Abstract
Gambling is a common recreational activity that becomes dysfunctional in a subset of individuals, with DSM “pathological gambling” regarded as the most severe form. During gambling, players experience a range of cognitive distortions that promote an overestimation of the chances of winning. Near-miss outcomes are thought to fuel these distortions. We observed previously that near misses recruited overlapping circuitry to monetary wins in a study in healthy volunteers (Clark et al., 2009). The present study sought to extend these observations in regular gamblers and relate brain responses to an index of gambling severity. Twenty regular gamblers, who varied in their involvement from recreational playing to probable pathological gambling, were scanned while performing a simplified slot machine task that delivered occasional monetary wins, as well as near-miss and full-miss nonwin outcomes. In the overall group, near-miss outcomes were associated with a significant response in the ventral striatum, which was also recruited by monetary wins. Gambling severity, measured with the South Oaks Gambling Screen, predicted a greater response in the dopaminergic midbrain to near-miss outcomes. This effect survived controlling for clinical comorbidities that were present in the regular gamblers. Gambling severity did not predict win-related responses in the midbrain or elsewhere. These results demonstrate that near-miss events during gambling recruit reward-related brain circuitry in regular players. An association with gambling severity in the midbrain suggests that near-miss outcomes may enhance dopamine transmission in disordered gambling, which extends neurobiological similarities between pathological gambling and drug addiction.
Introduction
Gambling is a form of entertainment that can become dysfunctional in some individuals: “pathological gambling” is a DSM-IV Impulse Control Disorder (American Psychiatric Association, 2000) with symptoms that include withdrawal and tolerance (Potenza, 2006). Accumulating data indicate neurobiological alterations in the brain reward system in problem gamblers (Reuter et al., 2005; Tanabe et al., 2007; Potenza, 2008). For example, an fMRI study using a guessing task with monetary wins and losses found an attenuation of win-related activity in the ventral striatum and medial prefrontal cortex (PFC) of pathological gamblers (Reuter et al., 2005). Similar changes have been described in drug abusers (Goldstein et al., 2007; Wrase et al., 2007) and are thought to indicate dysregulation of dopaminergic input to these structures. Dopaminergic involvement in gambling is supported by reports of problem gambling as a medication side effect in patients with Parkinson's disease (Dodd et al., 2005; Steeves et al., 2009).
Neuroimaging investigations of problem gambling to date have neglected the complex cognitions that gamblers frequently experience (Ladouceur and Walker, 1996). On games of chance like roulette or lottery, gamblers often misperceive some level of skill involvement (the “illusion of control”) (Langer, 1975). These cognitive distortions are more prevalent in problem gamblers (Miller and Currie, 2008) and are directly fostered by certain features of gambling games (Griffiths, 1993), including the presence of near misses: nonwin outcomes that are proximal to a jackpot. Near misses are able to promote continued gambling despite their objective nonwin (loss) status (Kassinove and Schare, 2001; Côté et al., 2003). The neural mechanisms that underpin near-miss effects have broader relevance to the understanding of reinforcement learning: on games of skill (e.g., soccer), near misses (e.g., hitting the post) provide a valid signal of skill acquisition and imminent reward, and thus a reinforcement learning system may usefully assign value to these outcomes. However, in games of chance, near misses do not indicate future success, and their potency suggests that gambling games may harness brain mechanisms that naturally handle skill situations (Clark, 2010).
Using a slot machine task in healthy volunteers, we found that near misses were associated with significant activity in brain regions (ventral striatum, anterior insula) that responded to monetary wins (Clark et al., 2009). The present study aimed to extend these observations in a group of regular gamblers. First, we aimed to corroborate our finding that near-miss outcomes would recruit components of the brain reward system in regular gamblers. Second, we sought to identify areas within this system in which brain activity during gambling was associated with gambling severity. Although previous fMRI studies have explored problem gambling using case control designs, it is increasingly recognized that disordered gambling is dimensional in nature: gamblers who do not meet DSM criteria frequently describe obvious gambling-related harms (e.g., debt, interpersonal conflict), and these harms increase steadily with gambling involvement (e.g., gambling frequency or expenditure) (Currie et al., 2006). To reflect this continuum of disordered gambling, we used voxelwise regression to identify brain areas in which win- and near-miss-related activity was predicted by individual variation in gambling severity.
Materials and Methods
Participants.
Regular gamblers (n = 24, 3 female) were recruited via advertisement. Four subjects were excluded from analysis due to excessive movement during scanning, leaving a reported group size of 20 (2 female). Subjects attended an fMRI scanning session at the Wolfson Brain Imaging Centre, Cambridge, United Kingdom. The protocol was approved by the Norfolk and Norwich Research Ethics Committee (COREC 06/Q0101/69) and all volunteers provided written informed consent. Volunteers were reimbursed £40 for participation and had the opportunity to win further money on the task (unbeknownst to subjects, this was a fixed amount of £15).
Gambling behavior was assessed using the SOGS (Lesieur and Blume, 1987), a 16-item self-report scale assessing core symptoms and negative consequences of gambling (e.g., loss chasing, borrowing money, lying about gambling, family conflict). Before the scanning session, subjects attended a screening session involving a structured psychiatric interview with a postdoctoral psychologist (Structured Clinical Interview for DSM-IV Axis I Disorders; SCID) (First et al., 1996). Given the high comorbidity between problem gambling and other mental health problems (Kessler et al., 2008), we opted to tolerate psychiatric comorbidities to avoid overselection of a clinically unrepresentative sample. Comorbidities were as follows: current dysthymia and/or drug-related mood disorder (n = 5), lifetime major depressive disorder (n = 4), current bipolar disorder (n = 1), anxiety or panic disorder (n = 2), lifetime drug dependence (n = 3), current alcohol/drug abuse (n = 8), and current alcohol dependence (n = 1). Three subjects were currently receiving psychotropic medication (antidepressant n = 2, benzodiazepine n = 1). In addition, urinalysis (SureStep) on the day of the fMRI scan detected positive tests for cannabis (tetrahydrocannabinol) in four participants. The following self-report questionnaire measures were used to quantify current psychiatric symptoms: Beck Depression Inventory (BDI; version 2) (Beck et al., 1996), Beck Anxiety Inventory (BAI) (Beck et al., 1988), Adult ADHD Self-Report Scale (ASRS) (Kessler et al., 2005), Padua Inventory for obsessive–compulsive disorder (OCD) symptoms (Burns et al., 1996), Barratt Impulsivity Scale (BIS) (Patton et al., 1995), and the Alcohol Use Questionnaire (AUQ) (Townshend and Duka, 2002).
Procedure.
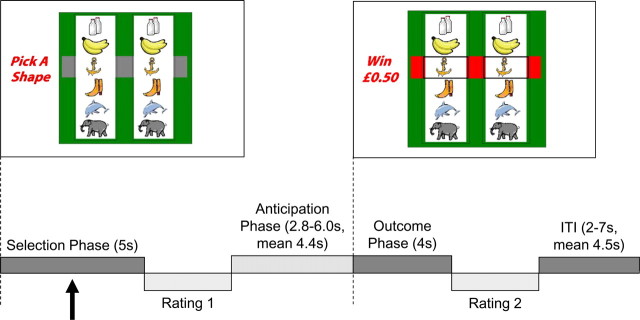
During the fMRI scan, subjects completed three blocks of 60 trials on the Slot Machine Task (Clark et al., 2009), lasting ∼45 min. Subjects were practiced on the task (10 trials with two hypothetical wins) before entering the scanner, and during scanning, responses were registered using a button box. Trial structure and display screen are displayed in Figure 1. On each trial, two reels are presented on the screen, with a horizontal “payline” visible centrally. Six icons are displayed on each reel in the same order. The six icons were selected from 16 alternatives at the start of the scan task, to heighten a sense of involvement.
Figure 1.
Task design. The slot machine task presents two reels, with the six identical play icons displayed on each reel, and a horizontal payline across the center of the screen. On trials with a white screen background (as displayed), the volunteer selected one icon on the left reel, using two buttons to scroll through the icons, and one button to select. On trials with a black screen background, the computer selected the play icon. Following icon selection, the right hand reel spun for a variable duration (2.8–6 s), and decelerated to a standstill. During outcome (4 s), if the right reel stopped on the selected icon (i.e., matching icons were aligned in the payline), the subject was awarded £0.50 (approximately $0.80); all other outcomes won nothing. Following the outcome phase, there was an intertrial interval of variable duration (2–7 s). On intermittent (1/3) trials, two ratings were acquired using an on-screen visual analog scale: following selection, subjects were asked “How do you rate your chances of winning?,” and following outcome, subjects were asked “How much do you want to continue to play the game?”
Each trial proceeded as follows: during the selection phase, one of the six icons was selected on the left reel (selection phase; 5 s fixed duration). Following selection, the right reel was spun for 2.8–6 s (anticipation phase) and decelerated to a standstill, beginning the outcome phase (4 s fixed). At the end of each trial, there was an intertrial interval of variable duration (2–7 s). In the outcome phase, if the right reel stopped on the selected icon (i.e., matching icons were displayed in the payline), a £0.50 win was delivered; all other outcomes won nothing. Trials in which the right reel stopped one position above or below the payline were designated near misses. Nonwin trials in which the reel stopped in one of the three remaining positions (i.e., more than one position from the payline) were designated full misses. During the selection phase, on trials with a white screen background, the participant selected the play icon using two buttons to scroll through the shapes and a third button to confirm selection (participant-chosen trials) within the 5 s window. On trials with a black screen background, the computer selected the play icon, and the subject was required to confirm the selection by pressing the third button within the 5 s window (computer-chosen trials). Participant-chosen (n = 90) and computer-chosen (n = 90) trials were presented in a fixed pseudorandom order. If selection/confirmation was not completed within the 5 s window, a “Too Late!” message was presented, followed by the intertrial interval. Outcomes were pseudorandomized to ensure a fair number of wins (1/6, total 30 = £15), near misses (2/6, total 60), and full misses (3/6, total 90).
On 1/3 trials, subjective ratings were acquired at two points during the trial, using onscreen 21-point visual analog scales. Following selection, subjects rated “How do you rate your chances of winning?” and following the outcome, subjects rated “How much do you want to continue to play the game?” No time limit was imposed for the subjective ratings. Data from the subjective ratings were converted to a standardized z score, based on each individual's mean and SD for that rating, to account for the variability in anchoring across subjects. Subjective ratings were analyzed using paired t tests (for “chances of winning”) and repeated-measures ANOVA (for “continue to play”) with outcome (three levels: win, near miss, full miss) and control (two levels: participant chosen, computer chosen) as factors.
Imaging procedure.
Scanning was performed on a Siemens TimTrio 3 tesla magnet using a 32-slice axial oblique sequence, with a repetition time of 2 s (echo time 30 ms, flip angle 78°, voxel size 3.1 × 3.1 × 3.0 mm, matrix size 64 × 64, field of view 201 mm × 201 mm, bandwidth 2232 Hz/Px). Three runs of 60 trials were completed (630 repetitions), with six dummy scans discarded at the start of each run to allow for equilibrium effects. A high-resolution magnetization-prepared rapid acquisition gradient-echo sequence (MP-RAGE) structural image was acquired after the functional runs for use in the spatial normalization.
FMRI data were analyzed using SPM5 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). Preprocessing consisted of slice timing correction, within-subject realignment, spatial normalization, and spatial smoothing using a 10 mm Gaussian kernel. Subjects' movement parameters were screened for excessive movement (defined as >5 mm within a run), resulting in exclusion of four participants (one female) from all analysis. Time series were high pass filtered (128 s). Volumes were normalized to the International Consortium for Brain Mapping (ICBM) templates that approximate to Talairach and Tournoux (1988) space, using a matrix calculated by normalizing the segmented MP-RAGE structural image for each subject onto the ICBM gray and white matter templates.
A canonical hemodynamic response function (HRF) was modeled to the onsets of the selection phase, the anticipation phase, and the outcome phase on each trial, to minimize unexplained variance in the design matrix. To analyze outcome-related brain responses, the events were classified into eight trial types, comprising a two (choice: participant chosen, computer chosen) by four (win, near miss before the payline, near miss past the payline, full miss) factorial design. Movement parameters from realignment were included in the design matrix as covariates of no interest. The HRF was used as a covariate in a general linear model, and a parameter estimate was obtained for each voxel, for each event type, reflecting the strength of covariance between the data and the canonical HRF. Contrast images were calculated between parameter estimates from different trial types, and individual contrast images were then taken to a second-level random-effects group analysis.
Four contrasts were computed to assess the outcome-related brain responses in the overall group of regular gamblers: (1) all monetary wins (i.e., both participant- and computer-chosen trials) minus all nonwin outcomes; (2) near misses (on both participant- and computer-chosen trials) minus full-miss outcomes (on both participant- and computer-chosen trials); (3) near miss by personal control interaction: areas differentially recruited by near misses compared with full misses as a function of participant versus computer control (i.e., 1, −1, −1, 1); and (4) win activity on participant-chosen trials minus win activity on computer-chosen trials. To explore these effects as a function of gambling severity, voxelwise univariate regressions were run using the SOGS score as a predictor variable. Given our a priori hypotheses about the role of brain reward circuitry in gambling distortions and problem gambling, we implemented the win contrast [all monetary wins minus all nonwins, thresholded at pFWE < 0.05 corrected (FWE is for familywise error)] from our previous study (Clark et al., 2009) as a mask for these contrasts, as well as the regression analyses, using the PickAtlas tool (Maldjian et al., 2003). These region-of-interest (ROI) analyses were thresholded at p < 0.05 corrected for multiple comparisons using random field theory (Worsley et al., 1996), i.e., FWE corrected, with a cluster threshold of 10 voxels to reduce the rate of false positives (Forman et al., 1995). This cluster threshold was selected on the grounds that the smallest region of a priori interest [midbrain proximal to substantia nigra/ventral tegmental area (SN/VTA)] has an estimated size of 20–25 voxels (Düzel et al., 2009). Signal change was extracted from activated foci using the MARSBAR tool (Brett et al., 2002) for the purposes of plotting the data. Whole-brain analyses are also presented using an exploratory threshold of p < 0.001 uncorrected.
Results
Variation in gambling severity
The regular gamblers were predominantly male (n = 18) with a mean age of 33.7 (SD 1.8), mean years of education of 14.5 (SD 0.5), and mean National Adult Reading Test-estimated full intelligence quotient of 111.5 (SD 7.3). The preferred form of gambling in the group was off-course sports betting (horse racing or soccer), but slot machines, cards, and lotteries were also common (supplemental Table 1, available at www.jneurosci.org as supplemental material). All but one subject were currently active gamblers, playing at least once a week on their preferred form of gambling; the participant who was no longer gambling had been abstinent for one year. Thirteen of the group met the SOGS threshold of ≥5 for probable pathological gambling (overall range 0–20, mean 7.25, median 6.5) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Maximum expenditure in a single day varied as follows: £10-£100 (n = 5), £100-£1000 (n = 8), £1000-£10,000 (n = 5), and more than £10,000 (n = 2). Descriptive data for the questionnaire measures of clinical symptoms are reported in supplemental Table 2 (available at www.jneurosci.org as supplemental material).
Subjective ratings during the slot machine task
The postselection ratings of “How do you rate your chances of winning?” were significantly higher on participant-chosen trials compared with computer-chosen trials (t(19) = 5.2, p < 0.001). This effect of personal control was attenuated as a function of gambling severity as measured by the SOGS (r20 = −0.53, p = 0.016). The postoutcome ratings of “How much do you want to continue to play?” were analyzed using two-way ANOVA to reveal a main effect of feedback (F(2,38) = 40.179, p < 0.001), no main effect of agency (F(1,19) < 1), and an agency-by-feedback interaction (F(2,38) = 3.604, p = 0.037) (supplemental Table 3, available at www.jneurosci.org as supplemental material). Participant-chosen wins were rated more highly than computer-chosen wins (t(19) = 2.199, p = 0.040), but personal control did not influence the ratings for near-miss (t(19)= −1.272, p = 0.217) or full-miss (t(19)= −0.998, p = 0.331) outcomes. “Continue-to-play” ratings were higher after wins compared with either kind of nonwin, regardless of personal control (t(19) > 3.889, p < 0.002 in all cases), whereas near misses and full misses did not differ on participant-chosen trials (t(19) = 1.104, p = 0.283) or computer-chosen trials (t(19) < 1). Thus, there was no detectable effect of the near-miss outcomes on self-report ratings in the regular gamblers.
fMRI responses to gambling outcomes
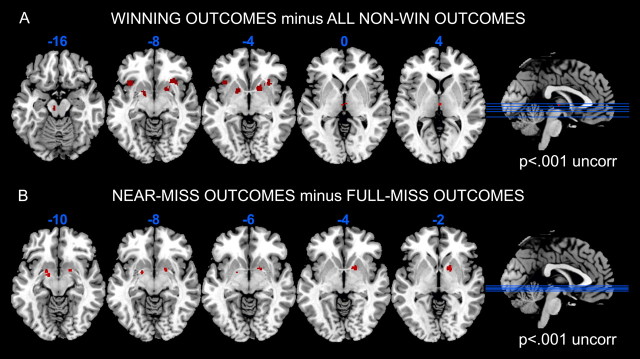
Brain regions sensitive to unpredictable monetary wins were identified by contrasting all winning outcomes against all nonwin outcomes, within an independent ROI defined from the win contrast in our previous study (Clark et al., 2009). Significant signal change was observed in a number of areas linked to reward and reinforcement learning: right ventral striatum (putamen) (peak voxel: x, y, z = 20, 10, −6, Z = 3.66, 133 voxels, pFWE = 0.029) and thalamus (x, y, z = 2, −6, 2; Z = 4.71, 14 voxels, pFWE = 0.001), with subthreshold foci in the left ventral striatum (x, y, z = −16, 2, −6, Z = 3.39, pFWE = 0.065), anterior insula bilaterally (x, y, z = 28, 20, −6, Z = 3.46, pFWE = 0.054; x, y, z = 36, 16, −8, Z = 3.36, pFWE = 0.070; x, y, z = −36, 18, −6, Z = 3.47, pFWE = 0.052), and midbrain proximal to SN/VTA (x, y, z = −8, −20, −14, Z = 3.36, pFWE = 0.071) (Fig. 2A, thresholded at p < 0.001 for display purposes). An independent contrast assessed brain responses to near-miss outcomes, compared with full misses. There was significant signal change in the right ventral striatum (putamen) (x, y, z = 18, 6, −2, Z = 3.67, 52 voxels, pFWE = 0.032) and the left parahippocampal gyrus (Brodmann area 28) bordering on the striatum (x, y, z = −16, −2, −10, Z = 4.32, 27 voxels, pFWE = 0.003) (Fig. 2B). The contrasts of participant-chosen wins minus computer-chosen wins, and the interaction contrast for near-miss activity as a function of personal control, did not yield any significant activation within the ROI mask.
Figure 2.
A, Win-related activation (win > nonwin outcomes) in the regular gamblers, using an ROI mask of win activity from an independent sample (Clark et al., 2009). Activity is displayed at p < 0.001 uncorrected, k = 10, to illustrate the anatomical extent of the activations and several responses falling just below the FWE threshold. B, Figure showing near-miss-related activation (near-miss events > full-miss events, within the ROI mask) across all participants, displayed at p < 0.001 uncorrected, k = 10. Activations are displayed on the ch2bet template, using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/).
Effects of gambling severity of fMRI responses to gambling outcomes
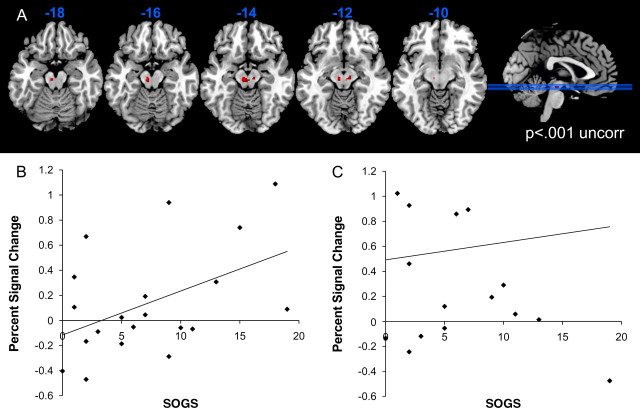
Gambling severity (SOGS score) was entered as a single regressor in the contrast of monetary wins minus all nonwins, using the win-sensitive ROI mask. There were no significant voxels in which SOGS score predicted either increases or decreases in win-related activity. However, a regression analysis for the near-miss minus full-miss contrast indicated that SOGS gambling severity was positively related to the brain response to near-miss outcomes in the midbrain (48 voxels: x, y, z = −6, −18, −16, Z = 4.99, pFWE < 0.001; x, y, z = 10, −18, −12, Z = 3.90, pFWE = 0.014) (Fig. 3). In addition, we also observed gambling severity was negatively related to the brain response to near-miss outcomes in the left caudate (x, y, z = −12, 8, 6, Z = 3.91, 11 voxels, pFWE = 0.013). This cluster lay at the dorsal tip of the ROI, overlapping the internal capsule, and we could not identify win (contrast 1)- or near-miss (contrast 2)-related activity at this focus in the present dataset, even at a liberal threshold (p < 0.005 uncorrected). Furthermore, the extracted signal from ventral striatum and midbrain clusters were positively correlated on both win (r20 = 0.72, p < 0.001) and near-miss (r20 = 0.43, p = 0.06) outcomes, as seen in previous studies (D'Ardenne et al., 2008; Schott et al., 2008; Kahnt et al., 2009). Therefore, although this caudate peak met our significance threshold, we are cautious about inferring a role for this region in gambling near misses.
Figure 3.
A, Effect of gambling severity (South Oaks Gambling Screen; SOGS) on near-miss-related activation, within the ROI mask (displayed at p < 0.001 uncorrected, k = 10). B, Extracted signal for the near-miss minus full-miss contrast in the midbrain, plotted against SOGS score. Midbrain signal was extracted from an independent region defined by the win minus nonwin contrast (contrast 1; peak voxel: x= −8, y= −20, z= −14). C, Extracted signal for the win minus nonwin contrast, plotted against SOGS score, for the same midbrain ROI as above.
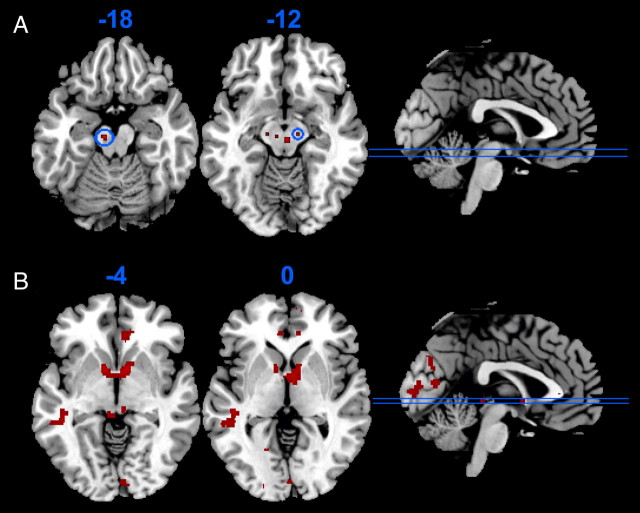
The smoothing kernel (10 mm) implemented in our primary analysis limited our ability to resolve activation within the midbrain. We remodeled the fMRI data using a smaller 4 mm smoothing kernel. In a whole-brain analysis using an exploratory threshold (p < 0.001 uncorrected), two activations in the midbrain (x = −8, y = −18, z = −18, Z = 3.37, p < 0.001; x = 12, y = −16, z = −12, Z = 3.28, p = 0.001) reflected the effect of SOGS gambling severity on near-miss-related activation (Fig. 4A, threshold of p < 0.005 uncorrected). These activations are consistent with a compound SN/VTA signal (Düzel et al., 2009).
Figure 4.
A, The association between gambling severity (SOGS score) and near-miss-related activation (near miss minus full miss) in the midbrain (z = −18 and z = −12), using a smaller (4 mm) smoothing kernel. Activity thresholded at p < 0.005 uncorrected for display purposes, with the clusters significant at the p < 0.001 threshold encircled in blue (x, y, z = −8, −18, −18 and 12, −16, −12). B, Between-groups comparison of the regular gamblers (n = 20) against 14 healthy volunteers with minimal gambling involvement (SOGS 0–2) from our previous experiment (Clark et al., 2009), illustrating reduced activity to monetary wins in the regular gamblers in the striatum and medial prefrontal cortex (p < 0.001 uncorrected, k = 10).
The regular gamblers displayed a number of clinical comorbidities that covaried moderately with their gambling severity. To examine whether the midbrain association was specifically associated with gambling severity rather than these comorbidities, we included continuous measures of depression (BDI), anxiety (BAI), ADHD symptomatology (ASRS), impulsivity (BIS), OCD symptoms (Padua scale) and alcohol use/abuse (AUQ scale) as additional covariate regressors in the SOGS regression. In each case, the midbrain activation (peak voxel: x = −6, y = −18, z = −16) for the SOGS association was detectable with a Z statistic between 2.20 and 2.56 (p = 0.014 to p = 0.005 uncorrected). In contrast, the negative association between SOGS and near-miss-related activity in the caudate did not survive controlling for depressive (BDI) and OCD (Padua scale) symptoms, at a liberal threshold of p < 0.05 uncorrected.
These data indicate that in a correlational design, a stronger midbrain response to near-miss outcomes was associated with disordered gambling. Previous case control studies of pathological gamblers indicate an overall attenuation of reward-related activity (Reuter et al., 2005). To investigate this apparent discrepancy, we conducted a post hoc between-groups analysis comparing the overall brain response to reward (wins minus nonwin outcomes) in our regular gamblers against the nongambling volunteers from our previous study (Clark et al., 2009). This was conducted as a whole-brain analysis using an exploratory significance threshold (p < 0.001 uncorrected). Consistent with the study by Reuter et al., the regular gamblers displayed a weaker response to monetary wins in several reward-sensitive regions including the striatum and rostral anterior cingulate cortex (Fig. 4B; supplemental Table 5, available at www.jneurosci.org as supplemental material), after covarying for group differences in age. There were no overall group differences in the near-miss response. A mixed-model ANOVA of the subjective ratings data in the regular gamblers and healthy nongamblers revealed no significant group differences, although notably, in the pooled group (n = 34) there was a marginally significant effect of participant-chosen near-miss outcomes to increase ratings of “continue to play” (t(33) = 1.87, p = 0.07) relative to participant-chosen full misses (supplemental Information and supplemental Table 6, available at www.jneurosci.org as supplemental material).
Discussion
The present study investigated brain responses during a computerized slot machine task in a group of regular gamblers who varied in their involvement from recreational, social playing to moderately severe probable pathological gambling. Unpredictable monetary wins on the task recruited a network of reward-sensitive regions including the ventral striatum. Our task further enabled the direct comparison of near-miss nonwins against full-miss nonwins, and this contrast revealed a response to near misses in striatal regions also responsive to wins, despite the objective nonwin status of these outcomes. This analysis in regular gamblers extends our recent findings in healthy volunteers with modest gambling involvement (Clark et al., 2009), highlighting the recruitment of brain reward circuitry by near-miss outcomes. The specific aim of the present study was to associate these fMRI responses with individual variation in gambling severity, to examine the relevance of these responses to an emerging literature on the neurobiology of problem gambling (Reuter et al., 2005; Potenza, 2008). Scores on our index of gambling severity (SOGS) ranged from 0 to 19 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material), with a score of 5 indicative of probable pathological gambling. This emphasizes the continuous nature of gambling harms in the nonclinical population (Currie et al., 2006), and indicates that a regression-based method of analysis is appropriate for exploring neural markers of disordered gambling. While the SOGS score was not associated with the brain response to monetary wins, gambling severity did predict the neural response to near-miss outcomes, in the midbrain. This activation was proximal to the dopaminergic nuclei in the SN/VTA, a finding that was further substantiated by a reanalysis of our data using a smaller (4 mm) smoothing kernel (Bunzeck and Düzel, 2006; D'Ardenne et al., 2008; Murray et al., 2008; Shohamy and Wagner, 2008; Düzel et al., 2009). Moreover, the association between midbrain activity to near misses and gambling severity was not readily explained by other clinical symptoms (depression, impulsivity, OCD, alcohol use) that are moderately prevalent in regular gamblers (Kessler et al., 2008).
The observed midbrain association is consistent with the role of dopamine transmission in disordered gambling, indicated by previous studies of peripheral markers (Bergh et al., 1997; Meyer et al., 2004), and the phenomenon of medication-induced pathological gambling in Parkinson's disease (Dodd et al., 2005; Steeves et al., 2009). This syndrome is particularly linked to D3-preferent dopamine agonist medications, and it is notable that D3 receptors are abundant in human SN (Gurevich and Joyce, 1999). The ability of near-miss outcomes to enhance dopamine transmission in more severe problem gamblers could underlie the potency of these outcomes to invigorate gambling (Kassinove and Schare, 2001; Côté et al., 2003; Clark et al., 2009). Electrophysiological studies recording from midbrain neurons have demonstrated the well known role for this system in signaling reward and coding reward prediction errors (Schultz, 2002; Montague et al., 2004). Human neuroimaging studies confirm midbrain blood oxygenation level-dependent (BOLD) responses in monetary reward tasks (Bjork et al., 2004; D'Ardenne et al., 2008; Schott et al., 2008), which correlate with a direct index of striatal dopamine release ([11C]raclopride displacement) (Schott et al., 2008). Certainly, it is likely that reward prediction errors were engendered on near-miss trials in the current task: a positive prediction error occurs as the reel decelerates and the subject anticipates a winning outcome. This is immediately followed by a negative prediction error, as the reel stops one position from the winning payline. Recent data indicate that midbrain BOLD signal may be particularly aligned with positive prediction errors (D'Ardenne et al., 2008), consistent with gamblers' more general style of overestimating their chances of winning (Ladouceur and Walker, 1996). Two further aspects of midbrain firing seen in electrophysiological data may be relevant to the current fMRI findings. First, midbrain neurons display generalization, in which they fire to stimuli that are similar to those predictive of reward (Tobler et al., 2005; Shohamy and Wagner, 2008). It is a testable hypothesis that problem gamblers show excessive generalization of reward-predictive stimuli, mediated by midbrain hyper-reactivity. Second, midbrain neurons may show adaptive coding within a task, in which their maximal response is scaled to the available reward (Tobler et al., 2005). This may explain why we did not observe a midbrain association with gambling severity on winning outcomes, despite an overall midbrain response to wins. However, we did not explicitly demonstrate a significant difference in the strength of the SOGS–midbrain association on near-miss and win trials. From the positive trendline in Figure 3C, it is conceivable that a SOGS–midbrain association may be detectable for winning outcomes in a larger sample.
A previous case control study in pathological gamblers reported reduced BOLD signal in ventral striatum and medial PFC in response to monetary wins (Reuter et al., 2005). This finding was interpreted as evidence for a reward deficiency account of pathological gambling, in which a hypoactive reward system confers vulnerability to a range of addictions (Bowirrat and Oscar-Berman, 2005). The task used in the Reuter et al. study was a simple two-choice guessing task that is unlikely to elicit the complex distortions of probability and skill perception that are central to gambling behavior (Ladouceur and Walker, 1996; Clark, 2010). We conducted a between-groups analysis comparing the regular gamblers from the present study against volunteers with modest gambling involvement from our previous study (Clark et al., 2009). Although the circuitry recruited by monetary wins was strikingly similar across the two groups, the regular gamblers displayed an attenuated response to winning that was significant in the ventral striatum and medial PFC, corroborating the study by Reuter et al. (2005). Critically, the present data show that this state of overall reward deficiency is coupled with excessive recruitment of brain reward circuitry under conditions of cognitive distortion (near misses), which varies as a function of gambling severity. It is likely that these two effects cancelled out in the between-groups comparison of near-miss activity, in which no differences were observed.
Two further points of comparison with our previous study are noteworthy. First, our earlier study reported an interaction between near misses and personal control in the medial PFC (Clark et al., 2009). We were unable to substantiate this interaction effect in the regular gamblers. Indeed, the regular gamblers did not display significant recruitment of this region even in the basic win contrast, and neuropsychological studies indicate specific impairments on probes of medial PFC integrity in problem gamblers (Goudriaan et al., 2006; Lawrence et al., 2009). Our previous study also hinted at a key role of the insula in the motivational effect of near misses. In the present study, insula activations were restricted to the overall win contrast, at a level just below FWE significance, and these responses did not covary with gambling severity. We believe these insula responses convey information about peripheral physiology (e.g., heart rate increases) during gambling (e.g., Craig, 2003), and it may be harder to engender this arousal in regular gamblers who have much experience with high-stimulation games. Psychophysiological studies in regular players have shown qualitative differences between gambling in laboratory settings versus naturalistic (e.g., casino) settings (Anderson and Brown, 1984; Meyer et al., 2004). Future work combining fMRI and psychophysiological monitoring is needed to assess the relationship between evoked arousal and brain activity during gambling (Critchley et al., 2001).
Some limitations of the current study should be noted. First, while we covaried for several common comorbidities, some relevant conditions including nicotine dependence and personality disorders (Cunningham-Williams et al., 1998) were not assessed. Second, the between-groups comparison against our earlier study was not planned and the groups were not well matched for age and gender. We covaried for age but not gender, as our group of regular gamblers was almost exclusively male. Disordered gambling is more prevalent in men (Kessler et al., 2008), but further studies are required to test whether our effects generalize to female gamblers. Third, the self-report ratings did not demonstrate a significant subjective effect of the near misses in the regular gamblers. This is likely an issue of statistical power given the fragility of visual analog ratings: in our previous study, the subjective effects were observed in a larger behavioral experiment in 40 volunteers. A marginally significant effect of the (participant-chosen) near misses to increase motivation to play was observed in a pooled analysis of the two fMRI datasets (n = 34) (supplemental Table 6, available at www.jneurosci.org as supplemental material). Finally, our inference that dopamine is involved in gambling near misses must be treated with a suitable degree of caution given the indirect nature of the BOLD signal and limited spatial resolution of fMRI (for review, see Düzel et al., 2009). Other neurotransmitters implicated in gambling behavior, including serotonin, are present in the midbrain and are modulated by motivational stimuli, albeit without phasic responses (Nakamura et al., 2008). Pharmacological challenge designs will be needed to explore these questions directly; for example, Zack and Poulos (2004) reported that the indirect dopamine agonist amphetamine increased urges to gamble and attentional bias in problem gamblers. One clinical implication of such findings is that drugs that reduce dopamine transmission may have therapeutic benefit in reducing cognitive distortions in problem gamblers.
Footnotes
This work was supported by a project grant from the Economic and Social Research Council and Responsibility in Gambling Trust to L.C. and T. W. Robbins (Professor of Cognitive Neuroscience, and Head, Department of Experimental Psychology, University of Cambridge) (RES-164-25-0010) and a grant from the Medical Research Council (G0802725). This work was completed within the Behavioural and Clinical Neuroscience Institute, supported by a consortium award from the Medical Research Council (United Kingdom) and the Wellcome Trust. We are grateful to the participants and the radiographic staff at the Wolfson Brain Imaging Centre, Cambridge, United Kingdom.
References
- American Psychiatric Association. Ed 4. Washington, DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders—text revision. [Google Scholar]
- Anderson G, Brown RI. Real and laboratory gambling, sensation-seeking and arousal. Br J Psychol. 1984;75:401–410. doi: 10.1111/j.2044-8295.1984.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bergh C, Eklund T, Södersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–475. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] Neuroimage. 2002;16 abstract 497. [Google Scholar]
- Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Burns GL, Keortge SG, Formea GM, Sternberger LG. Revision of the Padua Inventory of obsessive compulsive disorder symptoms: distinctions between worry, obsessions, and compulsions. Behav Res Ther. 1996;34:163–173. doi: 10.1016/0005-7967(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Clark L. Decision-making during gambling: an integration of cognitive and psychobiological approaches. Philos Trans R Soc Lond B Biol Sci. 2010;365:319–330. doi: 10.1098/rstb.2009.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté D, Caron A, Aubert J, Desrochers V, Ladouceur R. Near wins prolong gambling on a video lottery terminal. J Gambl Stud. 2003;19:433–438. doi: 10.1023/a:1026384011003. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Cunningham-Williams RM, Cottler LB, Compton WM, 3rd, Spitznagel EL. Taking chances: problem gamblers and mental health disorders–results from the St. Louis Epidemiologic Catchment Area Study. Am J Public Health. 1998;88:1093–1096. doi: 10.2105/ajph.88.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Hodgins DC, Wang J, el-Guebaly N, Wynne H, Chen S. Risk of harm among gamblers in the general population as a function of level of participation in gambling activities. Addiction. 2006;101:570–580. doi: 10.1111/j.1360-0443.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson's disease. Arch Neurol. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Washington DC: American Psychiatric; 1996. Structured clinical interview for DSM-IV axis I disorders, clinician version. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Griffiths M. Fruit machine gambling: the importance of structural characteristics. J Gambl Stud. 1993;9:101–120. [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci. 2009;21:1332–1345. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- Kassinove JI, Schare ML. Effects of the “near miss” and the “big win” on persistence at slot machine gambling. Psychol Addict Behav. 2001;15:155–158. doi: 10.1037//0893-164x.15.2.155. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, Ustun TB, Walters EE. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, Shaffer HJ. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur R, Walker M. A cognitive perspective on gambling. In: Salkovskis PM, editor. Trends in cognitive and behavioural therapies. Chichester, UK: Wiley; 1996. pp. 89–120. [Google Scholar]
- Langer EJ. The illusion of control. J Pers Soc Psychol. 1975;32:311–328. [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104:1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schwertfeger J, Exton MS, Janssen OE, Knapp W, Stadler MA, Schedlowski M, Krüger TH. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29:1272–1280. doi: 10.1016/j.psyneuen.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Miller NV, Currie SR. A Canadian population level analysis of the roles of irrational gambling cognitions and risky gambling practices as correlates of gambling intensity and pathological gambling. J Gambl Stud. 2008;24:257–274. doi: 10.1007/s10899-008-9089-5. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Murray GK, Clark L, Corlett PR, Blackwell AD, Cools R, Jones PB, Robbins TW, Poustka L. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry. 2008;8:34. doi: 10.1186/1471-244X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(Suppl 1):S142–S151. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, Rusjan P, Houle S, Strafella AP. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol Alcohol. 2002;37:187–192. doi: 10.1093/alcalc/37.2.187. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Ströhle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]