heterogeneity of S. aureus strains likely plays a role in this process. Heterogeneity among S. aureus strains develops in part as a consequence of its interaction with the mammalian host. Numerous putative and proven virulence factors, genes responsible directly for host adaptation, and toxins, are located on S. aureus MGEs [8, 13–22]. S. aureus contains many types of MGEs, including plasmids, transposons (Tn), insertion sequences (IS), bacteriophages, pathogenicity islands, and staphylococcal cassette chromosomes (Figs. 2 and 3). It is remarkable that most genes encoded by MGEs remain under the control of global regulators located within the core genome.
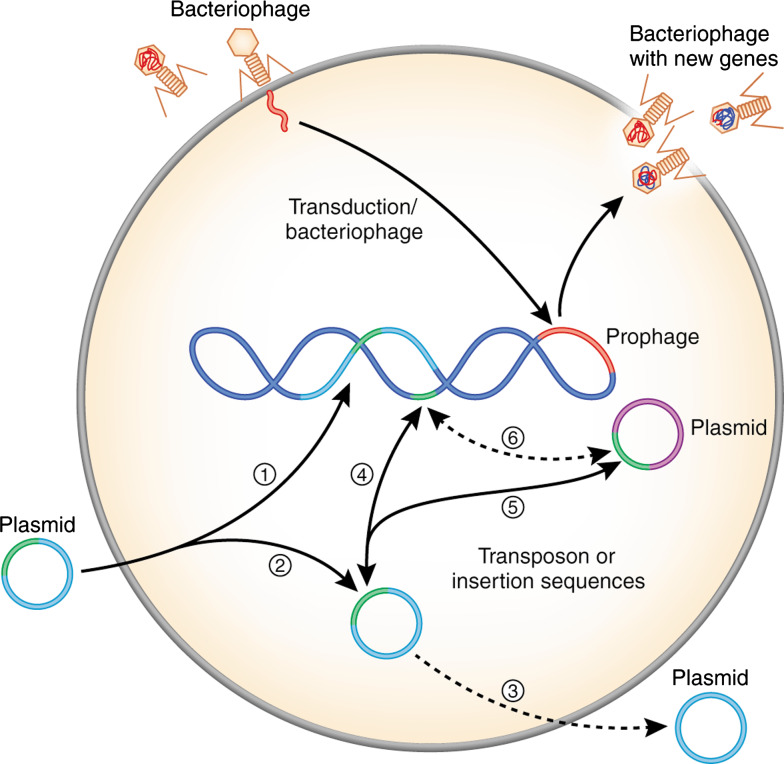
Fig. 2.
Acquisition of MGEs by S. aureus. 1 Incorporation of plasmids or plasmid elements into genomic DNA. 2 Plasmids can be maintained as free circular DNA. 3 Suicide plasmid. 4 Transfer of a transposon or an insertion sequence between plasmid and genomic DNA. 5 Transfer of a transposon or an insertion sequence between plasmids within the cell. 6 Transfer of a transposon or an insertion sequence from genomic DNA to another plasmid
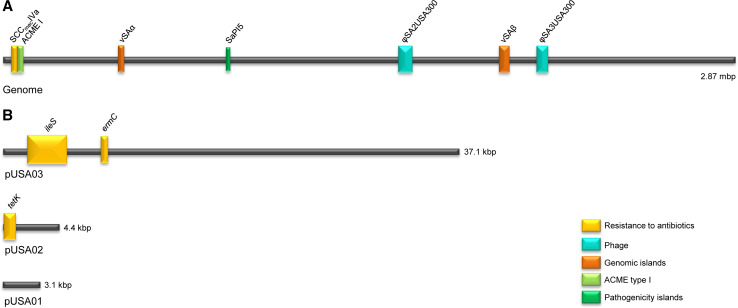
Fig. 3.
Linear schematic of the USA300 genome (strain FPR3757) and its major MGEs. a Genome. SCCmecIVa encodes methicillin resistance. νSAα encodes lpl, ssl and νSAβ encodes lukDE, spl, bsa. SaPI5 encodes seq2 and sek2, φSA2USA300 encodes lukS/F-PV, and φSA3USA300 encodes sak and chip. b Plasmids of FPR3757. pUSA03 contains genes encoding resistance to mupirocin (ileS) and MLSB (ermC). pUSA02 encodes resistance to tetracycline (tetK). pUSA01 is a cryptic plasmid
Plasmid-encoded antibiotic resistance
Plasmids are auto-replicating DNA molecules. Staphylococci typically carry one or more plasmids per cell and these plasmids have varied gene content. Staphylococcal plasmids can be classified into one of the three following groups: (1) small multicopy plasmids that are cryptic or carry a single resistance determinant; (2) larger (15–30 kb) low copy (4–6/cell) plasmids, which usually carry several resistance determinants; and (3) conjugative multiresistance plasmids [23]. Larger plasmids undergo theta replication (a DNA replication mechanism that resembles the Greek letter theta), whereas small plasmids usually replicate by the rolling-circle mechanism [24, 25]. As a consequence of the limited ability of S. aureus to acquire DNA from the environment (low natural competence) compared to bacteria such as Escherichia coli or Bacillus subtilis, most of the intercellular transfer of staphylococcal plasmids occurs by transduction or conjugation [26]. Upon entering the bacterial host, staphylococcal plasmids remain as free circularized DNA or linearize and integrate into the chromosome (Fig. 2).
Penicillin was the first antibiotic mass produced for use in humans. Although initially highly effective for treatment of S. aureus infections, today over 90% of human S. aureus strains are resistant to this antibiotic [27]. Penicillin resistance is conferred by β-lactamase, which hydrolyzes the β-lactam ring of penicillin thereby inactivating the antibiotic, and/or production of a low-affinity penicillin-binding protein (PBP2a) encoded by the mecA gene [12, 27, 28]. In S. aureus, β-lactamase is encoded by the blaZ gene and the closely linked regulatory genes, blaI and blaR [28]. Aside from plasmid encoded β-lactamase, bla genes may be located on transposons or within chromosomal DNA [27, 29].
More recently, S. aureus acquired vancomycin resistance elements from enterococci, resulting in the emergence of vancomycin-resistant S. aureus (VRSA) [30, 31]. Compared with vancomycin-intermediate S. aureus (VISA, MIC: 4–8 μg/ml), in which the mechanism of resistance is incompletely determined [32], high-level vancomycin resistance (that in VRSA) or VanA-mediated resistance is better characterized [30, 33, 34].
Tn1546 encodes the vancomycin resistance gene cluster within a conjugative plasmid. This MGE was most likely transferred to methicillin-resistant S. aureus (MRSA) from vancomycin-resistant enterococci (VRE) during co-infection [25, 30, 31, 35]. There are two predicted fates of the enterococcal plasmid upon entering staphylococci. On one hand, the enterococcal plasmid could simply be maintained, as occurred with strains VRSA-3, 5, and 6 [31, 36]. Alternatively, Tn1546 could be incorporated into a staphylococcal plasmid (VRSA-1, 7, 8, 9, and 10; plasmid pLW1043) in which case the original enterococcal plasmid functions as a suicide vector [31, 36]. Transposon Tn1546 encodes the vanA operon, which consists of vanA, vanH, vanX, vanS, vanR, vanY and vanZ [30, 38]. It is interesting that, for the second VRSA isolate reported in the US (VRSA-2), the van operon is located within a truncated Tn1546 on a 120-kbp plasmid, which is an unusually large plasmid for S. aureus [37]. vanA and vanH are responsible for synthesis of a D-Ala-D-Lac precursor that has much lower affinity to glycopeptide antibiotics than the original D-Ala-D-Ala. vanX encodes a dipeptidase that plays a role in the elimination of wild-type D-Ala-D-Ala targets by hydrolysis [39]. Expression of vancomycin resistance genes occurs only in the presence of vancomycin, a process mediated by a two-component signal transduction system encoded by vanS and vanR. vanY and vanZ encode an accessory protein that could play a role in teicoplanin resistance [34, 40].
In addition to genes encoding antibiotic resistance and molecules involved in metabolism, staphylococcal plasmids encode resistance to a variety of organic and inorganic ions, such as cadmium, mercury, arsenate, etc., which are highly toxic for living cells (Table 1) [41]. Staphylococcal plasmids may also encode toxin genes. For example, a large 37.5-kb S. aureus plasmid, pRW001, contains genes encoding exfoliative toxin B, bacteriocin, and bacteriocin immunity [42]. Staphylococcal exfoliative toxins (ETs) are associated with strains isolated from patients with staphylococcal scaled-skin syndrome (SSSS) or bullous impetigo [43–45]. ET isoforms A, B and D are serine proteases that specifically cleave host desmoglein 1, resulting in loss of cell–cell adhesion in the epidermal layer of skin, thereby causing blister formation and exfoliation [43, 46]. In addition to pRW001, genes encoding exfoliative toxins are located on phages (φETA, φETA2, and φETA3), a genomic island (νSAγ, former etdPI), and at least one other plasmid (pETB) (Table 2) [21, 42, 44, 45].
Table 1.
Resistance determinants encoded on non-SCCmec staphylococcal MGEs
| MGE | Resistance determinant | Antibiotic/heavy metal | Mechanism of action | Reference |
|---|---|---|---|---|
| Plasmid | aadD | Neomycin, kanamycin, paromomycin, and tobramycin | Aminoglycoside adenyltransferase | [100, 101] |
| ant 4 | Tobramycin | Aminoglycoside nucleotidyltransferase | [102] | |
| arsRBC | Arsenate, antimonite | Efflux ATPase | [21, 103, 104] | |
| blaZ, blaI, blaR1 | Penicillin (β-lactam antibiotics) | β-lactamase | [105, 106] | |
| ble | Bleomycin | Bleomycin-binding protein prevents DNA damage by binding bleomycin | [107, 108] | |
| cadA,B | Cadmium resistance and probably zinc | Cadmium efflux ATPase | [109, 110] | |
| cadD,X | Cadmium resistance | Efflux | [21, 111] | |
| cat | Chloramphenicol | Chloramphenicol acetyltransferase | [112, 113] | |
| cfr | Chloramphenicol, florfenicol, and clindamycin | Methylation of 23S subunit of bacterial ribosome | [114, 115] | |
| dfrA, dfrK | Trimethoprim | Dihydrofolate reductase | [101, 116] | |
| ermB,C | MLSB resistance (macrolides: erythromycin, lincosamides: clindamycin, streptogramin B) | Methylation of 23S subunit of bacterial ribosome | [117, 118] | |
| fusB | Fusidic acid | Ribosome protection mechanism | [119, 120] | |
| ileS-2 | High‐level resistance to mupirocin (pseudomonic acid A) | Isoleucyl RNA synthetase | [121, 122] | |
| mer operon | Mercury | Reduction of mercury ions to elementary Hg | [123] | |
| mphBM | Macrolide antibiotics | Putative phosphorylase | [124] | |
| msrA | Macrolide antibiotics | Active efflux | [124] | |
| mupA | High‐level mupirocin resistance | Novel isoleucyl RNA synthetase | [122, 125] | |
| qacA,B and smr (qacC/D) | Quaternary ammonium compounds, biocides | Drug efflux pump | [126–128] | |
| str | Streptomycin | Streptomycin adenyltransferase | [113] | |
| tetK, tetL | Tetracyclines | Active efflux of tetracycline | [129–131] | |
| vat | Streptogramins type A | Acetylation of the antibiotic | [132] | |
| vga | Streptogramins type A, lincosamides, and pleuromutilins | Efflux | [101] | |
| vgb | Streptogramins type B | Inactivation by virginiamycin B lyase | [133] | |
| Transposon | aacA-aphD | Gentamycin, kanamycin, tobramycin | Antibiotic modification by aminoglycoside acetyltransferase and aminoglycoside phosphotransferase | [82, 86, 90] |
| blaZ, blaI, blaR1 | β-Lactam antibiotics | Hydrolysis of β-lactam ring | [134] | |
| cadB, cadC | Cadmium resistance | Efflux | [135] | |
| ermA,B | MLSB resistance (macrolides: erythromycin, lincosamides: clindamycin, streptogramin B) | Methylation of 23S subunit of bacterial ribosome | [118] | |
| fexA | Florfenicol, chloramphenicol | Efflux | [114] | |
| merA, B | Respectively, inorganic and organic mercury resistance | Ion transport | [89, 136, 137] | |
| sat4 | Streptothricin | Streptothricin acetyltransferase | [115] | |
| spc(ant9) | Spectinomycin | Spectinomycin adenyltransferase | [102] | |
| tetM | Tetracycline, minocycline | Protection of ribosome binding site for tetracycline | [129, 131] | |
| vanRSHAXYZ a | Vancomycin | Production of low affinity pepdydoglican precursor with terminal D-Ala-D-Lac | [30, 31, 34, 35, 40] | |
| SCC476 | far1 | Fusidic acid resistance | [18] | |
| SCCmercury | mer operon | Mercury | Ion transport | [69] |
aVancomycin resistance is encoded on the Tn1546 transposon but transferred by conjugative plasmid
Table 2.
S. aureus virulence determinant encoded on MGEs
| Toxin/virulence determinant (gene) | MGE | Disease/mechanism of action | Reference |
|---|---|---|---|
| Adhesion protein Bap (bap) | SaPIbov2 | Specific adhesion to bovine mammary mucosa | [55] |
| Bacteriocin (bsa) | νSAβ | Bactericidal activity against other bacteria | [13] |
| Capsular polysaccharide protein | SCCcap1 | Inhibits phagocytosis | [75] |
| Chemotaxis inhibitory protein of S. aureus (chip) | φ13, φtp310-3, φN315, φ252B, φNM3, φMu3A, φSa3USA300, φSa3JH1, φSa3mw, φSa3 ms, φSa3JH9, φβC-USA300_TCH1516 | Blocks C5a and fMLP-induced neutrophil activation and chemotaxis; blocks C5a and formylated peptide receptor | [50, 138] |
| Epidermal cell differentiation inhibitor B (edin-B) | νSAγ (etdPI) | ADP-ribosyltransferase; inhibits morphological differentiation of keratinocytes in vitro and modifies eukaryotic Rho GTPase | [44] |
| Epidermal cell differentiation inhibitor C (edin-C) | pETB | ADP-ribosyltransferase, inhibits morphological differentiation of keratinocytes in vitro and modifies eukaryotic Rho GTPase | [45] |
| Exfoliative toxin A (eta) | φETA, φETA2, φETA3 | Causes staphylococcal scalded skin syndrome (SSSS), Ritter disease, and bulbous impetigo in neonates | [21, 44] |
| Exfoliative toxin B (etb) | pETB, pRW001 | Causes SSSS, Ritter disease, and bulbous impetigo in neonates | [42, 45] |
| Exfoliative toxin D (etd) | νSAγ (etdPI) | Causes SSSS, Ritter disease, and bulbous impetigo in neonates | [44, 45] |
| Enterotoxin A (sea) | φSa3 ms, φSa3, φSa3mw, φ252B,φNM3, φMu50A, | Super antigen (SAg), causes food poisoning | [13] |
| Enterotoxin B (seb) | SaPI1, SaPI3,pZA10 | SAg, causes food poisoning | [13, 139, 140] |
| Enterotoxin C (sec) | SaPIbov1 | SAg, causes food poisoning | [13, 141] |
| Enterotoxin C1 (sec1) | SaPI4, pZA10 | SAg, causes food poisoning | [13, 139] |
| Enterotoxin C3 (sec3) | SaPIn1/m1 | SAg, causes food poisoning | [13] |
| Enterotoxin C4 (sec4) | SaPImw2, SaPIm3 | SAg, causes food poisoning | [13] |
| Enterotoxin D (sed) | pIB485 | SAg, causes food poisoning | [142] |
| Enterotoxin G (seg) | φSa3, νSAβ (SaPIn3/m3) | SAg, causes food poisoning | [13] |
| Enterotoxin I (sei) | νSAβ (SaPIn3/m3) | SAg, causes food poisoning | [13] |
| Enterotoxin J (sej) | pIB485 | SAg, causes food poisoning | [143] |
| Enterotoxin K (sek) | φSa3 ms, φSa3mw, SaPIbov1, SaPI1, SaPI3, SaPI5 | SAg, causes food poisoning | [56, 144] |
| Enterotoxin K2 (sek2) | φSa3 | SAg, causes food poisoning | [145] |
| Enterotoxin L (sel) | SaPI1, SaPIbov1, SaPI3, SaPIn1/m1, SaPI4 | SAg, causes food poisoning | [54, 55, 144] |
| Enterotoxin L2 (sel2) | SaPImw2, SaPIm3, | SAg, causes food poisoning | [13] |
| Enterotoxin M (sem) | νSAβ (SaPIn3/m3) | SAg, causes food poisoning | [13] |
| Enterotoxin N (sen) | νSAβ (SaPIn3/m3) | SAg, causes food poisoning | [13, 146] |
| Enterotoxin O (seo) | νSAβ (SaPIn3/m3) | SAg, causes food poisoning | [13] |
| Enterotoxin P (sep) | φN315, φMu50A | SAg, causes food poisoning | [146, 147] |
| Enterotoxin Q (seq) | φSa3 ms, φSa3mw, SaPI1, SaPI3, SaPI5 | SAg, causes food poisoning | [56] |
| Ferrichrome operon (fhuD) | SaPI3, SaPIm4 | Iron up-take | [148] |
| α-hemolysin (hla) | νSAγ (etdPI) | Pore-forming cytolytic toxin | [149, 150] |
| Hyaluronate lyase (hysA) | νSAβ | Degradation of mucopolysaccharide hyaluronic acid | [13, 151] |
| Leukocidin (lukM, lukF) | φPV83 | Pore-forming leukocyte toxin | [152] |
| Leukotoxin D, E (lukD, lukE) | νSAβ | Pore-forming leukocyte toxin | [13, 153] |
| Lipoprotein-like (lpl) | νSAα | Induce inflammatory response of host immune system | [13, 65] |
| Lysophospholipase | pAvX (poultry strains) | Hypothetical role in virulence | [99] |
| Pantone-Valentine leukocidin (lukF-PV, lukS-PV) | φSa2mw, φPVL108, φSa2, φSa2USA300, φSLT, φPVL, φSLT-USA300_TCH1516, φtp310-1, φ2958PVL | Pore-forming leukocyte toxin, linked by epidemiology to necrotic infections | [154–158] |
| Pathogenicity island protein (ear) | SaPImw2; SaPI1, SaPI3, SaPI4, SaPI5 | Unknown | [54] |
| Phenol-soluble modulin located within SCCmec (psm-mec) | SCCmec | Pro-inflammatory and cytolytic activity | [159] |
| Phenol-soluble modulins (psmβ) | νSAγ (etdPI) | Possible pro-inflammatory activity | [16, 160, 161] |
| Plasmin‐sensitive surface protein (pls) | SCCmec I | Decreases the invasiveness of MRSA strains, acts as an adhesin | [162] |
| Serine protease-like protein (spl) | νSAβ (SaPIn3/m3) | Hypothetical role in virulence | [13, 163] |
| Staphopain A (scpA) | pAvX | Edematous and necrotic dermatitis in chickens | [99, 164] |
| Staphylococcal inhibitor of complement (scn) | φ13, φtp310-3, φN315, φSa3mw, φ252B, φNM3, φMu50A, φSa3JH1, φSa3 ms, φSa3JH9, φMu3A, φSa3USA300, φβC-USA300_TCH1516 | Inhibits phagocytosis of S. aureus by human neutrophils; blocks formation of C3b | [50, 165] |
| Staphylococcal superantigen-like, SSL (former, staphylococcal enterotoxin-like, set) | νSAα (SaPIn2/m2) | Targeting elements of innate immune response | [13, 166] |
| Staphylokinase (sak) | φN315, φMu50A, φSa2, φSa3mw, φ6390, φ13, φ252B, φNM3, φMu3A, φSa3 ms, φtp310-3, φβC-USA300_TCH1516, φSa3USA300 φSa3JH1, φSa3JH9, | Proteolytic destruction of host tissue; activates conversion of plasminogen to plasmin; inhibits opsonization by degradation of IgG and C3b, promotes resistance to defensins | [147, 167–169] |
| TSST-1 (tst) | SaPI1, SaPI2, SaPIbov1, SaPI3, SaPIn1/m1 | Causes toxic shock syndrome (TSS) | [46, 55, 170, 171] |
Genomic islands: νSAα, νSAβ, and νSAγ (etdPI)
Pathogenicity islands: SaPIbov1 and SaPIbov2, SaPI1- SaPI5, SaPIn1/m1, SaPIn3/m3, SaPImw2, SaPIm3, and SaPIm4
Phages: φ13, φtp310-3, φN315, φSa3, φSa3mw, φ252B, φNM3, φMu50A, φSa3JH1, φSa3 ms, φSa3JH9, φMu3A, φSa3USA300, φβC-USA300_TCH1516, φETA, φETA2, φETA3, φPV83, φPVL108, φSLT, φPVL, φSLT-USA300_TCH1516, φtp310-1, and φ2958PVL
Plasmids: pAvX, pIB485, pZA10, pETB, and pRW001
SCC Staphylococcal cassette chromosome
Bacteriophages and virulence
Bacteriophages (phages) or bacterial viruses seem to have the greatest impact on staphylococcal diversity and evolution. All phages are classified into one of three distinctive groups: lytic, temperate, and chronic. Lytic phages are members of the Myoviridae family that have been used in phage therapy, because bacteria lyse completely during release of progeny phages. Bacteria infected with chronic phages release progeny into the extracellular environment without killing the host, which allows bacteria to grow and divide. Temperate phages, which are members of the Siphoviridae family, form the most numerous group among all phages. Temperate phages have the ability to lyse bacteria after infection, but they typically form a long-term relationship with the host cell, whereby the phage DNA integrates into the staphylococcal genome as a prophage [47, 48]. Phages can impact expression of virulence determinants by either positive or negative lysogenic conversion. Following positive lysogenic conversion, bacteria express prophage-encoded virulence determinants. Negative lysogenic conversion occurs when there is insertional inactivation of genes (e.g., β-hemolysin of S. aureus) by integration of the phage DNA into the bacterial chromosome [47, 49]. Although there is loss of β-hemolysin during lysogeny, these prophages contain genes encoding immune-modulator proteins, such as staphylokinase (Sak), staphylococcal inhibitor of complement (SCIN), and chemotaxis inhibitory protein of S. aureus (CHIPS) [49, 50]. Other S. aureus prophages encode virulence molecules such as enterotoxins and Panton-Valentine leukocidin (PVL) (Table 2). PVL belongs to a group of bi-component, pore-forming cytolytic toxins that are specific for myeloid cells [51].
Prophages and prophage-encoded molecules also work in concert with other MGEs within staphylococci. For example, prophages create mobility for some staphylococcal pathogenicity islands. The most common example is the ability of helper phage 80α to mediate excision and transfer of SaPI1 to other staphylococci [52, 53]. Some phages also have the ability to transfer antibiotic resistance by transduction of plasmids or plasmid elements previously incorporated into chromosomal DNA. Plasmid pS194 with a chloramphenicol resistance determinant and pI258 containing erythromycin resistance are transduced by phages φ11 and φ11de, respectively [41].
Pathogenicity islands
Staphylococcal pathogenicity islands (SaPIs) are MGEs of 14–17 kb in size (Table 2). To date, at least 16 SaPIs have been sequenced and SaPI1 is considered as the prototype [53, 54]. SaPIs form a coherent family with highly conserved core genes [53, 55]. Core genes include two open reading frames encoding transcriptional regulatory proteins and a region encoding intergrase, Rep protein, and terminase. In addition to core genes, almost all SaPIs encode enterotoxins or toxic shock syndrome toxin (TSST) [56]. SaPIbov2 is an exception to this rule, and instead contains Bap adhesion protein, which plays a role in bovine chronic mastitis infections [57, 58].
Staphylococcal pathogenicity islands are integrated in one of six different specific sites on the chromosome (att s) and each is always in the same orientation [53]. SaPIs can be mobilized following infection by certain staphylococcal bacteriophages or by induction of endogenous prophages [59, 60], such as induced excision of SaPI1 by phage 80α [54]. Several hypotheses to explain the origin and evolution of SaPIs exist [56]. For example, Yarwood et al. [56] proposed the existence of a common ancestral genetic element—probably a prophage—for all SaPIs that then generated diversity of islands through modular recombination events.
Genomic islands
Three families of genomic islands exist among the S.aureus strains whose genomes have been sequenced [8, 13, 16, 61]. These genomic islands, named νSAα, νSAβ, and νSAγ (Table 2), are flanked by a broken transposase gene upstream and partial restriction-modification system (RM) type I downstream. Given the composition of genomic islands (remnant transposase genes and a G + C content that differs from the core genome), a current notion is that genomic islands were once mobile elements acquired by HGT [62]. A complete RM type I comprises host specificity determinant genes hsdR, hsdM, and hsdS, but only hsdM and hsdS are found juxtaposed to the S. aureus genomic islands [13, 61, 63]. Both flanking DNA segments contribute to the stability of genomic islands within the S. aureus chromosome. A lipoprotein gene cluster (lpl) and staphylococcal superantigen-like genes (ssl) are located on νSAα [64]. νSAβ (also known as SaPIn3/m3) encodes bacteriocin, enterotoxins, hyaluronate lyase, and a serine protease gene cluster [13, 18, 65]. The third staphylococcal genomic island, νSAγ, contains genes encoding β-type phenol-soluble modulins and a cluster of ssl genes similar to that present within νSAα [16].
Staphylococcal cassette chromosome
Staphylococcal cassette chromosomes (SCCs) are relatively large fragments of DNA that always insert into the orfX gene on the S. aureus chromosome. SCC can encode antibiotic resistance and/or virulence determinants. Considering that many SCCs encode the methicillin resistance gene (mecA), SCCs can be classified into staphylococcal cassette chromosome mec (SCCmec) or non-SCCmec groups.
SCCmec
The first MRSA strain was reported in 1961, 2 years after the introduction of methicillin for treatment of penicillin-resistant S. aureus infections [12, 66]. All MRSA strains contain SCCmec, which encodes the mecA gene, thus conferring resistance to methicillin and all β-lactam antibiotics (reviewed in [12]). SCCmec may have been acquired by S. aureus from S. sciuri [67, 68]. Resistance to β-lactam antibiotics is maintained by production of a low-affinity penicillin-binding protein (PBP2a), which fails to bind methicillin and other β-lactam antibiotics. As a result, these antibiotics do not inhibit the ability of PBPs (transpeptidase enzymes) to cross-link peptidoglycan polymers of the bacterial cell wall. In addition to the mecA gene, SCCmec encodes the repressor MecI, transmembrane β-lactam signal transducer MecR1, recombinases CcrAB and CcrC, and joining (formerly junkyard) regions J, which may also encode additional antibiotic resistance (Fig. 4). Integration and excision of SCCmec by the recombinases occurs within a specific attachment site (attBscc) on the S. aureus chromosome at the 3′ end of orfX [61].
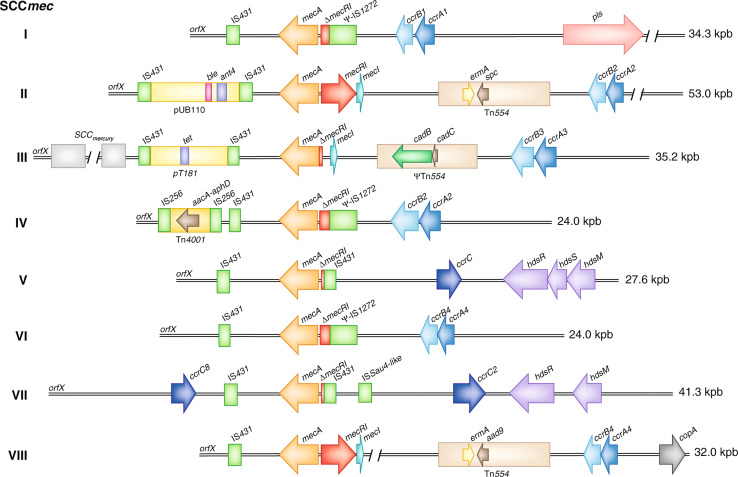
Fig. 4.
Comparison of S. aureus SCCmec types. Class A SCCmec contains a complete mecA regulon (mec1-mecR1-mecA). Class B and class C SCCmec contain regulatory genes that are disrupted by IS, IS1272-ΔmecR1-mecA and IS431-ΔmecR1-mecA, respectively. Tn554 encodes erythromycin (ermA) and streptomycin/spectinomycin resistance (aad9 or spc); copA encodes a putative copper-transport ATPase; hsdR, hsdM, and hsdS encode a partial restriction-modification system (RM) type I; Tn4001 encodes an aminoglycoside resistance operon (aacA-aphD); plasmid pT181 encodes tetracycline resistance (tet); ΨTn554 encodes cadmium resistance (cadB, cadC); and plasmid pUB110 encodes bleomycin (ble) and tobramycin resistance (ant4′). pls Plasmin‐sensitive surface protein
Based on the organization of mec and associated genes within the SCCmec complex, five (A–E) different classes of SCCmec have been defined, of which three (A–C) are the most common in S. aureus [69–71]. Only class A SCCmec consists of the complete mecA regulon (mec1-mecR1-mecA), as the regulatory genes are disrupted by insertional sequences in class B and C, SCCmec–IS1272-ΔmecR1-mecA in class B and IS431-ΔmecR1-mecA in class C SCCmec elements [61, 70]. Three classes of the mec complex and four different ccr allotypes define at present eight SCCmec types (I–VIII) (Fig. 4). However, SCCmec types can be further differentiated into subtypes depending on variations in the J regions. Interestingly, community-associated MRSA (CA-MRSA) strains typically carry SCCmecIV, V, or VII elements [72], whereas HA-MRSA typically contain the larger SCCmecI, II, III, VI, or VIII elements that may encode resistance determinants in addition to mecA [12, 13, 69, 72]. These additional resistance determinants are often encoded by plasmids, transposons, or insertion sequences incorporated into the J regions of SCCmec [61]. For example, the J1 region of SCCmecVIII encodes a putative copper-transport ATPase (copA) and the J2 region has a Tn554 transposon encoding erythromycin (ermA) and streptomycin/spectinomycin resistance (aad9) genes (for more details, see Table 1; Fig. 4) [73, 74].
Non-mec SCC
Staphylococcal cassette chromosomes can be complex and are thus not limited to encoding methicillin resistance. Non-mec SCC and ψSCC (without or no functional recombinase) contain virulence or fitness/survival determinants. A methicillin-susceptible S. aureus strain, MSSA476, contains a mec-like element (SCC476) that encodes fusidic acid resistance [18]. SCCmercury encodes resistance to mercury chloride that was probably obtained from coagulase-negative staphylococci (CoNS) by integration of a plasmid that carried the resistance determinant or by direct transfer of the SCCmercury element [69].
Some S. aureus strains produce capsular polysaccharide 1, which has been reported to confer resistance to phagocytosis [75]. The genes encoding synthesis of capsular polysaccharide 1 are located on a special SCC element named SCCcap1 [75]. Although SCCcapI resembles type III of SCCmec, it is immobile because it lacks an active ccrA homologue and the ccrB homologue contains a nonsense mutation [75, 76].
Arginine catabolic mobile element
The arginine catabolic mobile element (ACME) was discovered by sequencing the complete genome of USA300, the most prominent CA-MRSA strain of North America [15]. ACME encodes a complete arginine deiminase pathway that converts L-arginine to carbon dioxide, ATP, and ammonia. A cluster of six genes, arcRADBC (arc locus) and opp3 (oligopeptide permease system), constitute type I ACME present in the USA300 strain [15]. Type I ACME is associated with specific SCCmec subtypes (Fig. 3). It is present in clinical isolates belonging to multilocus sequence type (MLST or ST) 8 containing SCCmecIVa, but not in SCCmecIVb, IVc, or IVmisc [77]. An ACME variant that lacks the opp3 operon and varies in DNA sequence has also been found in ST8 MSSA, ST5 (USA100, SCCmecII), and ST59 (USA1000) strains [77–79]. An ACME variant has also been detected in MRSA ST97 strains carrying SCCmecV [77].
The arc cluster contained within ACME is distinct from the other S. aureus arc cluster encoded within the core genome [15]. ACME is adjacent to SCCmec and integrated at the same attB site within orfX [15]. Therefore, it is likely that the recombinases that mediate excision of SCCmec also mobilize ACME [15, 80].
The role played by ACME in the success of USA300 remains unknown. Diep et al. suggest it enhances fitness of S. aureus, possibly by facilitating colonization and/or hematogenous dissemination to target organs [15, 80]. On the other hand, Montgomery et al. [81] found no significant difference between ACME-positive and ACME-negative USA300 strains in a rat model of necrotizing pneumonia and a mouse model of skin infection. Further studies are needed to better understand the importance of this interesting MGE.
Other transposable elements
Both insertion sequences (IS) and transposons (Tn) are widely distributed among the S. aureus genome. They may be present in a single copy or multiple copies on the chromosome or in association with other MGEs.
Insertion sequences
Although insertion sequences (IS) can exist independently in the S. aureus genome, they often present as pairs constituting a composite transposon [82]. IS insert into various loci and may cause changes in the expression of genes in the core chromosome. In addition, IS inactivate genes by direct insertion or by having a polar effect on the transcription of nearby genes [83, 84]. Activation of genes within the vicinity of an IS is usually mediated by promoters carried by IS elements or by forming a hybrid promoter with the native promoter of particular gene [85]. IS256 and IS257, in addition to constituting composite transposons Tn4001 and Tn4003, form a hybrid promoter for the aminoglycoside resistance operon (aacA-aphD) and the gene encoding resistance to trimethoprim (dfrA), respectively [82, 86, 87].
Transposons
Transposons (Tn) predominantly encode antibiotic resistance genes in S. aureus (Table 1). The smaller transposons are usually presented in multiple copies in the staphylococcal genome, either inserted into the chromosome or into MGEs, such as SCC or plasmids. This group includes Tn554 and Tn552, which encode resistance to MLSB antibiotics and spectinomycin or penicillinase, respectively [41, 61, 88].
By comparison, larger transposons (>18 kbp) are present in single copies and encode resistance to antibiotics such as tetracycline [89], trimethoprim [87], aminoglycosides [82, 90], or vancomycin [30, 31, 35].
Concluding remarks
A wide range of environmental conditions, including interspecies competition within particular ecological niche and antibiotic selective pressure, select for organisms that have acquired MGEs—those that are presumably advantageous for survival—by HGT. Production of antibiotics by microorganisms is mirrored (countered) by development of resistance to these molecules and is a naturally occurring phenomenon. Antibiotics are toxins produced by bacteria and fungi to compete with other microorganisms for a specific ecological niche. Unfortunately, the level of antibiotic resistance among bacteria continues to increase, consistent with the high use of antibiotics by humans. Sub-inhibitory concentrations of antibiotics also create an environment conducive to acquisition of resistance [91].
Antibiotics that interfere with bacterial DNA replication and induce an SOS response also induce excision and transduction of prophages and staphylococcal pathogenicity islands in the bacterial genome, resulting in high-frequency of horizontal gene transfer [60, 92, 93]. Consequentially, this process promotes dissemination of determinants encoding antibiotic resistance molecules and virulence factors. MGEs can be species-specific, and, therefore, differences exist in MGEs of S. aureus strains that have a tropism for humans or animals [94]. Nevertheless, some S. aureus strains transmit from animals to humans or vice versa [95–98]. Transfer of staphylococci from one host species to another provides an additional means to acquire new genetic material, often encoded by MGEs [99].
In summary, although MGEs constitute only ~25% of the staphylococcal genome [8], they encode many putative virulence factors and antibiotic determinants and thus play an important role in bacterial adaptability and survival.
Acknowledgments
We thank James M. Musser (The Methodist Hospital Research Institute, Houston TX, USA) for critical reading of the manuscript. This article was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClintock B. Chromosome organization and genetic expression. Cold Spring Harb Symp Quant Biol. 1951;16:13–47. doi: 10.1101/sqb.1951.016.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Frost LS, Leplae R, Summers AO, Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 4.Siefert JL. Defining the mobilome. Methods Mol Biol. 2009;532:13–27. doi: 10.1007/978-1-60327-853-9_2. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Rivera MC, Lake JA. Horizontal gene transfer among genomes: the complexity hypothesis. Proc Natl Acad Sci USA. 1999;96:3801–3806. doi: 10.1073/pnas.96.7.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet. 2008;9:605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- 7.Hacker J, Kaper JB. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol. 2000;54:641–679. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay J, Holden M. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 2004;12:378–385. doi: 10.1016/j.tim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Belkum A. Staphylococcal colonization and infection: homeostasis versus disbalance of human (innate) immunity and bacterial virulence. Curr Opin Infect Dis. 2006;19:339–344. doi: 10.1097/01.qco.0000235159.40184.61. [DOI] [PubMed] [Google Scholar]
- 11.Weems JJ. The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad Med. 2001;110:24–26. doi: 10.3810/pgm.2001.10.1042. [DOI] [PubMed] [Google Scholar]
- 12.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K-I, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 14.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008;190:300–310. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep B, Gill S, Chang R, Phan T, Chen J, Davidson M, Lin F, Lin J, Carleton H, Mongodin E, Sensabaugh G, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus . Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 16.Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. Molecular correlates of host specialization in Staphylococcus aureus . PLoS One. 2007;2:e1120. doi: 10.1371/journal.pone.0001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holden MTG, Feil EJ, Lindsay JA, Peacock SJ, Day NPJ, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher C, Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, Mayes R, Moule S, Mungall K, Ormond D, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Sharp S, Simmonds M, Stevens K, Whitehead S, Barrell BG, Spratt BG, Parkhill J. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, Batra R, Parkhill J, Bentley SD, Edgeworth JD. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW) J Bacteriol. 2010;192:888–892. doi: 10.1128/JB.01255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K-I, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R-I, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus . Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 22.Musser JM, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray RA. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SA. Plasmid rolling-circle replication: highlights of two decades of research. Plasmid. 2005;53:126–136. doi: 10.1016/j.plasmid.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay JA. Genomic variation and evolution of Staphylococcus aureus . Int J Med Microbiol. 2010;300:98–103. doi: 10.1016/j.ijmm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa K, Inose Y, Okamura H, Maruyama A, Hayashi H, Takeyasu K, Ohta TA. New staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Gen Cell. 2003;8:699–712. doi: 10.1046/j.1365-2443.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsen JE, Christensen H, Aarestrup FM. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J Antimicrob Chemother. 2006;57:450–460. doi: 10.1093/jac/dki492. [DOI] [PubMed] [Google Scholar]
- 28.Hackbarth CJ, Chambers HF. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 1993;37:1144–1149. doi: 10.1128/aac.37.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidhu MS, Heir E, Leegaard T, Wiger K, Holck A. Frequency of disinfectant resistance genes and genetic ginkage with ß-lactamase transposon Tn552 among clinical staphylococci. Antimicrob Agents Chemother. 2002;46:2797–2803. doi: 10.1128/AAC.46.9.2797-2803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus . Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. Vancomycin-resistant Staphylococcus aureus isolates associated with inc18-like vanA plasmids in Michigan. Antimicrob Agents Chemother. 2008;52:452–457. doi: 10.1128/AAC.00908-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, Tomasz A. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci USA. 2007;104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–155. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 34.Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus . Future Microbiol. 2007;2:323–334. doi: 10.2217/17460913.2.3.323. [DOI] [PubMed] [Google Scholar]
- 35.Ballard SA, Pertile KK, Lim M, Johnson PDR, Grayson ML. Molecular characterization of vanB elements in naturally occurring gut anaerobes. Antimicrob Agents Chemother. 2005;49:1688–1694. doi: 10.1128/AAC.49.5.1688-1694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:4580–4587. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover FC, Weigel LM, Appelbaum PC, McDougal LK, Chaitram J, McAllister S, Clark N, Killgore G, O’Hara CM, Jevitt L, Patel JB, Bozdogan B. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob Agents Chemother. 2004;48:275–280. doi: 10.1128/AAC.48.1.275-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saha B, Singh AK, Ghosh A, Bal M. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia) J Med Microbiol. 2008;57:72–79. doi: 10.1099/jmm.0.47144-0. [DOI] [PubMed] [Google Scholar]
- 39.Lessard IA, Walsh CT. Mutational analysis of active-site residues of the enterococcal D-ala-D-Ala dipeptidase VanX and comparison with Escherichia coli D-ala-D-Ala ligase and D-ala-D-Ala carboxypeptidase VanY. Chem Biol. 1999;6:177–187. doi: 10.1016/S1074-5521(99)89009-7. [DOI] [PubMed] [Google Scholar]
- 40.Courvalin P. Vancomycin resistance in Gram‐positive cocci. Clin Infect Dis. 2006;42:S25–S34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 41.Jensen SO, Lyon BR. Genetics of antimicrobial resistance in Staphylococcus aureus . Future Microbiol. 2009;4:565–582. doi: 10.2217/fmb.09.30. [DOI] [PubMed] [Google Scholar]
- 42.Jackson MP, Iandolo JJ. Cloning and expression of the exfoliative toxin B gene from Staphylococcus aureus . J Bacteriol. 1986;166:574–580. doi: 10.1128/jb.166.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishifuji K, Sugai M, Amagai M. Staphylococcal exfoliative toxins: “Molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci. 2008;49:21–31. doi: 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, Nishifuji K, Sasaki M, Fudaba Y, Aepfelbacher M, Takata T, Ohara M, Komatsuzawa H, Amagai M, Sugai M. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect Immun. 2002;70:5835–5845. doi: 10.1128/IAI.70.10.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi T, Hayashi T, Takami H, Ohnishi M, Murata T, Nakayama K, Asakawa K, Ohara M, Komatsuzawa H, Sugai M. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADP-ribosyltransferase, EDIN-C. Infect Immun. 2001;69:7760–7771. doi: 10.1128/IAI.69.12.7760-7771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plano LR. Staphylococcus aureus exfoliative toxins: how they cause disease. J Invest Dermatol. 2004;122:1070–1077. doi: 10.1111/j.1523-1747.2004.22144.x. [DOI] [PubMed] [Google Scholar]
- 47.Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, Broker BM, Doskar J, Wolz C. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J Bacteriol. 2009;191:3462–3468. doi: 10.1128/JB.01804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mann NH. The potential of phages to prevent MRSA infections. Res Microbiol. 2008;159:400–405. doi: 10.1016/j.resmic.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Coleman DC, Sullivan DJ, Russell RJ, Arbuthnott JP, Carey BF, Pomeroy HM. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: molecular mechanism of triple conversion. J Gen Microbiol. 1989;135:1679–1697. doi: 10.1099/00221287-135-6-1679. [DOI] [PubMed] [Google Scholar]
- 50.van Wamel W, Rooijakkers S, Ruyken M, van Kessel K, van Strijp J. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald JR, Monday SR, Foster TJ, Bohach GA, Hartigan PJ, Meaney WJ, Smyth CJ. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J Bacteriol. 2001;183:63–70. doi: 10.1128/JB.183.1.63-70.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick R, Subedi A. The SaPIs: mobile pathogenicity islands of staphylococcus. Chem Immunol Allergy. 2007;93:42–57. doi: 10.1159/000100857. [DOI] [PubMed] [Google Scholar]
- 54.Novick RP. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus . Plasmid. 2003;49:93–105. doi: 10.1016/s0147-619x(02)00157-9. [DOI] [PubMed] [Google Scholar]
- 55.Ubeda C, Tormo MA, Cucarella C, Trotonda P, Foster TJ, Lasa I, Penades JR. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol. 2003;49:193–210. doi: 10.1046/j.1365-2958.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 56.Yarwood JM, McCormick JK, Paustian ML, Orwin PM, Kapur V, Schlievert PM. Characterization and expression analysis of Staphylococcus aureus pathogenicity island 3. J Biol Chem. 2002;277:13138–13147. doi: 10.1074/jbc.M111661200. [DOI] [PubMed] [Google Scholar]
- 57.Carles U, Ma Angeles T, Carme C, Pilar T, Timothy JF, Inigo L, Jose RP. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile. Mol Microbiol. 2003;49:93–210. doi: 10.1046/j.1365-2958.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 58.Tormo MA, Knecht E, Gotz F, Lasa I, Penades JR. Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer? Microbiology. 2005;151:2465–2475. doi: 10.1099/mic.0.27865-0. [DOI] [PubMed] [Google Scholar]
- 59.Tormo MA, Ferrer MD, Maiques E, Ubeda C, Selva L, Lasa I, Calvete JJ, Novick RP, Penades JR. Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. J Bacteriol. 2008;190:2434–2440. doi: 10.1128/JB.01349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ubeda C. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol. 2005;56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 61.Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist Update. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 62.Dobrindt U, Hochhut B, Hentschel U, Hacker J. Genomic islands in pathogenic and environmental microorganisms. Nat Rev Micro. 2004;2:414–424. doi: 10.1038/nrmicro884. [DOI] [PubMed] [Google Scholar]
- 63.Waldron D, Lindsay J. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol. 2006;188:5578–5585. doi: 10.1128/JB.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lina G, Bohach Gregory A, Nair Sean P, Hiramatsu K, Jouvin‐Marche E, Mariuzza R. Standard nomenclature for the superantigens expressed by Staphylococcus. J Infect Dis. 2004;189:2334–2336. doi: 10.1086/420852. [DOI] [PubMed] [Google Scholar]
- 65.Tsuru T, Kobayashi I. Multiple genome comparison within a bacterial species reveals a unit of evolution spanning two adjacent genes in a tandem paralog cluster. Mol Biol Evol. 2008;25:2457–2473. doi: 10.1093/molbev/msn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jevons MP, Rolinson GN, Knox R. “Celbenin”-resistant staphylococci. BMJ. 1961;1:124–126. [Google Scholar]
- 67.Severin A, Wu SW, Tabei K, Tomasz A. High-level β-lactam resistance and cell wall synthesis catalyzed by the mecA homologue of Staphylococcus sciuri introduced into Staphylococcus aureus . J Bacteriol. 2005;187:6651–6658. doi: 10.1128/JB.187.19.6651-6658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu S, Piscitelli C, De Lencastre H, Tomasz A. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri . Microb Drug Resist. 1996;2:435–441. doi: 10.1089/mdr.1996.2.435. [DOI] [PubMed] [Google Scholar]
- 69.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, Jamklang M, Chavalit T, Song J-H, Hiramatsu K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006;50:1001–1012. doi: 10.1128/AAC.50.3.1001-1012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr Opin Microbiol. 2007;10:428–435. doi: 10.1016/j.mib.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, Daum RS, Hiramatsu K. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sitthisak S, Knutsson L, Webb JW, Jayaswal RK. Molecular characterization of the copper transport system in Staphylococcus aureus . Microbiol. 2007;153:4274–4283. doi: 10.1099/mic.0.2007/009860-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang K, McClure J-A, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class a mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:531–540. doi: 10.1128/AAC.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luong TT, Ouyang S, Bush K, Lee CY. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J Bacteriol. 2002;184:3623–3629. doi: 10.1128/JB.184.13.3623-3629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanssen AM, Sollid JUE. SCCmec in staphylococci: genes on the move. FEMS Immunol Med Microbiol. 2006;46:8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 77.Ellington MJ, Yearwood L, Ganner M, East C, Kearns AM. Distribution of the ACME-arcA gene among methicillin-resistant Staphylococcus aureus from England and Wales. J Antimicrob Chemother. 2008;61:73–77. doi: 10.1093/jac/dkm422. [DOI] [PubMed] [Google Scholar]
- 78.Goering RV, McDougal LK, Fosheim GE, Bonnstetter KK, Wolter DJ, Tenover FC. Epidemiologic distribution of the arginine catabolic mobile element among selected methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:1981–1984. doi: 10.1128/JCM.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miragaia M, de Lencastre H, Perdreau-Remington F, Chambers HF, Higashi J, Sullam PM, Lin J, Wong KI, King KA, Otto M, Sensabaugh GF, Diep BA. Genetic diversity of arginine catabolic mobile element in Staphylococcus epidermidis . PLoS One. 2009;4:e7722. doi: 10.1371/journal.pone.0007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages S-A, Jones A, Palazzolo‐Ballance AM, Perdreau‐Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin‐resistant. J Infect Dis. 2008;197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 81.Montgomery CP, Boyle-Vavra S, Daum RS. The arginine catabolic mobile element is not associated with enhanced virulence in experimental invasive disease caused by the community-associated methicillin-resistant Staphylococcus aureus USA300 genetic background. Infect Immun. 2009;77:2650–2656. doi: 10.1128/IAI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Byrne ME, Rouch DA, Skurray RA. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989;81:361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- 83.Fiandt M, Szybalski W, Malamy MH. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119:223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- 84.Jansen A, Türck M, Szekat C, Nagel M, Clever I, Bierbaum G. Role of insertion elements and yycfg in the development of decreased susceptibility to vancomycin in Staphylococcus aureus . Int J Med Microbiol. 2007;297:205–215. doi: 10.1016/j.ijmm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Jaurin B, Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli . Cell. 1983;32:809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 86.Rouch DA, Byrne ME, Kong YC, Skurray RA. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987;133:3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- 87.Rouch DA, Messerotti LJ, Loo LSL, Jackson CA, Skurray RA. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257 . Mol Microbiol. 1989;3:161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 88.Phillips S, Novick RP. Tn554—a site-specific repressor-controlled transposon in Staphylococcus aureus . Nature. 1979;278:476–478. doi: 10.1038/278476a0. [DOI] [PubMed] [Google Scholar]
- 89.Soge OO, Beck NK, White TM, No DB, Roberts MC. A novel transposon, Tn6009, composed of a Tn916 element linked with a Staphylococcus aureus mer operon. J Antimicrob Chemother. 2008;62:674–680. doi: 10.1093/jac/dkn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange CC, Werckenthin C, Schwarz S. Molecular analysis of the plasmid-borne aacA/aphD resistance gene region of coagulase-negative staphylococci from chickens. J Antimicrob Chemother. 2003;51:1397–1401. doi: 10.1093/jac/dkg257. [DOI] [PubMed] [Google Scholar]
- 91.Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 92.Carles U, Elisa M, Erwin K, Inigo L, Richard PN, José RP. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol. 2005;56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 93.Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, Barbe J, Penades JR. β-Lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus . J Bacteriol. 2006;188:2726–2729. doi: 10.1128/JB.188.7.2726-2729.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sung JM-L, Lloyd DH, Lindsay JA. Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology. 2008;154:1949–1959. doi: 10.1099/mic.0.2007/015289-0. [DOI] [PubMed] [Google Scholar]
- 95.Belkum A, Melles DC, Peeters JK, Leeuwen WB, Duijkeren E, Huijsdens XW, Spalburg E, de Neeling AJ, Verbrugh HA. Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg Infect Dis. 2008;14:479–483. doi: 10.3201/eid1403.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, Voss A, Kluytmans J. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13:1834–1839. doi: 10.3201/eid1312.070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vitale CB, Gross TL, Weese JS. Methicillin-resistant Staphylococcus aureus in cat and owner. Emerg Infect Dis. 2006;12:1998–2000. doi: 10.3201/eid1212.060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weese JS, Archambault M, Willey BM, Hearn P, Kreiswirth BN, Said-Salim B, McGeer A, Likhoshvay Y, Prescott JF, Low DE. Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 2000–2002. Emerg Infect Dis. 2005;11:430–435. doi: 10.3201/eid1103.040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lowder BV, Guinane CM, Ben Zakour NL, Weinert LA, Conway-Morris A, Cartwright RA, Simpson AJ, Rambaut A, Nübel U, Fitzgerald JR. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus . Proc Natl Acad Sci USA. 2009;106:19545–19550. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Byrne ME, Gillespie MT, Skurray RA. 4′, 4″ adenyltransferase activity on conjugative plasmids isolated from Staphylococcus aureus is encoded on an integrated copy of pUB110. Plasmid. 1991;25:70–75. doi: 10.1016/0147-619x(91)90008-k. [DOI] [PubMed] [Google Scholar]
- 101.Kadlec K, Schwarz S. Novel ABC transporter gene, vga(C), located on a multiresistance plasmid from a porcine methicillin-resistant Staphylococcus aureus ST398 strain. Antimicrob Agents Chemother. 2009;53:3589–3591. doi: 10.1128/AAC.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lelievre H, Lina G, Jones ME, Olive C, Forey F, Roussel-Delvallez M, Nicolas-Chanoine M-H, Bebear CM, Jarlier V, Andremont A, Vandenesch F, Etienne J. Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics. J Clin Microbiol. 1999;37:3452–3457. doi: 10.1128/jcm.37.11.3452-3457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Broer S, Ji G, Broer A, Silver S. Arsenic efflux governed by the arsenic resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1993;175:3480–3485. doi: 10.1128/jb.175.11.3480-3485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji G, Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992;174:3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaase M, Lenga S, Friedrich S, Szabados F, Sakinc T, Kleine B, Gatermann SG. Comparison of phenotypic methods for penicillinase detection in Staphylococcus aureus . Clin Microbiol Infect. 2008;14:614–616. doi: 10.1111/j.1469-0691.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- 106.Hou Z, Meng J-R, Zhao J-R, Hu B-Q, Liu J, Yan X-J, Jia M, Luo X-X. Inhibition of beta-lactamase-mediated oxacillin resistance in Staphylococcus aureus by a deoxyribozyme. Acta Pharmacol Sin. 2007;28:1775–1782. doi: 10.1111/j.1745-7254.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 107.Gennimata D, Davies J, Tsiftsoglour S. Bleomycin resistance in Staphylococcus aureus clinical isolates. J Antimicrob Chemother. 1996;37:65–75. doi: 10.1093/jac/37.1.65. [DOI] [PubMed] [Google Scholar]
- 108.McElgunn CJ, Zahurul M, Bhuyian A, Sugiyama M. Integration analysis of pSK41 in the chromosome of a methicillin-resistant Staphylococcus aureus K-1. J Basic Microbiol. 2002;42:190–200. doi: 10.1002/1521-4028(200206)42:3<190::AID-JOBM190>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 109.Crupper SS, Worrell V, Stewart GC, Iandolo JJ. Cloning and expression of cadD, a new cadmium resistance gene of Staphylococcus aureus . J Bacteriol. 1999;181:4071–4075. doi: 10.1128/jb.181.13.4071-4075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nies DH. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992;27:17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 111.Massidda O, Mingoia M, Fadda D, Whalen MB, Montanari MP, Varaldo PE. Analysis of the beta-lactamase plasmid of borderline methicillin-susceptible Staphylococcus aureus: focus on bla complex genes and cadmium resistance determinants cadD and cadX. Plasmid. 2006;55:114–127. doi: 10.1016/j.plasmid.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Projan SJ, Novick R. Comparative analysis of five related staphylococcal plasmids. Plasmid. 1988;19:203–221. doi: 10.1016/0147-619x(88)90039-x. [DOI] [PubMed] [Google Scholar]
- 113.Projan SJ, Moghazeh S, Novick RP. Nucleotide sequence of pS194, a streptomycin-resistance plasmid from Staphylococcus aureus . Nucl Acids Res. 1988;16:2179–2188. doi: 10.1093/nar/16.5.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kehrenberg C, Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant staphylococcus isolates. Antimicrob Agents Chemother. 2006;50:1156–1163. doi: 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev. 2004;28:519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 116.Tennent JM, Young H-K, Lyon BR, Amyes SGB, Skurray RA. Trimethoprim resistance determinants encoding a dihydrofolate reductase in clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci. J Med Microbiol. 1988;26:67–73. doi: 10.1099/00222615-26-1-67. [DOI] [PubMed] [Google Scholar]
- 117.Otsuka T, Zaraket H, Takano T, Saito K, Dohmae S, Higuchi W, Yamamoto T. Macrolide-lincosamide-streptogramin B resistance phenotypes and genotypes among Staphylococcus aureus clinical isolates in Japan. Clin Microbiol Infect. 2007;13:325–327. doi: 10.1111/j.1469-0691.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 118.Westh H, Hougaard D, Vuust J, Rosdahl V. Prevalence of erm gene classes in erythromycin-resistant Staphylococcus aureus strains isolated between 1959 and 1988. Antimicrob Agents Chemother. 1995;39:369–373. doi: 10.1128/aac.39.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jappe U, Heuck D, Strommenger B, Wendt C, Werner G, Altmann D, Witte W. Staphylococcus aureus in dermatology outpatients with special emphasis on community-associated methicillin-resistant strains. J Invest Dermatol. 2008;128:2655–2664. doi: 10.1038/jid.2008.133. [DOI] [PubMed] [Google Scholar]
- 120.O’Brien FG, Price C, Grubb WB, Gustafson JE. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J Antimicrob Chemother. 2002;50:313–321. doi: 10.1093/jac/dkf153. [DOI] [PubMed] [Google Scholar]
- 121.Oliveira NEM, Cavalcanti EDAC, Laport MS, Bastos MDCDF, Giambiagi-deMarval M. Constitutive expression of the ileS-2 gene responsible for high-level mupirocin resistance in Staphylococcus aureus. J Med Microbiol. 2009;58:1582–1584. doi: 10.1099/jmm.0.013912-0. [DOI] [PubMed] [Google Scholar]
- 122.Patel JB, Gorwitz RJ, Jernigan JA. Antimicrobial resistance: mupirocin resistance. Clin Infect Dis. 2009;49:935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 123.Laddaga RA, Chu L, Misra TK, Silver S. Nucleotide sequence and expression of the mercurial-resistance operon from Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci USA. 1987;84:5106–5110. doi: 10.1073/pnas.84.15.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsuoka M, Endou K, Kobayashi H, Inoue M, Nakajima Y. A plasmid that encodes three genes for resistance to macrolide antibiotics in Staphylococcus aureus . FEMS Microbiol Lett. 1998;167:221–227. doi: 10.1111/j.1574-6968.1998.tb13232.x. [DOI] [PubMed] [Google Scholar]
- 125.Antonio M, McFerran N, Pallen MJ. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2002;46:438–442. doi: 10.1128/AAC.46.2.438-442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Littlejohn TG, DiBerardino D, Messerotti LJ, Spiers SJ, Skurray RA. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus . Gene. 1991;101:59–66. doi: 10.1016/0378-1119(91)90224-y. [DOI] [PubMed] [Google Scholar]
- 127.Liu Q, Liu M, Wu Q, Li C, Zhou T, Ni Y. Sensitivities to biocides and distribution of biocide resistance genes in quaternary ammonium compound tolerant Staphylococcus aureus isolated in a teaching hospital. Scan J Infect Dis. 2009;41:403–409. doi: 10.1080/00365540902856545. [DOI] [PubMed] [Google Scholar]
- 128.Nakaminami H, Noguchi N, Nishijima S, Kurokawa I, Sasatsu M. Characterization of the pTZ2162 encoding multidrug efflux gene qacB from Staphylococcus aureus . Plasmid. 2008;60:108–117. doi: 10.1016/j.plasmid.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 129.Bismuth R, Zilhao R, Sakamoto H, Guesdon JL, Courvalin P. Gene heterogeneity for tetracycline resistance in Staphylococcus spp. Antimicrob Agents Chemother. 1990;34:1611–1614. doi: 10.1128/aac.34.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guay GG, Khan SA, Rothstein DM. The tet(K) gene of plasmid pT181 of Staphylococcus aureus encodes an efflux protein that contains 14 transmembrane helices. Plasmid. 1993;30:163–166. doi: 10.1006/plas.1993.1045. [DOI] [PubMed] [Google Scholar]
- 131.Trzcinski K, Cooper BS, Hryniewicz W, Dowson CG. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus . J Antimicrob Chemother. 2000;45:763–770. doi: 10.1093/jac/45.6.763. [DOI] [PubMed] [Google Scholar]
- 132.Korczynska M, Mukhtar TA, Wright GD, Berghuis AM. Structural basis for streptogramin B resistance in Staphylococcus aureus by virginiamycin B lyase. Proc Natl Acad Sci. 2007;104:10388–10393. doi: 10.1073/pnas.0701809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mukhtar TA, Koteva KP, Hughes DW, Wright GD. Vgb from Staphylococcus aureus inactivates streptogramin B antibiotics by an elimination mechanism not hydrolysis†. Biochem. 2001;40:8877–8886. doi: 10.1021/bi0106787. [DOI] [PubMed] [Google Scholar]
- 134.Rowland S-J, Dyke KGH. Tn552, a novel transposable element from Staphylococcus aureus . Mol Microbiol. 1990;4:961–975. doi: 10.1111/j.1365-2958.1990.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 135.Dubin DT, Chikramane SG, Inglis B, Matthews PR, Stewart PR. Physical mapping of the mec region of an Australian methicillin-resistant Staphylococcus aureus lineage and a closely related American strain. J Gen Microbiol. 1992;138:169–180. doi: 10.1099/00221287-138-1-169. [DOI] [PubMed] [Google Scholar]
- 136.Babich K, Engle M, Skinner JS, Laddaga RA. Deletion mutant analysis of the Staphylococcus aureus plasmid pI258 mercury-resistance determinant. Can J Microbiol. 1991;37:624–631. doi: 10.1139/m91-106. [DOI] [PubMed] [Google Scholar]
- 137.Bogdanova E, Minakhin L, Bass I, Volodin A, Hobman JL, Nikiforov V. Class II broad-spectrum mercury resistance transposons in Gram-positive bacteria from natural environments. Res Microbiol. 2001;152:503–514. doi: 10.1016/s0923-2508(01)01224-4. [DOI] [PubMed] [Google Scholar]
- 138.Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JAG, de Haas CJC, van Kessel KPM. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the c5a and formylated peptide receptor. J Immunol. 2004;172:6994–7001. doi: 10.4049/jimmunol.172.11.6994. [DOI] [PubMed] [Google Scholar]
- 139.Altboum Z, Hertman I, Sarid S. Penicillinase plasmid-linked genetic determinants for enterotoxins b and c1 production in Staphylococcus aureus . Infect Immun. 1985;47:514–521. doi: 10.1128/iai.47.2.514-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frea JI, McCoy E, Strong FM. Purification of type b staphylococcal enterotoxin. J Bacteriol. 1963;86:1308–1313. doi: 10.1128/jb.86.6.1308-1313.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Avena RM, Bergdoll MS. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochem. 1967;6:1474–1480. doi: 10.1021/bi00857a033. [DOI] [PubMed] [Google Scholar]
- 142.Bayles KW, Iandolo JJ. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989;171:4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang S, Iandolo JJ, Stewart GC. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiol Lett. 1998;168:227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]
- 144.Chiang Y-C, Chang L-T, Lin C-W, Yang C-Y, Tsen H-Y. PCR primers for the detection of staphylococcal enterotoxins K, L, and M and survey of staphylococcal enterotoxin types in Staphylococcus aureus isolates from food poisoning cases in Taiwan. J Food Protect. 2006;69:1072–1079. doi: 10.4315/0362-028x-69.5.1072. [DOI] [PubMed] [Google Scholar]
- 145.Sumby P, Waldor MK. Transcription of the toxin genes present within the staphylococcal phage phiSa3 ms is intimately linked with the phage’s life cycle. J Bacteriol. 2003;185:6841–6851. doi: 10.1128/JB.185.23.6841-6851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiang Y-C, Liao W-W, Fan C-M, Pai W-Y, Chiou C-S, Tsen H-Y. PCR detection of staphylococcal enterotoxins (SEs) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poisoning cases in Taiwan. Int J Food Microbiol. 2008;121:66–73. doi: 10.1016/j.ijfoodmicro.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 147.Brussow H, Canchaya C, Hardt W-D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cabrera G, Xiong A, Uebel M, Singh VK, Jayaswal RK. Molecular characterization of the iron-hydroxamate uptake system in Staphylococcus aureus . Appl Environ Microbiol. 2001;67:1001–1003. doi: 10.1128/AEM.67.2.1001-1003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Essmann F, Bantel H, Totzke G, Engels IH, Sinha B, Schulze-Osthoff K, Janicke RU. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 2003;10:1260–1272. doi: 10.1038/sj.cdd.4401301. [DOI] [PubMed] [Google Scholar]
- 150.Highlander S, Hulten K, Qin X, Jiang H, Yerrapragada S, Mason E, Shang Y, Williams T, Fortunov R, Liu Y, Igboeli O, Petrosino J, Tirumalai M, Uzman A, Fox G, Cardenas A, Muzny D, Hemphill L, Ding Y, Dugan S, Blyth P, Buhay C, Dinh H, Hawes A, Holder M, Kovar C, Lee S, Liu W, Nazareth L, Wang Q, Zhou J, Kaplan S, Weinstock G. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus . BMC Microbiol. 2007;7:99. doi: 10.1186/1471-2180-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Makris G, Wright JD, Ingham E, Holland KT. The hyaluronate lyase of Staphylococcus aureus—a virulence factor? Microbiol. 2004;150:2005–2013. doi: 10.1099/mic.0.26942-0. [DOI] [PubMed] [Google Scholar]
- 152.Zou D, Kaneko J, Narita S, Kamio Y. Prophage, phiPV83-pro, carrying panton-valentine leukocidin genes, on the Staphylococcus aureus P83 chromosome: comparative analysis of the genome structures of phiPV83-pro, phiPVL, phi11, and other phages. Biosci Biotech Biochem. 2000;64:2631–2643. doi: 10.1271/bbb.64.2631. [DOI] [PubMed] [Google Scholar]
- 153.Barrio MB, Rainard P, Prévost G. LukM/LukF’-PV is the most active Staphylococcus aureus leukotoxin on bovine neutrophils. Microb Infect. 2006;8:2068–2074. doi: 10.1016/j.micinf.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 154.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 155.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 156.Panton PN, Valentine FCO. Staphylococcal toxin. Lancet. 1932;219:506–508. [Google Scholar]
- 157.Tristan A, Bes M, Meugnier H, Lina G, Bozdogan B, Courvalin P, Reverdy ME, Enright MC, Vandenesch F, Etienne J. Global distribution of Panton-Valentine leukocidin—positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis. 2007;13:594–600. doi: 10.3201/eid1304.061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 159.Queck SY, Khan BA, Wang R, Bach T-HL, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, DeLeo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mehlin C, Headley CM, Klebanoff SJ. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vuong C, Dürr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004;6:753–759. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 162.Werbick C, Becker K, Mellmann A, Juuti KM, Eiff C, Peters G, Kuusela PI, Friedrich AW, Sinha B. Staphylococcal chromosomal cassette mec type I, spa type, and expression of pls are determinants of reduced cellular invasiveness of methicillin‐resistant. J Infect Dis. 2007;195:1678–1685. doi: 10.1086/517517. [DOI] [PubMed] [Google Scholar]
- 163.Stec-Niemczyk J, Pustelny K, Kisielewska M, Bista M, Boulware KT, Stennicke HR, Thogersen IB, Daugherty PS, Enghild JJ, Baczynski K, Popowicz GM, Dubin A, Potempa J, Dubin G. Structural and functional characterization of splA, an exclusively specific protease of Staphylococcus aureus . J Biochem. 2009;419:555–564. doi: 10.1042/BJ20081351. [DOI] [PubMed] [Google Scholar]
- 164.Takeuchi S, Matsunaga K, Inubushi S, Higuchi H, Imaizumi K, Kaidoh T. Structural gene and strain specificity of a novel cysteine protease produced by Staphylococcus aureus isolated from a diseased chicken. Vet Microbiol. 2002;89:201–210. doi: 10.1016/s0378-1135(02)00171-2. [DOI] [PubMed] [Google Scholar]
- 165.Rooijakkers SHM, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJB, van Kessel KPM, van Strijp JAG. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 166.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 167.Bokarewa M, Tarkowski A. Human alpha-defensins neutralize fibrinolytic activity exerted by staphylokinase. Thromb Haemost. 2004;91:991–999. doi: 10.1160/TH03-11-0696. [DOI] [PubMed] [Google Scholar]
- 168.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172:1169–1176. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 169.Rooijakkers SHM, van Wamel WJB, Ruyken M, van Kessel KPM, van Strijp JAG. Anti-opsonic properties of staphylokinase. Microb Infect. 2005;7:476–484. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 170.Kreiswirth BN, Projan SJ, Schlievert PM, Novick RP. Toxic shock syndrome toxin-1 is encoded by a variable genetic element. Rev Infect Dis. 1989;11:S75–S82. doi: 10.1093/clinids/11.supplement_1.s83. [DOI] [PubMed] [Google Scholar]
- 171.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus . Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]