Abstract
Transplant arteriosclerosis (TA) is the hallmark of chronic allograft dysfunction (CAD) affecting transplanted organs in the long term [1,2]. These fibroproliferative lesions lead to neointimal thickening of arteries in all transplanted allografts [2]. Luminal narrowing then leads to graft ischemia and organ demise. To date, there are no known tolerance induction strategies that prevent TA [3,4]. Therefore, this study was designed to test the hypothesis that human regulatory T cells (Treg cells) expanded ex vivo could prevent TA. Here we show the comparative capacity of Treg cells, sorted via two separate strategies, to prevent TA in a clinically relevant chimeric humanized mouse system. We found that the in vivo development of TA in human arteries was prevented with the treatment of ex vivo expanded human Treg cells. Additionally, we show that Treg cells sorted based on the low expression of CD127 (IL-7Rα) provide a more potent therapy to conventional Treg cells. Our results demonstrate, for the first time, that human Treg cells can inhibit TA by impairing effector function and graft infiltration. We anticipate our findings to serve as a foundation for the clinical development of therapeutics targeting TA in both allograft transplantation and other immune-mediated causes of vasculopathy [5].
Investigating the mechanisms driving the development of TA in situ in transplant recipients is challenging. While protocol biopsies and intravascular imaging provide snapshots of the process and enable the evolution of CAD to be described[6,7], the development of a chimeric humanized mouse system has enabled the mechanisms and impact of novel interventions to be studied in vivo[8]. Allogeneic peripheral blood mononuclear cells (PBMC) were found to elicit immune-mediated rejection of transplanted human arterial segments mimicking the TA seen in human transplantation. Interestingly, IFN-γ elicited the same response in the absence of leukocytes[8], supporting the concept that both immune and non-immune mechanisms contribute to the process. In this report, we investigated the possibility of attenuating the fibroproliferative lesions seen with TA using ex vivo expanded human Treg cellular therapy, compared the capacity of CD25hiCD4+ vs. CD127loCD25+CD4+ cells to suppress allogeneic immune responses, and evaluated the impact of Treg cells on IFN-γ production.
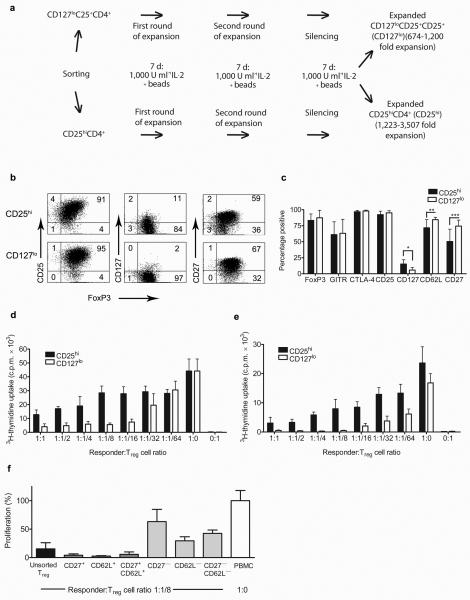
Human Treg cells were FACS-sorted from healthy donor PBMC as CD25hiCD4+ cells (designated CD25hi) or CD127loCD25+CD4+ cells (designated CD127lo) to greater than 94% purity (n=5 independent experiments; Supplementary Fig. 1a). After sorting, the cells were expanded in vitro using beads coated with CD3 and CD28-specific antibodies and recombinant human IL-2 (Fig. 1a)[9,10]. Both populations consistently expanded to greater than 600-fold (n=5 expansions; Supplementary Fig. 1b). After expansion, both subsets retained expression of Treg cell markers including CD25, FoxP3, GITR, CTLA-4 (Fig. 1b,c and Supplementary Fig. 1c)[11]. Interestingly, significant differences were observed in the expression of CD127, CD62L and CD27. Moreover, in vitro suppression assays consistently revealed a higher suppressive capacity in CD127lo sorted cells towards allo-stimulated autologous PBMC (n = 5) as well as CD25−CD4+ effector cells (n = 3) after expansion (Fig. 1d,e). Expanded CD127lo cells expressing CD62L and CD27 suppressed PBMC much more efficiently than cells expressing low levels of CD62L, CD27, or both (Fig. 1f). The molecular mechanism of suppression by expanded Treg cells in vitro was not dependent on IL-10 or CTLA-4 (Supplementary Fig. 2a) and required cell-cell contact (Supplementary Fig. 2b). Moreover, both CD25hiCD4+ and CD127loCD4+ expanded Treg cells inhibited dendritic cell maturation as demonstrated by the reduced increase in CD86 expression (Supplementary Fig 2c,d). Importantly, the expanded Treg populations could be frozen for storage without loss of functional activity (Supplementary Fig. 3a) and exerted their suppressive ability even over HLA-mismatched PBMC (Supplementary Fig. 3b).
Figure 1. Sorted and ex vivo expanded human CD25hiCD4+ and CD127loCD4+ cells retain characteristic features of Treg cells and differ in suppressive activity in vitro.
(a) Schematic representation of the expansion protocol and (b) a typical staining of CD25hi and CD127lo populations from the same blood donor is shown. (c) Expression of FOXP3, GITR, CTLA-4, CD25, CD127, CD62L and CD27 after cell expansion is depicted. The graph represents the percentage of cells positive for each indicated marker. * P = 0.004 **P = 0.0128 ***P = 0.0218. (d) Autologous PBMC or (e) ex vivo expanded CD25−CD4+ T cells were co-cultured with irradiated allogeneic PBMC and serial dilutions of expanded Treg cells. Data for cells expanded from the same blood donor are shown, with 4 replicates per test. (f) Expanded Treg cells were sorted for CD27, CD62L or both markers and co-cultured with autologous PBMC and irradiated allogeneic PBMC (as in c). Results are expressed as a percentage of allo-stimulated PBMC proliferation. Error bars in all panels represent the means ± s.d.
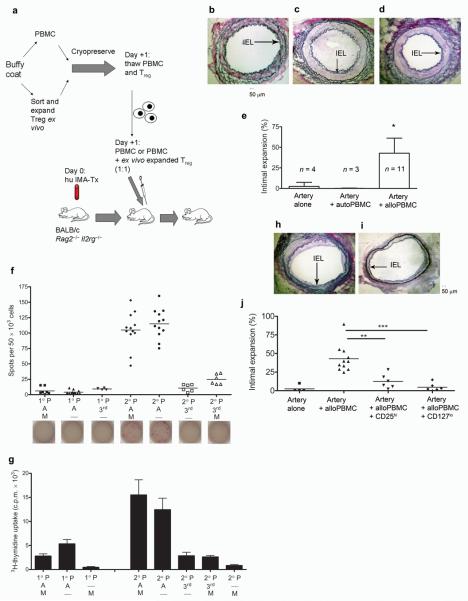
Next, the functional activity of the CD25hiCD4+ and CD127loCD4+ expanded cells, from five separate PBMC donors, was tested in vivo. Human internal mammary arterial (IMA) sidebranches, procured from patients undergoing coronary artery bypass surgery, were transplanted into the infrarenal aorta of BALB/c.Rag2−/−.Il2rg−/− mice deficient in T, B, and NK cells. The following day 10 × 106 allogeneic or autologous PBMC were used to inoculate mice bearing human arterial grafts. Transplanted human arterial grafts were maximally dilated and harvested 30 days after transplantation (Fig. 2a). In mice reconstituted with allogeneic PBMC (defined as >1% splenic human leukocyte chimerism; Supplementary Fig. 4) a significant vascular intimal hyperplasia and a decrease in luminal diameter was observed in the transplanted vessels as compared to that of vessels transplanted into animals that either did not receive human PBMC, or those reconstituted with PBMC autologous to the arterial graft (Fig. 2b–e; Supplementary Table 1). Importantly, the neointimal proliferation seen in rejecting arterial grafts was allo-specific to the human vessel as the native mouse aorta prior to the suture line was not affected (Supplementary Fig. 5a). Moreover, xeno-responses were confirmed to play only a minor role in this system as evidenced by in vitro mixed lymphocyte reactions (MLR); wherein, allo-human responses predominated over any and all xeno-responses (Supplementary Fig. 5b). In vitro analysis of precursor frequency, measured by IFN-γ ELISPOT or proliferation, demonstrated increased frequency of donor alloantigen specific responding cells upon restimulation, irrespective of the presence or absence of mouse splenocytes (Fig. 2f,g).
Figure 2. TA mediated by allogeneic human PBMC in human arterial interposition grafts is attenuated with human Treg cells.
(a) A schematic representation of the in vivo protocol. (b–d) EvG staining of a human artery procured from mice transplanted with an artery alone and not reconstituted with PBMC (b); or reconstituted with either 10 × 106 allogeneic human PBMC (c) or 10 × 106 autologous PBMC taken from the arterial donor (d). (e) Percentage of intimal expansion in human arteries from all experiments. (f) Results of ELISPOT for human IFN-γ and (g) MLR performed with cells from both the primary (1°) and secondary (2°) cultures (f and g, 2 independent experiments with ≥ 4 replicates; P denotes responding PBMC, A – irradiated allogeneic PBMC, 3rd – irradiated 3rd party PBMC, M – irradiated mouse splenocytes). (h,i) EvG staining of human artery procured from mice inoculated with PBMC + CD25hi Treg cells (h) or PBMC + CD127lo Treg cells (i) at a 1:1 ratio (10 × 106 cells). Histological quantification of intimal expansion in mice treated with Treg cell therapy (j). Error bars are represented as means ± s.d. * P < 0.02 in the artery + allo-PBMC group when compared to controls, ** P = 0.0013, ***P = 0.0007. IEL denotes internal elastic lamina.
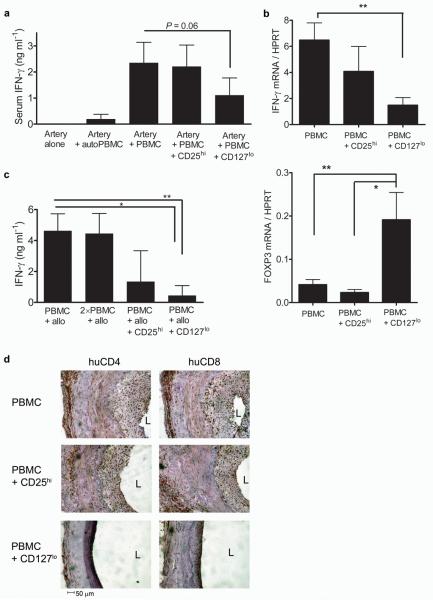
We then reconstituted mice transplanted with human vessels with human PBMC and ex vivo expanded CD25hi or CD127lo cells from the same allogeneic blood donor at a 1:1 ratio. Here we show that both CD25hi and CD127lo populations have a significant impact on the development of TA in vivo without inhibiting the rate of reconstitution (Fig. 2h–j and Supplementary Fig. 4b–d). Analysis of vessels transplanted into mice reconstituted with human Treg cells only was not feasible as the level of reconstitution achieved using fractionated CD4+ T cells alone was <1% (data not shown). The vasculopathy seen in chimeric humanized mice treated with CD25hi Treg cellular therapy was significantly less than that in mice reconstituted with allo-PBMC alone (P = 0.013); however, TA is still evident in these grafts at varying degrees (Fig. 2h,j). In contrast, animals treated with CD127lo ex vivo expanded Treg cells reveal almost complete abrogation of TA without such variation between different PBMC and vessel donors, corroborating our in vitro results (Fig. 1 and Fig. 2 i,j). Moreover, in the presence of Treg cell therapy IFN-γ production is markedly diminished when compared to stimulation of PBMC alone with alloantigen either in vivo (Fig. 3a,b and Supplementary Fig. 6a) or in vitro (Fig. 3c and Supplementary Fig. 6b).
Figure 3. Treg cells impair effector cell function.
(a) ELISA for human IFN-γ performed on serum samples harvested from mice transplanted with an arterial graft reconstituted with allogeneic PBMC in the presence or absence of ex vivo expanded CD25hi or CD127lo Treg cells. (b) Expression of IFN-γ and FOXP3 mRNA measured by real-time RT-PCR in transplanted grafts from mice inoculated with allogeneic PBMC or allogeneic PBMC and Treg cells (n = 4–6, *P < 0.05 **P < 0.01). (c) Human IFN-γ ELISA on supernatants from cultures of PBMC 5 days after stimulation with irradiated allogeneic PBMC in the presence or absence of Treg cells (* P = 0.0260 ** P = 0.0022). (d) Infiltration of human CD4 and CD8 T cells into the adventitial and neointimal layers of the arterial grafts in mice reconstituted with allogeneic PBMC alone or PBMC and Treg cells. (n = 3 separate experiments; L = lumen). Error bars represent the means ± s.d.
The ability of Treg cells to inhibit the secretion of IFN-γ in vivo, thought to be integral to the development of TA[8,12,13], was confirmed by testing serum samples procured from human leukocyte engrafted mice; upregulation of human IFN-γ by human PBMC alone was substantially inhibited by CD127lo Treg cellular therapy (Fig. 3a). Importantly, the expression of IFN-γ within the graft itself was also significantly inhibited by treatment with CD127lo Treg cells as indicated by real-time PCR analysis (Fig. 3b; P < 0.01). Suppression of IFN-γ expression within the allograft correlated with increased detection of intragraft FOXP3 mRNA in animals treated with CD127lo Treg cells (Fig. 3b), indicating that CD127lo Treg cells may infiltrate the graft. Histological assessment revealed that virtually all human graft infiltrating cells were CD3+ T cells (854 ± 280 hCD45+ versus 845 ± 318 hCD3+ in the neointima in the PBMC group; mean ± SD). Notably, human CD4 and CD8 infiltration of both the graft adventitia and neointima[14] was markedly decreased in mice treated with PBMC + CD127lo Treg cell therapy compared to that in mice reconstituted with PBMC alone or with PBMC + CD25hi cells (Fig. 3d). Taken together, these data suggest that treatment with Treg cells may inhibit the development of TA by preventing the effector response of PBMC and mobilization of IFN-γ producing cells to an allograft [15,16].
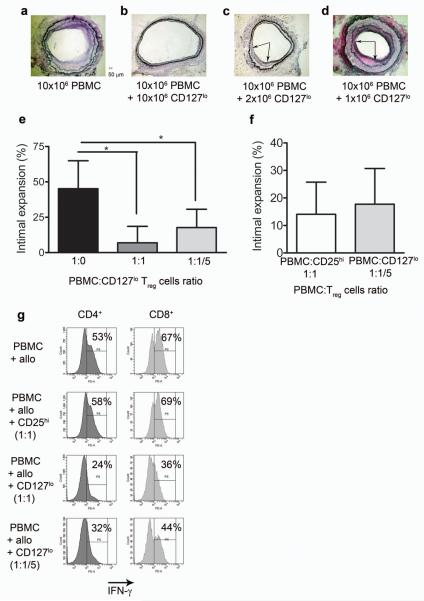
We then investigated the potency of CD127lo Treg cells on the development of TA in the human-mouse chimera. Interestingly, when 2 × 106 CD127lo Treg cells were transferred with 10 × 106 PBMC, we also observed a significant decrease in the development of arteriosclerotic disease when compared to transplanted animals inoculated with PBMC alone (Fig. 4a–c,e; P < 0.05). However, the efficacy of regulation was lost when 1 × 106 CD127lo Treg cells were co-transferred with 10 × 106 PBMC (Fig. 4d). The level of TA seen in animals receiving five-fold less CD127lo Treg cells was similar to that of animals receiving PBMC + CD25hi Treg cells at a 1:1 ratio (Fig. 4f). Additionally, CD4+ and CD8+ lymphocytes produced less IFN-γ in vitro when co-cultured with CD127lo Treg cells at a 1:1 and a 1:1/5 ratio confirming the increased potency of CD127lo Treg cells (Fig. 4g). Taken together, these data suggest that titrated levels of ex vivo expanded Treg cells sorted based on CD127lo expression are five fold more potent than that of CD25hi Treg cells.
Figure 4. Ex vivo expanded CD127lo Treg cells are more potent than CD25hi Treg.
(a–d) Representative EvG staining of human arterial grafts from mice reconstituted with 10 × 106 allogeneic PBMC (a, n = 9), with PBMC + 10 × 106 CD127lo Treg (1:1 ratio) (b, n = 7), with PBMC and 2 × 106 CD127lo Treg (1:5 ratio) (c, n = 3) and PBMC + 1 × 106 CD127lo Treg cells (1:10 ratio) (d; n = 3). (e) Calculated percentage of intimal expansion at different PBMC to CD127lo Treg ratios (* P < 0.05). (f) Percentage of intimal expansion at 1:1/5 PBMC to CD127lo Treg ratio compared to PBMC with CD25hi Treg at a 1:1 ratio (n = 4). (g) Intracellular IFN-γ production in CD4+ and CD8+ T cells from in vitro cultures of PBMC ± Treg cells (n = 3 independent experiments). Error bars represented as standard deviations.
These data demonstrate the unique ability of ex vivo expanded human Treg cells to successfully abrogate the development of TA in vivo. Over the past decade, both thymus derived and antigen induced Treg cells, initially identified by Sakaguchi et. al., as CD25+CD4+, have been highlighted for their suppressive capabilities in vitro and in vivo[11,17,18] and therapeutic potential in the regulation of autoimmunity, allergy and immune-mediated transplant rejection. Although, the results presented here are promising and exciting, they must also be interpreted with caution. As seen in Supplementary Fig. 7, mice treated with Treg cell therapy lose medial architecture in the graft when compared to native arterial sections or animals treated with PBMC alone.
Therefore, translation to the clinic, is likely dependent upon the isolation of pure populations of suppressive cells that can reproducibly and safely abrogate disease. Critical to this process is the identification of markers that permit the isolation and enrichment of viable Treg cells for therapeutic transfer. The recent observation of the inverse correlation between the IL-7 Rα (CD127) subunit and the suppressive capacity of CD25hiCD4+ Treg cells allows for an additional potential target to isolate and expand cells with increased suppressive function ex vivo[19-21]. In mouse models, only the subpopulation of CD25+CD4+ Treg cells expressing CD62L+ were found responsible for protection against lethal acute GVHD[22]. Therefore, the higher expression of CD62L on the ex vivo expanded CD127lo Treg cells used here may be advantageous in clinical situations allowing for more efficient homing to secondary lymph nodes, as well as in the chimeric humanized in vivo model used here, based on the high conservation of the CD62L molecule between species[23]. Additionally, the downregulation of the IL-7Rα along with the upregulation of CD27 (Fig. 1) have been correlated previously to a highly suppressive subset of Treg cells believed to exert their effects locally (i.e. allograft or draining lymph node) rather than re-circulating systemically[19,24]. The paradigm of disease that has been proposed to explain development of TA is one of local effector cell-mediated recruitment of rapidly proliferating vascular smooth muscle cells leading to neointimal formation and arterial luminal occlusion[3]. With our findings, it seems that immunoregulation of these locally destructive effector cells may be feasible with the use of Treg cellular therapy. However, further tests of the efficacy and safety of these cells are essential.
Methods
Mice
We obtained BALB/c.rag2−/−.Il2rg −/−(H2d) mice from Charles River Laboratories and housed the animals under specific pathogen-free conditions. We performed all experiments using protocols approved by the Committee on Animal Care and Ethical Review at Oxford University and in accordance with the UK Animals (Scientific Procedures) Act 1986. We used mice between the ages of 6-10 weeks.
The vast majority of mice (over 75%) reconstituted with human PBMC showed >1% splenic engraftment defined as fully reconstituted. The level of reconstitution was routinely over 10% and did not correlate with amount of arteriosclerosis seen (SNN, GW, KJW unpublished observations).
Procurement of human arterial side branches
We obtained perforating side branches of the IMA during procurement of the left and right IMA in a skeletonized fashion and immediately placed in a 4.0% phenoxybenzamine and normal saline solution[25]. Human tissue used for this study was obtained under the ethical reference number 04/Q1605/89. Study was approved by UK National Research Ethics Service, Oxfordshire REC B. Informed consent was obtained from all patients.
Aortic interposition grafting
Aortic interposition grafts were performed with human IMA sidebrancehes into the abdominal aorta of BALB/c.rag2−/−.Il2rg −/− mice with an end-to end anastomosis using a technique described by Koulack et al.[26]
Sorting and expansion of human CD25hi T cells and CD127lo T cells
We isolated PBMC from buffy coats provided by the National Blood Service (NBS) by Ficoll-Paque (GE Healthcare) gradient centrifugation. CD4+ T cells were then enriched using the Human CD4+ Lymphocyte Enrichment Set (BD), stained with appropriate antibodies (CD4 APC and CD25 PE or CD127 PE, CD25 APC and CD4 PerCP, all BD) and sorted using a BD FacsARIA cell sorter (BD). CD4+ T cells expressing high levels of CD25 (CD25hiCD4+) and low levels of CD127 (CD127lo CD25+CD4+) were subsequently sorted and recovered. In a limited number of experiments we stained whole PBMC for sorting giving a similar purity after each sort (more than 96%). Cells were cultured in RPMI 1640 media supplemented with L-glutamine, penicillin/streptomycin, sodium pyruvate and 10% of human AB pooled serum. For expansion, sorted cells were cultured in the presence of recombinant human IL-2 (1,000 U ml−1) (Chiron) and Human T-Expander CD3/CD28 Dynabeads (Invitrogen) in a 1:2 cell per bead ratio for two rounds of expansion, 7 days each. After the second round of expansion, beads were removed and cells were silenced for 2 days in medium containing 200 U ml−1 of IL-2 (Fig. 1a). After each expansion, the phenotype of cells were assessed by staining for FoxP3 (eBiosciences), GITR (R&D Systems) CD25, CTLA-4, CD127, CD27 and CD62L (BD).
Flow cytometry
Analysis of in vivo experiments on post-operative day 30:
We generated single cell suspensions from spleen and performed a red cell lysis was performed. For phenotypic analysis, antibodies against human CD3 (eBioscience), CD4 (Caltag), CD8, CD25, CD45, and CD127 (BD) were used.
Intracellular cytokine staining
For cytokine analysis, cells were stimulated with irradiated allogeneic PBMC for 5 days and re-stimulated for 5 h with phorbol 12-myristate 13-acetate (PMA, 100 ng ml−1, Sigma), ionomycin (1 μg ml−1, Sigma) and monensin (GolgiStop, 1 μl ml−1, BD) before being stained with antibodies to CD4, CD8, CD3 (BD), and the viability dye 7-AAD (eBiosciences). We then fixed and permeabilized cells and stained with antibody to intracellular IFN-γ (BD). .
In vitro suppression assays
We tested the suppressive activity of expanded CD25hi or CD127lo cells by using an in vitro suppression assays. Autologous PBMC (1 × 105) were incubated with irradiated allogeneic PBMC (1 × 105) and varying numbers of expanded CD25hi or CD127lo cells. We then assessed proliferation after 7 days by addition of 3H-thymidine for the last 16 h of culture.
Adoptive transfer of human cells
In all experiments, human PBMC were obtained from random donors and the data pooled. 10 × 106 Ficoll-Paque purified human PBMC were injected intraperitoneally into recipient mice. Alternatively, human PBMC were adoptively transferred in the same fashion with an equal number of CD25hi or CD127lo ex vivo expanded human T cells. In the described titration experiments, 2 × 106 CD127lo or 1 × 106 CD127lo were co-transferred with 10 × 106 PBMC.
ELISA for human IFN-γ
As per manufacturer's protocol (eBioscience ELISA Ready-SET-Go! Kit)
Cytokine Bead Array
Cytokine multiplex for the following TH1 and TH2 cytokines were stained for using two color (PE and FITC) bead array systems manufactured by Bender Medsystems (Vienna, Austria). The assay was carried out using manufacturer's protocols. Cytokines detected were: IL-6, IL-8, IL-10, IL-2, IFN-γ, IL-12p70, TNF-β, TNF-α, IL-5, and IL-4.
ELISPOT
We performed IFN-γ ELISPOT (BD Biosciences) in 3–6 repeats with 5 × 104 responder cells and 1 × 105 irradiated stimulatory cells per well incubated for 15 h. The assay was carried out following manufacturer's protocol.
Real-time RT-PCR
Total RNA was isolated from arterial grafts using NucleoSpin RNA XS kit (Fisher Scientific) and followed by cDNA synthesis using High Capacity RNA-to-cDNA kit (Applied Biosystems). Real-time quantification was performed using Stratagene Mx3000P (Agilent Technologies) using the HPRT probe and primers as described in [27] and TaqMan Gene Expression Assays (Applied Biosystems) for IFN-γ and FOXP3. Quantification of the gene of interest was given by ΔCt method.
Histology
After thirty days post transplantation, we procured aortic grafts which were then snap- frozen in OCT embedding medium prior to cryostat sectioning at a thickness of 6–10 μm. After drying, sections were fixed in 100% acetone for 10 min at 4 °C and stored in −80 °C until further use. We then blocked all sections with 10% BSA and 5% mouse serum at room temperature for 30 min. Primary antibodies, mouse anti-human CD4 (BD) and mouse anti-human CD8 (BD) were then applied. Following several washes, HRP-conjugated secondary antibody (DAKO Cytomation) was applied, and sections were visualized with diaminobenzidene (Sigma).
Computational and statistical analyses
We analysed data from mice displaying >1% human CD45+ live leukocytes in the spleen as measured by flow cytometry. Morphometric analysis of transplant arteriosclerosis on EvG-stained sections were measured as described[18]. Briefly, percentage intimal expansion (IE) was calculated using the following formula: % intimal expansion = (AI- AL/ AI) × 100; where AI is the area within the internal elastic lamina and AL is the luminal area.
Statistical evaluations were performed using Graphpad Prism software. Statistical significance was determined using Mann-Whitney U test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank the staff of the Biomedical Services Unit at the John Radcliffe Hospital for expert animal care; M. Barnardo and the staff at the Clinical Transplant Immunology Laboratory, The Oxford Transplant Centre for molecular HLA typing; M. Carvalho-Gaspar for advice on real-time PCR; F. Issa and R. Goto for assistance with some experiments; A. Bushell and N. Jones for valuable discussions. The cardiac surgical registrars and operating theatre staff for assistance with the procurement of vessels. This work was supported by grants from The Wellcome Trust, European Union Integrated Project, RISET (www.RISETfp6.org), Medical Research Council UK and Garfield Weston Trust. SNN is an International Society for Heart and Lung Transplantation Research Fellow; JW is a RISET investigator, GW is a DFG Fellow; WZ was supported by an unrestricted grant from Becton Dickinson; AS was supported by the Swedish Heart and Lung Foundation and the Swedish Research Council.
Footnotes
Competing financial interest policy – Authors declare no competing financial interest
References
- 1.Pucci AM, Forbes RD, Billingham ME. Pathologic features in long-term cardiac allografts. J Heart Transplant. 1990;9:339–345. [PubMed] [Google Scholar]
- 2.Hillebrands JL, Rozing J. Chronic transplant dysfunction and transplant arteriosclerosis: new insights into underlying mechanisms. Expert Rev Mol Med. 2003;2003:1–23. doi: 10.1017/S146239940300557X. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell RN, Lichtman AH. The link between IFN-gamma and allograft arteriopathy: is the answer NO? J Clin Invest. 2004;114:762–764. doi: 10.1172/JCI22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult heart transplantation report--2006. J Heart Lung Transplant. 2006;25:869–879. doi: 10.1016/j.healun.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Tullius SG, et al. Contribution of early acute rejection episodes to chronic rejection in a rat kidney retransplantation model. Kidney Int. 1998;53:465–472. doi: 10.1046/j.1523-1755.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 7.Kobashigawa JA, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Tellides G, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey WR, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 11.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 13.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 14.Cuffy MC, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 15.Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol. 2007;179:3578–3587. doi: 10.4049/jimmunol.179.6.3578. [DOI] [PubMed] [Google Scholar]
- 16.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002;3:1208–1213. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 18.Warnecke G, Bushell A, Nadig SN, Wood KJ. Regulation of transplant arteriosclerosis by CD25+CD4+ T cells generated to alloantigen in vivo. Transplantation. 2007;83:1459–1465. doi: 10.1097/01.tp.0000265446.61754.d2. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putnam AL, et al. Expansion of Human Regulatory T Cells from Patients with Type 1 Diabetes. Diabetes. 2008;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ermann J, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 23.Jutila MA, Watts G, Walcheck B, Kansas GS. Characterization of a functionally important and evolutionarily well-conserved epitope mapped to the short consensus repeats of E-selectin and L-selectin. J Exp Med. 1992;175:1565–1573. doi: 10.1084/jem.175.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenen HJ, Fasse E, Joosten I. CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol. 2005;174:7573–7583. doi: 10.4049/jimmunol.174.12.7573. [DOI] [PubMed] [Google Scholar]
- 25.Mussa S, Choudhary BP, Taggart DP. Radial artery conduits for coronary artery bypass grafting: current perspective. J Thorac Cardiovasc Surg. 2005;129:250–253. doi: 10.1016/j.jtcvs.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Koulack J, et al. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 1995;16:110–113. doi: 10.1002/micr.1920160213. [DOI] [PubMed] [Google Scholar]
- 27.Trzonkowski P, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8:338–347. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.