Abstract
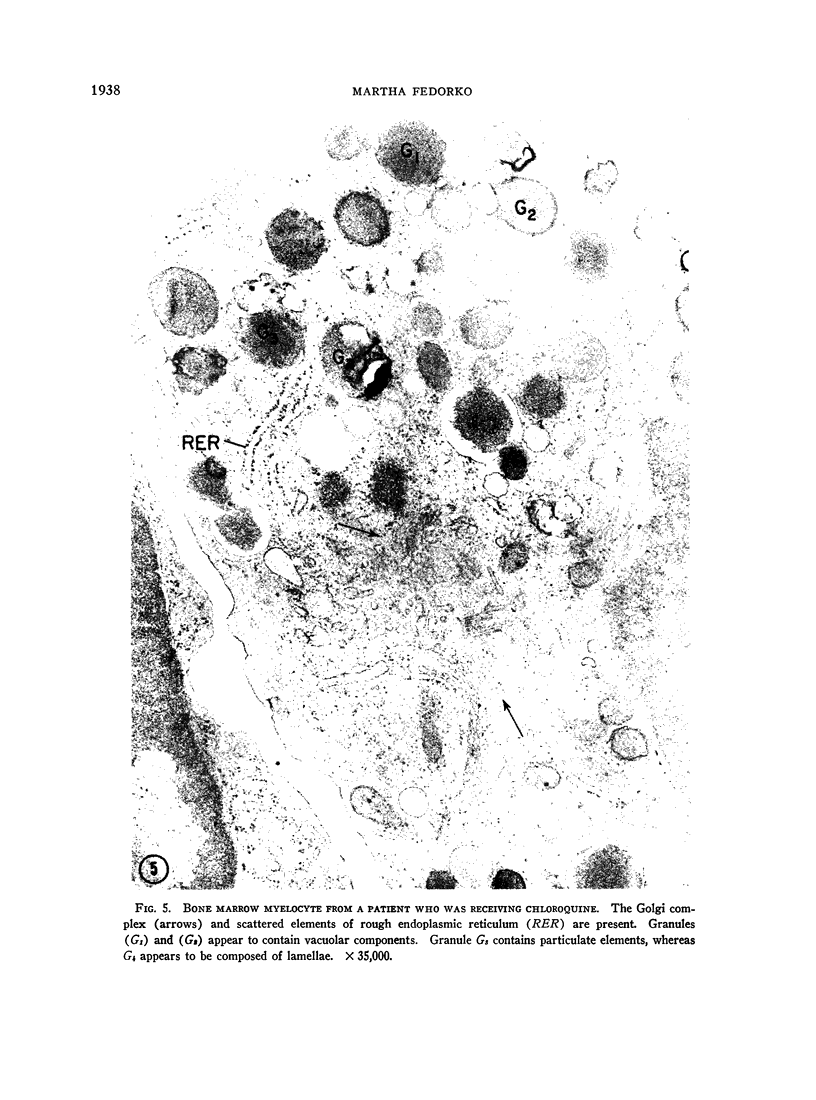
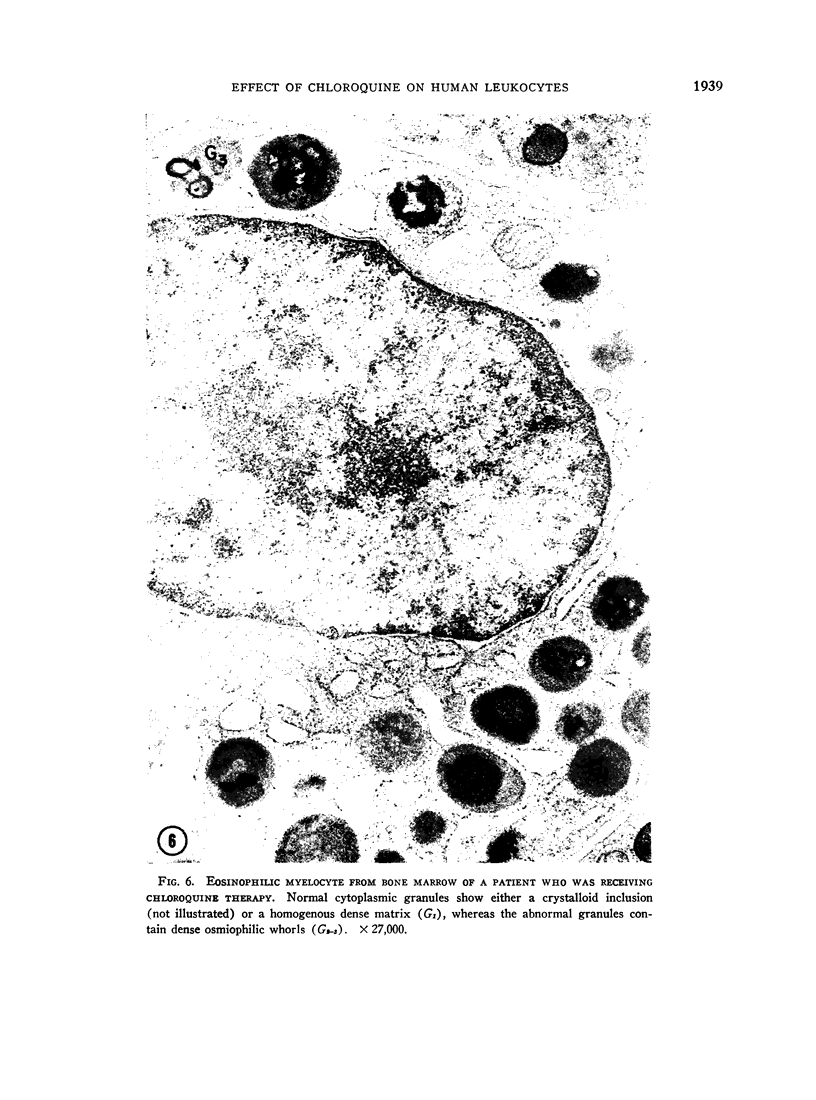
Bone marrow and peripheral blood from patients who had received chloroquine phosphate were studied to determine the effect of this drug on the ultrastructure of cytoplasmic granules in leukocytes. Neutrophils from approximately one-half of the patients who were treated developed abnormal cytoplasmic granules. Vacuolar, lamellar, and particulate components within abnormal, large granules were present in myelocytes from certain patients who received chloroquine therapy. Mature neutrophils and lymphocytes from these patients showed variable numbers of large, membrane-bounded structures containing myelin figures. Cytoplasmic granules in eosinophilic myelocytes from patients treated with chloroquine did not contain the usual crystalloid structure, but instead contained small whorls of osmiophilic material. The granules in abnormal mature eosinophils were replaced by large vacuoles which contained amorphous material. The abnormal granules seen in these various white cells after chloroquine therapy may either reflect defective granule formation or autophagy.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT B. J., KELLY L. S. Autoradiographic studies of leukocyte formation. Proc Soc Exp Biol Med. 1958 Dec;99(3):681–684. doi: 10.3181/00379727-99-24462. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOOT E. C. EOSINOPHIL TURNOVER IN THE NORMAL RAT. Br J Haematol. 1965 Jul;11:439–445. doi: 10.1111/j.1365-2141.1965.tb06606.x. [DOI] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G. Cytoplasmic granule formation in myelocytes. An electron microscope radioautographic study on the mechanism of formation of cytoplasmic granules in rabbit heterophilic myelocytes. J Cell Biol. 1966 May;29(2):307–316. doi: 10.1083/jcb.29.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER J., NENNA A., COUTURE J. ETUDE COMPARATIVE DE LA CIRCULATION DANS LE SANG ET DE L''ELIMINATION URINAIRE DE LA CHLOROQUINE BASE ET DU SULFATE DE CHLOROQUINE. Bull World Health Organ. 1963;29:417–421. [PMC free article] [PubMed] [Google Scholar]
- SWIFT H., HRUBAN Z. FOCAL DEGRADATION AS A BIOLOGICAL PROCESS. Fed Proc. 1964 Sep-Oct;23:1026–1037. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]