Abstract
Peripheral blood monocytes represent the rapid response component of mononuclear phagocyte host defense, generating vigorous but finite antibacterial responses. We investigated the fate of highly purified primary human monocytes following phagocytosis of different bacteria. Exposure to high bacterial loads resulted in rapid loss of cell viability and decreased functional competence. Cell death typically involved classical apoptosis. Exposure to high numbers of Escherichia coli and Klebsiella pneumoniae induced non-apoptotic death with loss of cell membrane integrity, marked disruption of phagolysosomes and caspase-1 activation, while a subset of cells also released caspase 1 regulated extracellular traps. Classical apoptosis increased if extracellular bacterial replication was reduced and decreased if intracellular ATP levels were reduced during these infections. Both classical apoptosis and the alternative forms of cell death allowed monocytes, whose functional competence was exhausted, to down-regulate reactive oxygen species (ROS) and pro-inflammatory cytokine responses. In contrast, sustained stimulation of glycolytic metabolism and mitochondrial oxidative phosphorylation, with associated HIF-1α upregulation, maintained intracellular ATP levels and prolonged monocyte functional longevity, as assessed by maintenance of phagocytosis, ROS production and pro-inflammatory cytokine generation. Monocyte innate responses to bacteria are short-lived and are limited by an intrinsic program of apoptosis, a response that is subverted by overwhelming infection with E. coli and K. pneumoniae or bacterial stimulation of cell metabolism. In this regard, the fate of monocytes following bacterial challenge more closely resembles neutrophils than macrophages.
Keywords: Monocytes/Macrophages, Bacterial, Apoptosis, Cytokines
Introduction
Circulating peripheral blood monocytes represent a pleiotropic population of cells which are essential for innate immune responses to pathogenic micro-organisms in the blood, mucosal surfaces and tissues (1). Peripheral blood monocytes, derived from bone marrow precursors, are recruited to sites of inflammation during bacterial infection (2). In tissues they function as recruited phagocytes but, being less numerous than polymorphonuclear phagocytes, their principal roles are in tissue homeostasis and in maintenance of the pool of resident tissue macrophages (3). For certain infections monocytes may be the key mononuclear phagocyte mediating cell mediated immunity (4). Monocytes demonstrate marked phenotypic differences from differentiated macrophages but their roles in the context of high bacterial loads, as occurs in sepsis, are incompletely characterized (2).
During bacterial infection monocytes perform distinct roles in the innate host response. Monocytes actively phagocytose bacteria and promptly kill ingested organisms, as part of the rapid response component of the mononuclear phagocyte limb of innate immunity (5). They demonstrate high-output secretion of pro-inflammatory cytokines, such as IL-1β and TNF-α, in response to engagement of TLR4 or other pattern recognition receptors. Ingestion of particles by phagocytic cells is ATP-dependent and is associated with a fall in intracellular ATP (6, 7). Lysosomal-mediated intracellular degradation of particles is also ATP dependent (8). In comparison to tissue macrophages, monocytes have a lower concentration of mitochondria, which places them at risk of depleting intracellular ATP levels rapidly during antibacterial innate responses (9). Monocytes thus have a greater reliance on constitutive glycolytic metabolism for maintenance of intracellular ATP, in comparison to tissue macrophages, such as alveolar macrophages, which rely to a greater extent on oxidative phosphorylation by mitochondria (10).
During the interaction of myeloid cells with bacterial pathogens a variety of cell death processes have been described, which frequently benefit host defense. Apoptosis is characterized by nuclear condensation and fragmentation, DNA cleavage, cell shrinkage, and preservation of membrane integrity, and is frequently, though not always, viewed as an anti-inflammatory death process (11, 12). In contrast, pyroptosis which also involves nuclear condensation and DNA cleavage, is associated with caspase 1 activation and membrane rupture and may therefore be viewed as a more inflammatory death process (11). More recently, neutrophils have been shown to utilise a death program termed extracellular trap (ET)osis in which nuclear DNA, histones and serine proteases are released as an antimicrobial strategy (13). Factors which may influence the death process include the bacterial strain, bacterial load and the activation state of the cell, with apoptotic and non-apoptotic death processes occurring after the same infection depending on the prevailing conditions (11, 14).
The paradigm of phagocyte cell death during antibacterial host defense is provided by neutrophils, which undergo apoptosis after a period of sustained phagocytosis and killing (15). In contrast to tissue macrophages monocytes have enhanced susceptibility to apoptosis (16-19) and a relatively short lifespan of 1-2 days in the circulation (20, 21). Pro-inflammatory stimuli and growth factors, however, are potent pro-survival stimuli for monocytes and reverse the intrinsic susceptibility to constitutive apoptosis (22, 23). Phagocytosis and killing of bacteria can induce a delayed program of apoptosis in macrophages as a component of innate host defense (24). Alternatively, early cell death of macrophages may be a pathogen-driven mechanism by which the immune system is evaded and can take various forms ranging between apoptosis, pyroptosis or necroptosis, with varying consequences for the overall inflammatory response (11). In some forms of severe sepsis, for example meningococcal disease, extremely high counts of bacteria can be present within the bloodstream, and the highest counts are associated with the most severe manifestations of disease (25). Comparatively little is known concerning the fate of highly purified primary human monocytes following high dose bacterial challenge and their altered susceptibility to apoptosis suggests responses may differ from differentiated macrophages.
In view of their central role in the inflammatory response to overwhelming bacterial infection, we have investigated the cell fate of monocytes following exposure to a range of bacteria and demonstrate rapid cell death either by classical apoptosis, or by an alternative death processes, concomitant with exhaustion of functional competence and downregulation of pro-inflammatory responses. In contrast, bacterial stimulation of monocyte metabolism prevents apoptosis, enabling cell survival and prolonging pro-inflammatory responses.
Materials and Methods
Monocyte isolation and culture
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Paque (GE healthcare) density centrifugation of whole blood, donated by healthy volunteers. The South Sheffield Research Ethics Committee approved the studies, and subjects gave written, informed consent. Monocytes were enriched from freshly isolated PBMC using MACS Monocyte Isolation Kit II and MACS LS Columns (Miltenyi Biotec), yielding an average 98% purity. Purified monocytes were cultured for 1h in RPMI 1640 culture medium (Lonza) with 2mM L-glutamine (Gibco BRL) containing 10% human AB serum (First Link (UK) LTD) in 24-well plates (Costar®, Sigma-Aldrich) with or without coverslips at 5×105 cells/well or 25cm2 flasks (Costar®, Sigma-Aldrich) at 2×106cells/flask to allow monocyte adherence. The media was then replaced with RPMI 1640 with 2mM L-glutamine containing 10% heat inactivated fetal calf serum (FCS; Bioclear), maintained at 5% CO2 at 37°C and cells used within 12h.
Bacterial infection
Neisseria meningitidis (MC58, B:15:P1.7.16b serogroup B), Klebsiella pneumoniae subsp. pneumoniae (ATCC 43816), Escherichia coli (C29, group 2 capsular serotype K54) (26), Neisseria lactamica (Y92-1009 (sequence type 3493 complex 613) and serotype 2 Streptococcus pneumoniae (D39 strain, NCTC 7466 were the strains studied. In specific experiments the unencapsulated strain of MC58, ¢13, was examined to increase bacterial internalization (27). All bacteria were grown overnight on blood agar with the exception of N. lactamica which was grown on chocolate blood agar. Individual colonies were selected and grown in liquid media to mid-log phase (28). N. lactamica was grown in tryptone broth (Oxoid), E. coli in Brain Heart Infusion and K. pneumoniae or N. meningitidis in Mueller-Hinton broth (Oxoid). S. pneumoniae stocks were prepared and opsonized as described previously (28). Adherent monocytes were infected for various time periods with each bacterium at a multiplicity of infection (MOI) of 10 colony forming units (CFU) per monocyte (unless otherwise stated) (28). Intracellular colony counts were determined using a gentamicin killing assay (29).
Flow cytometry
All flow cytometric measurements were made using a four colour FACSCalibur (BD Biosciences, Mountain View, CA). Forward and side scatter light was used to identify ‘viable’ monocyte populations based on size and granularity. 10,000 events were recorded and all data was analysed using FlowJo software, version 8.8.4 (Tree Star Inc.).
Brightfield and fluorescence microscopy
Brightfield and fluorescent images of whole cell morphology, nuclear morphology and extracellular traps were captured using a Zeiss LSM 510 confocal microscope with a Zeiss 63×/1.4 oil objective. Extracellular DNA structures were stained with 5μM Hoechst 33342 (Invitrogen) and fixed in 2% paraformaldehyde then incubated with a 1: 200 dilution of rabbit anti-human histone H2A.Z antibody (#2718, Cell Signalling Technology, Inc.) at 4°C overnight before staining with a 1:100 dilution of a goat anti-rabbit IgG fluorescein isothiocyanate-conjugated secondary antibody (Sigma-Aldrich) for 1h at room temperature. Images were processed by LSM software and AxioVision 4.7.2 software. Quantification was provided by analyzing 300 cells per field. In certain experiments 10μM of the caspase-1 inhibitor z-YVAD-fmk (Calbiochem) or DMSO vehicle control was added 30 min prior to infection. In select experiments fluorescence microscopy was also performed with a Leica DMRB microscope with a 40x/0.7 objective lens. Image processing involved use of LAS AF software.
Transmission electron microscopy (TEM)
2×106 monocytes were mock infected or challenged with bacteria for 12h. Cells were centrifuged at 1000g for 5min, washed three times in PBS and fixed in ice cold 3% Glutaraldehyde, 0.1M Phosphate buffer overnight at 4°C. The cell pellets were then washed in 0.1M Phosphate buffer twice. Secondary fixation was carried out in 2% aqueous osmium tetroxide for 2h at room temperature and washed twice in 0.1M Phosphate buffer. Specimens were then dehydrated through a graded series of ethanol; 75% ethanol for 15min; 95% ethanol for 15min; 100% ethanol for 15min twice followed by 100% ethanol dried over anhydrous copper sulphate for 15min. The specimens were then placed in propylene oxide for 15min twice. Infiltration was accomplished by placing the pellets in a 0.5:0.5 mixture of propylene oxide and Araldite resin overnight at room temperature. The pellets were then left in Araldite resin for 6h at room temp and finally embedded in fresh Araldite resin for 48h at 60°C. Sections (85nm) were cut on a Reichert Ultracut E ultramicrotome and stained with 1% Toluidine blue in 1% Borax. Sections were examined using a FEI Tecnai Transmission Electron Microscope at an accelerating voltage of 80Kv and micrographs were taken using a Gatan digital camera.
Determination of monocyte viability
The cell culture media from mock infected or bacteria exposed monocytes were analyzed for LDH release using a commercially available kit (Cytotox 96, Promega) following the manufacturer instructions. For Trypan blue dye exclusion, mock and infected monocytes were harvested by cell scraping and stained with 0.2% w/v Trypan blue dye (Sigma-Aldrich). Cell counts were made using a haemocytometer by brightfield microscopy.
Morphological analysis of cell death
Monocytes on coverslips were fixed in 2% w/v paraformaldehyde (Sigma-Aldrich). Monocytes were stained by TUNEL using an ApopTag® Fluorescein Direct In Situ Apoptosis Detection kit (Chemicon International, Inc.) following the manufacturer instructions, and counterstained with DAPI containing mounting medium (Vector Labs, Inc.) (28). Nuclear morphology was analyzed by fluorescence microscopy, assessing 300 cells per field.
Measurement of loss of inner mitochondrial trans-membrane potential (ΔΨm)
Monocytes were stained with 10 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; Molecular Probes) for 30min at 37°C, 4h and 12h post infection to measure loss of inner mitochondrial transmembrane potential (ΔΨm) by flow cytometry (29). Loss of ΔΨm was determined by the loss of red fluorescence.
Analysis of loss of lysosomal acidification
Monocyte cultures, in the presence or absence of gentamicin, were washed three times with PBS at 4h after infection, incubated at 37°C in RPMI containing 5μM acridine orange (Sigma-Aldrich) for 15 min, washed and re-suspended in ice cold PBS for analysis by flow cytometry with loss of orange fluorescence recorded as a marker of loss of lysosomal acidification.
Latex bead phagocytosis
Green fluorescent carboxylate-modified latex beads with a mean diameter of 1μm (L3030; Sigma-Aldrich) were opsonized in 10% human AB serum with RPMI 1640 for 30min at 37°C and incubated with monocytes for 1h to allow phagocytosis. To account for extracellular binding, f-actin dependent phagocytosis was inhibited by adding 2μM cytochalasin D (Sigma-Aldrich) to control samples for 30min (37°C, 5% CO2) prior to infection. (30). The extent of phagocytosis was determined by flow cytometry with an increase in fluorescence associated with internalized latex beads.
Determination of intracellular ATP concentration
Infected monocytes were treated with 100μg/ml gentamicin for 1h to kill extracellular bacteria. The media was then replaced with serum free RPMI 1640 for 5, 10, 15 and 30min and the cells lysed in 100μl 1% v/v NP-40. Cell lysates were boiled for 10mins to inactivate ATPase activity. Intracellular ATP was determined using a commercially available bioluminescent kit following the manufacturer instructions (Adenosine 5′-Triphosphate bioluminescent assay kit, Sigma-Aldrich) with results normalized to numbers of ‘viable’ cells for each culture, as assessed by the numbers of adherent cells without altered cell morphology (31). Luminesence was measured using a Fusion™ Universal Microplate Analyzer (Packard Instrument Company) and analysed by Fusion Instrument Control Application software, version 4.00. To determine the effect of oxidative phosphorylation or glycolysis on ATP generation cultures were treated with either 50μM oligomycin (Sigma-Aldrich), an inhibitor of mitochondrial respiration, 10mM 2-deoxy-d-glucose (Sigma-Aldrich), an inhibitor of glycolysis, or a combination of both inhibitors 30min prior to cell lysis (or at the indicated time if different).
Detection of Reactive oxygen species (ROS)
Production of intracellular ROS was measured using the cell permeable molecule 2′, 7′-dichloro-dihydrofluorescein diacetate (DCF; Sigma-Aldrich) (32). Monocytes were pre-incubated with DCF for 30min before infection for the indicated time periods. Cells were washed in PBS then analyzed by flow cytometry. To determine extracellular ROS production, monocytes pre-incubated with DCF for 30min were infected for 12h, supernatants centrifuged at 8000g for 10min then transferred to a white walled, 96 well plate (Costar®; Sigma-Aldrich) and fluorescence detected using a Fusion™ Universal Microplate Analyzer (Packard Instrument Company) and analysed by Fusion Instrument Control Application software, version 4.00. As a positive control for ROS monocytes were treated with 1mM of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) for 1h before analysis and to measure the production of ROS by S. pneumoniae, bacteria were cultured in the absence of monocytes, using the same dose of bacteria as used to infect the monocytes.
Cytokine bead array
Monocyte culture supernatants were collected 12h post infection then centrifuged at 8000g for 10min to remove monocyte and bacterial debris. Cytokines were analysed using a BD™ CBA Flex set (IFN-γ, TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12p70) and measured using a BD FACSArray™ Bioanalyser (BD Biosciences, Mountain View, CA). The limits of detection were 1.8pg/ml (IFN-γ), 1.2pg/ml (TNF-α), 2.3pg/ml (IL-1β), 1.6pg/ml (IL-6), 1.2pg/ml (IL-8), 0.13pg/ml (IL-10) and 0.6pg/ml (IL-12p70).
SDS PAGE and Western blotting
Infected monocytes grown in T25 flasks were lysed for protein as previously described (29). Protein concentration was determined using a modified Lowry assay (DC protein assay, Bio-Rad) and equal protein was added to all lanes. Protein samples were separated by SDS PAGE (12%) and blotted on to nitrocellulose membrane (Bio-Rad). Blots were incubated with anti caspase-1 (Abcam), anti HIF1α (Abcam) or anti actin (Sigma-Aldrich) antibodies for 12h at 4°C. Protein was detected using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulins (Dako), and enhanced chemiluminescence (EZ-ECL, Geneflow). Cell lysates from MCF-7 cells cultured for 24h in normoxia (normal incubator 5% CO2) or hypoxia (3 kPa O2), maintained using an invivo400 hypoxic work station (Ruskinn, UK) with a 5% CO2/balance N2 gas mix, were used as negative and positive controls for HIF-1α.
Statistics
All data was recorded as mean ± standard error of the mean (SE) unless otherwise stated. Statistical testing was performed using Prism® 5.02 software (GraphPad Software Inc.) with relevant statistical tests described in the figure legends. Significance was defined as p < 0.05.
Results
Bacterial infection is associated with loss of micromolecule transport by monocyte cell membranes
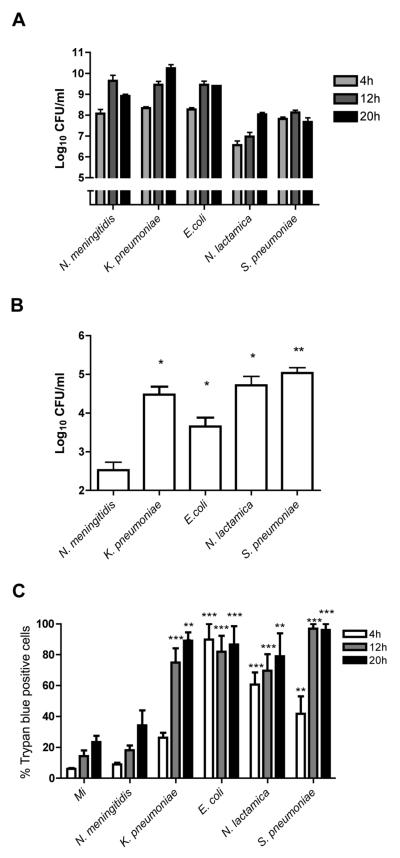
We first examined whether the initial interaction with bacteria altered permeability of monocytes to trypan blue, an assay often regarded as a marker of loss of cell viability but which strictly measures altered cell membrane transport of micromolecules (33). The bacteria studied included pathogens which are leading causes of invasive disease and sepsis, as well as a commensal organism, Neisseria lactamica, which is shielded from the adaptive immune system by a polyclonal IgM response and rarely causes invasive disease. This organism was used for comparison with N. meningitidis (34-36). All these bacteria are phagocytosed and killed by mononuclear phagocytes without intracellular persistence. We found increasing numbers of extracellular bacteria with time in each culture, from 4-20h after infection (Figure 1A). We demonstrated that monocytes could internalize each bacterium, as shown by intracellular bacterial colony counts 4h after infection (Figure 1B), although this required opsonization of Streptococcus pneumoniae to stimulate phagocytosis as previously shown (28). Internalization was lowest for N. meningitidis. We found that all microorganisms tested, with the exception of N. meningitidis, significantly reduced monocyte cell membrane transport of micromolecules 4-12h post-infection, as shown by trypan blue staining (Figure 1C). The levels and timing of this effect varied between bacteria, but these findings suggested altered cellular homeostasis after sustained bacterial challenge in most infections.
Figure 1. Bacterial challenge results in loss of monocyte viability.
Monocytes were mock infected (Mi) or exposed to the indicted bacteria at an MOI of 10 for 4, 12 or 20 h and (A) Extracellular colony forming units (CFU) were estimated (B) Intracellular colony counts were estimated at 4h by gentamicin killing assay and (C) Monocyte trypan blue exclusion assessed by brightfield microscopy. The percentage of cells staining positive with trypan blue are indicated, n=4 per group, * p< 0.05, **p<0.01, *** p<0.001, all comparisons vs. Mi by ANOVA with Dunnett's post-test comparison.
Bacterial infection can induce apoptotic and non-apoptotic cell death
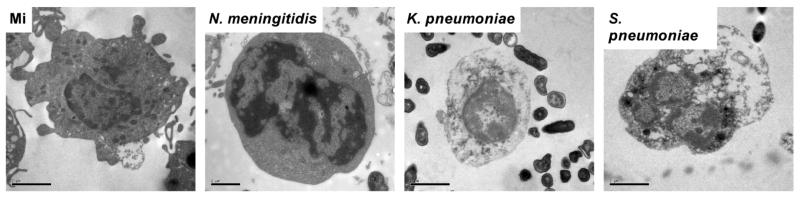
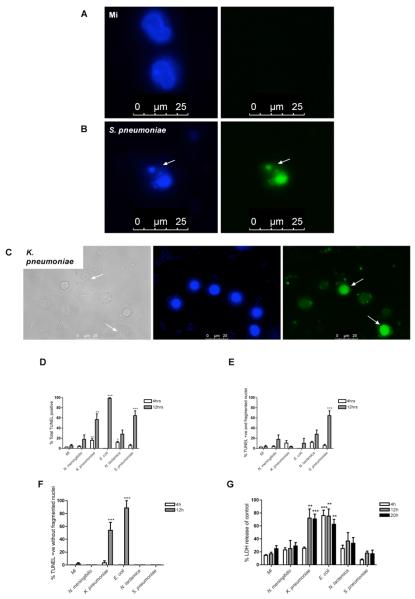
We hypothesized that altered levels and kinetics of trypan blue staining might reflect the existence of distinct monocyte cell death mechanisms after bacterial challenge and that responses to specific bacteria might favor particular forms of cell death. To address this we focused on morphological and biochemical changes, recognising that while both apoptosis and pyroptosis induce nuclear condensation and DNA fragmentation, apoptosis frequently involves nuclear fragmentation while pyroptosis induces prominent disruption of the cell membrane (11, 12). Examination of nuclear morphology by electron microscopy revealed that some bacteria, for example S. pneumoniae, induce features of apoptosis such as nuclear fragmentation but that other infections, such as K. pneumoniae, do not (Figure 2). In keeping with the cell viability data we observed monocytes exposed to N. meningitidis had normal cell morphology. Further assessment with fluorescence microscopy enabled the combined assessment of nuclear morphology and DNA strand breaks, as detected by terminal UTP nick end-labelling (TUNEL) staining. At 12h post-infection with S. pneumoniae we observed nuclear fragmentation (Figure 3A-B). In contrast, K. pneumoniae infection produced TUNEL-positive cells but not nuclear fragmentation (Figure 3C). The percentage of monocytes with DNA strand breaks and fragmented (apoptotic) nuclei and with DNA strand breaks but without nuclear fragmentation were quantified after each infection (Figure 3D-F). As shown, most of the TUNEL-positive monocytes following S. pneumoniae or N. lactamica exposure had nuclear fragmentation (Figure 3D-F). The combination of nuclear fragmentation with DNA strand breaks in these infections was consistent with apoptotic cell death. In contrast, after exposure to E. coli or K. pneumoniae, the majority of monocytes were TUNEL-positive but lacked fragmented apoptotic nuclear morphology (Figure 3F).
Figure 2. Nuclear morphology by transmission electron microscopy in monocytes exposed to bacteria.
Representative transmission electron microscopy images of monocytes, stained with toluidine blue, following mock infection (original magnification ×11,500) or infection with N. meningitidis (original magnification ×14,000), K. pneumoniae (original magnification ×11,500) or S. pneumoniae (original magnification ×11,500) at an MOI of 10 for 12h obtained using a FEI Tecnai Transmission Electron Microscope.
Figure 3. Varying forms of cell death are observed in monocytes exposed to bacteria.
(A-B) Representative fluorescent images of DAPI (blue images) and TUNEL (green images) stained monocytes following mock infection (A) or exposure to S. pneumoniae (B) at an MOI of 10 for 12h. The arrow illustrates a fragmented nucleus. (C) Representative brightfield (greyscale image) or fluorescent images of DAPI (blue image) and TUNEL (green image) stained monocytes following K. pneumoniae infection at an MOI of 10 for 12h. Arrows indicate cells showing membrane rupture and TUNEL positive nuclei without fragmentation. All images were obtained with a Zeiss LSM 510 confocal microscope with a Zeiss 63×/1.4 oil objective. (D-F) Following mock infection or exposure to N. meningitidis, K. pneumoniae, E. coli, N. lactamica, or S. pneumoniae at an MOI of 10 for 4 and 12 h, monocytes were stained with TUNEL and DAPI and observed using fluorescence microscopy. The total percentage of TUNEL positive monocytes (D), the percentage of TUNEL positive monocytes with fragmented nuclei (E) and the percentage of TUNEL positive monocytes without fragmented nuclei (F) were recorded, n=3. (G) Monocytes were mock infected or exposed to bacteria, as above, and the percentage LDH release compared to positive control at each time point, n=4 per group, * p< 0.05, **p<0.01, *** p<0.001, all comparisons vs. mock infected by ANOVA with Dunnett's post-test comparison. Bacterial colony forming units in these experiments are shown in the supplemental table 1A. MI, mock infected; +ve positive.
We next determined the integrity of the cell membrane after exposure to each bacterium. As shown in Figure 3C, some bacteria were associated with permeabilization of the cell membrane. For example, K. pneumoniae infection resulted in marked disruption to the cell membrane, as assessed by light microscopy. To formally evaluate the effect of each bacterial species on the integrity of the cell membrane we measured LDH release (Figure 3G), a marker of significant membrane disruption (37). Only E. coli and K. pneumoniae exposure resulted in significant LDH release from monocytes. This suggested these infections were associated with a non-apoptotic cell death mechanism, with absence of nuclear fragmentation and membrane permeabilization being features of pyroptosis (11).
The monocyte death processes following exposure to E. coli and K. pneumoniae are influenced by bacterial numbers
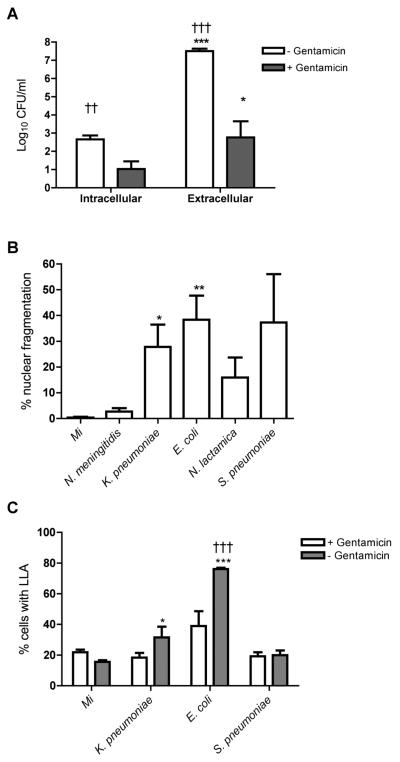
Different forms of programmed cell death can occur following identical stimuli (38) and we next investigated whether the non-apoptotic death process seen with E. coli and K. pneumoniae exposure was related to bacterial numbers. We repeated experiments in which bacterial numbers were reduced by early addition of antimicrobials. As shown for E. coli this approach reduced both extracellular and intracellular bacterial burden (Figure 4A). Addition of antimicrobials resulted in similar levels of nuclear fragmentation following S. pneumoniae, E. coli or K. pneumoniae challenge (Figure 4B).
Figure 4. Large numbers of K. pneumoniae and E. coli cause cell death with features distinct from classical apoptosis.
(A) The intracellular and extracellular colony forming units (CFU) per ml of monocyte culture lysate or culture supernatant were estimated 6h after exposure to E. coli in the presence or absence of gentamicin added 2h post infection. n=4, * p<0.05, *** p<0.001, unpaired t test comparing extracellular vs. intracellular; †† p<0.01, ††† p<0.001, unpaired t test comparing with gentamicin vs. without gentamicin. (B) Monocytes were mock infected (Mi) or exposed to N. meningitidis, K. pneumoniae, E. coli, N. lactamica, or S. pneumoniae at an MOI of 10 and gentamicin was added at 4h post infection. The percentage of monocytes with fragmented nuclei was recorded at 12h, n=3, * p<0.05, ** p<0.01, ANOVA with Dunnett's post-test vs. Mi. (C) Monocytes were mock infected or exposed to bacteria as in (B) in the presence or absence of gentamicin, added 1h post-infection. The percentage of monocytes with loss of lysosomal acidification (LLA) was recorded at 4h by flow cytometry, n=4, *p<0.05, *** p<0.001, ANOVA with Dunnett's post-test vs. Mi; ††† p<0.001 two-way ANOVA with Bonferroni post-test with gentamicin vs. without gentamicin.
Following E. coli and K. pneumoniae exposure in the absence of antimicrobials the degree of membrane disruption (37) and the lack of apoptotic nuclear morphology suggested a death mechanism distinct from classical apoptosis (39). Loss of lysosomal acidification can be detected by a reduction in orange fluorescence of acridine orange and this may be more extensive after some non-apoptotic death process (12, 40). We observed a greater percentage of cells with loss of lysosomal acidification after exposure to E. coli and K. pneumoniae (Figure 4C). Antimicrobial treatment also reduced the percentage of cells with loss of lysosomal acidification at 4h following E. coli or K. pneumoniae challenge.
Monocytes release extracellular traps after exposure to some bacteria
Disruption of the cell membrane with extracellular release of DNA is a feature of neutrophil ETosis (13). We performed fluorescence microscopy of monocytes using the DNA-binding agent Hoechst 33342 and anti-histone monoclonal antibodies to determine whether there were any features of this death process in our cultures. Column-purified monocytes, which lacked neutrophil contamination, demonstrated release of extracellular DNA and histones following exposure to E. coli (Figure 5A) or K. pneumoniae (data not shown). Large amounts of extracellular material were noted emanating from cell nuclei and were associated with extracellular bacteria and the frequency of cells showing this feature increased as the infectious challenge was increased (Figure 5 B). This finding is reminiscent of the extracellular traps that have been observed for neutrophils and mast cells but have not been observed in monocytes (13, 41, 42). Increased extracellular bacterial numbers therefore contributed to the percentage of monocytes showing features of pyroptosis or ETosis after exposure to E. coli or K. pneumoniae.
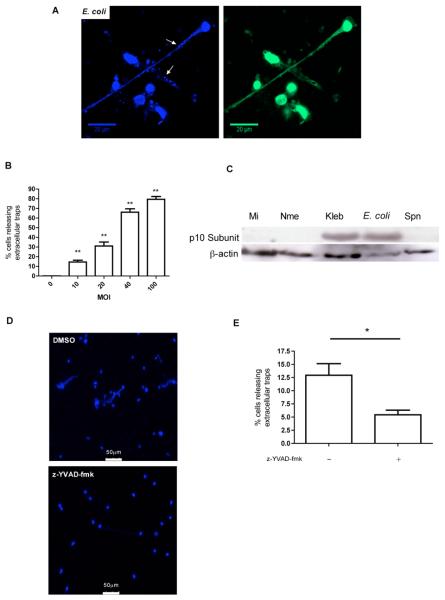
Figure 5. E. coli can trigger extracellular trap release from monocytes.
(A) Representative fluorescent image of DNA stained with Hoechst 33342 and anti-histone Ab H2A.Z 1h post infection with E. coli at an MOI of 10. Arrows indicate bacteria trapped in extracellular DNA. The image was obtained with a Zeiss LSM 510 confocal microscope with a Zeiss x63/1.4 oil objective. (B) Percentage of monocytes releasing extracellular traps 1h after challenge with E. coli at the indicated MOIs n=3, *p<0.05, ** p<0.01, ANOVA with Dunnett's post-test vs. MOI =0. (C) Western blot probed for the active p10 sub-unit of caspase-1 and β-actin from monocytes 12h after mock infection (Mi) or N. meningitidis (Nme), K. pneumoniae (Kpn), E. coli (Eco), or S. pneumoniae (Spn) infection at an MOI of 10. The blot is representative of three independent experiments. (D) Representative fluorescent images of DNA stained with Hoechst 33342 1h after infection with E. coli at an MOI of 10 in the presence or absence of the caspase 1 inhibitor z-YVAD-fmk. The images were obtained using a Leica DMRB microscope with a x40/0.7 objective. (E) Percentage of monocytes releasing extracellular traps 1h after infection with E. coli in the presence or absence of the caspase 1 inhibitor z-YVAD-fmk, n=4, * p<0.05, (Students t-test).
Some non-apoptotic death programs, associated with bacterial infection, involve intracellular caspase-1 activation and this is a key feature of pyroptosis but not usually of classical apoptosis (11). We performed western blots on cell lysates for the active (p10) fragment of caspase 1 and were able to demonstrate E. coli and K. pneumoniae infection was associated with marked caspase 1 activation 12h after challenge, unlike other infections (Figure 5C). Inhibition of caspase 1 markedly reduced the appearance of extracellular traps (Figure 5D-E).
Preservation of intracellular ATP prolongs monocyte phagocytic capacity
Having shown that early changes in the transport of micromolecules were associated with the development of a variety of death processes following bacterial challenge we next investigated if changes in cell homeostasis involved cell metabolism, initially measuring the inner mitochondrial transmembrane potential (ΔΨm), as a marker of mitochondrial homeostasis (43) and measuring intracellular ATP. One hour after exposure some bacterial infections induced loss of ΔΨm in some cells but no infection was associated with a marked reduction in the concentration of intracellular ATP in the population of cells (supplemental Figure 1). After 4-12h all of the bacteria studied induced significant loss of ΔΨm, Figure 6A. Despite this N. meningitidis infection differed from other infections in that intracellular ATP concentration in the population of ‘viable’ cells 12h after infection was maintained at levels similar to those observed in mock infected cells, after normalization of the number of ‘viable’ cells to the level of those detected in the mock infected culture (Figure 6B). ‘Viable’ monocytes after N. lactamica exposure also maintained intracellular ATP levels, but at lower levels than after N. meningitidis exposure. We addressed whether ATP preservation was dependent on glycolytic metabolism or oxidative phosphorylation. Mock infected monocytes required the simultaneous inhibition of both oxidative phosphorylation and glycolysis to deplete intracellular ATP and to inhibit ATP-dependent functions such as phagocytosis (Figure 6C-D). Intracellular ATP was maintained at levels close to normal following inhibition of either oxidative phosphorylation or glycolytic metabolism in monocytes exposed to N. meningitidis. Maintenance of intracellular ATP following exposure to N. meningitidis occurred in association with upregulation of HIF-1α (Figure 6E), an important factor upregulating a range of metabolic pathways in mononuclear phagocytes (44).
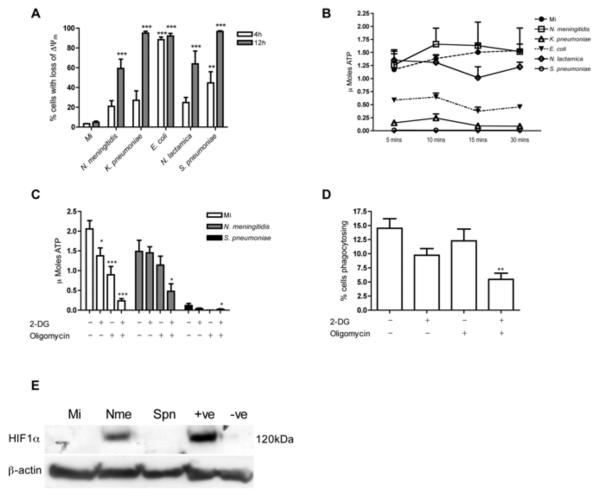
Figure 6. Monocytes exposed to N. meningitidis preserve intracellular ATP levels and retain phagocytic capacity.
(A) Monocytes were mock infected (Mi) or exposed to N. meningitidis, K. pneumoniae, E. coli, N. lactamica, or S. pneumoniae at an MOI of 10 and at 4 or 12h the percentage of cells with loss of inner-mitochondrial transmembrane potential (Δψm) was estimated by flow cytometry n=6, **p<0.01, ***p<0.001 vs. Mi, ANOVA with Dunnett's post-test. (B) Monocytes were mock infected or exposed to bacteria as in (A) and 12h post infection intracellular ATP levels were estimated by bioluminescence. K. pneumoniae (5min, p<0.01), S. pneumoniae (5min p<0.001) and E. coli (10min, p<0.05) were significantly different to Mi. At 30min there was no significant difference between N. meningitidis or N. lactamica and Mi n=3, two-way ANOVA with Bonferroni post-test (C) Monocytes were mock infected or challenged with N. meningitidis or S. pneumoniae at an MOI of 10 for 12h. For the last 30 min of incubation 2-deoxyglucose (2-DG) and/or oligomycin was added to the indicated wells prior to lysis and intracellular ATP levels were estimated by bioluminescence, n=6, * p<0.05, *** p<0.001, all comparisons vs. samples without inhibitors for that infection, ANOVA with Dunnett's post-test. (D) The percent monocytes phagocytosing opsonised fluorescent latex beads 1h following treatment with or without 2-DG and oligomycin, as in (C), n=3, ** p<0.01 vs. samples without inhibitors, ANOVA with Dunnett's post-test. (E) Representative Western blot probed for hypoxia-inducible factor 1α (HIF-1α) and β-actin from monocytes following 12h mock infection or exposure to N. meningitidis (Nme) or S. pneumoniae (Spn) at an MOI of 10. The positive control (+ve) was a lysate from MCF-7 cells cultured in normoxia and the negative control (−ve) a lysate from MCF-7 cells cultured in hypoxia. The blot is representative of three independent experiments. Bacterial colony forming units (CFU) in these experiments are shown in supplementary Table 1B. −ve, negative control; +ve, positive control.
Levels of bacterial internalization were lower for N. meningitidis than for non-Neisserial spp. (Figure 1B). To exclude the possibility that maintenance of intracellular ATP merely reflected differing rates of delivery of bacteria to phagolysosomes, and hence reduced ATP requirements for phagocytosis and killing, we utilised an unencapsulated mutant, since N. meningitidis capsule has been shown to decrease adherence and phagolysosomal compartmentalization, and increased the infecting dose (45). The unencapsulated mutant resulted in comparable intracellular ATP concentrations, despite increased internalization, and also comparable low levels of apoptosis to the parental strain, suggesting capsular inhibition of internalization did not explain the monocyte survival (supplemental Figure 2). To determine if there was any relationship between ATP levels and the mechanism of cell death we studied monocytes exposed to K. pneumoniae incubated in the presence or absence of gentamicin. To some of these cultures we added inhibitors of glycolytic metabolism and oxidative phosphorylation. As shown in supplemental Figure 3, addition of gentamicin increased the apoptotic morphology (as in Figure 4B), in association with a less marked drop in intracellular ATP than seen in the absence of gentamicin. Inhibitors of ATP generation increased the percentage of cells with the pyroptotic appearance, but reduced the apoptotic appearance of cells with fragmented nuclei, despite the presence of antibiotics.
Prolonged monocyte survival allows sustained innate immune responses
In view of the sustained metabolic competence of monocytes after exposure to N. meningitidis we next addressed whether these cells also showed sustained evidence of innate immune function, measuring their capacity to phagocytose opsonized latex beads, generate reactive oxygen species (ROS), as detected by flow cytometry with DCF, and express cytokines, as determined by cytometric bead array (CBA). All infections were associated with comparable phagocytosis of latex beads to the mock infected cultures at 1h (supplemental Figure 4A). We found that only after exposure to N. meningitidis and N. lactamica were monocytes able to continue to phagocytose particles at levels comparable to the mock infected cultures 12h post-infection (Figure 7A). All infections stimulated generation of reactive oxygen species (ROS) from monocyte cultures early after bacterial challenge (Figure 7B). In the case of S. pneumoniae there was also evidence of extracellular ROS expression, in keeping with the known capacity of this organism to generate ROS (46), (supplemental Figures 4B-5). By 12 h ROS production remained noteworthy in the N. meningitidis exposed cultures (Figure 7C), in keeping with the ongoing capacity of these cultures to phagocytose bacteria. Other bacterial infections differed in their capacity to stimulate intracellular ROS production at this time. Cultures associated with monocyte apoptosis (e.g. S. pneumoniae and N. lactamica) also stimulated ROS production in the cells retaining viability. E. coli or K. pneumoniae exposure resulted in no detectable intracellular ROS in cultures 12h after challenge even though intracellular ROS was detectable at earlier time points following exposure to these bacteria.
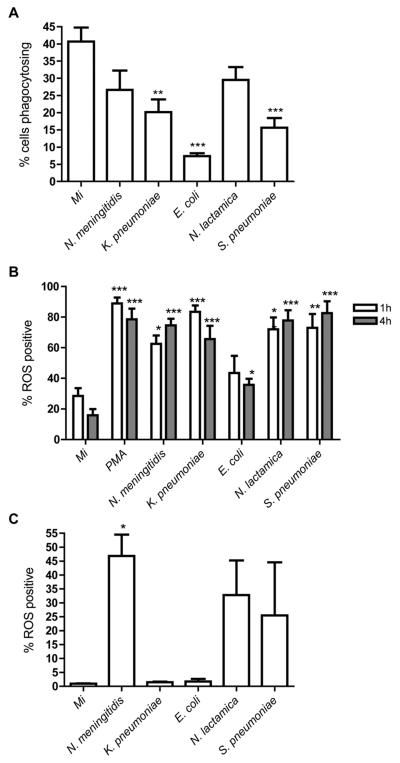
Figure 7. N. meningitidis exposed monocytes demonstrate sustained phagocytosis and production of reactive oxygen species (ROS).
(A) The percent monocytes phagocytosing opsonised fluorescent latex beads following 12h mock infection (Mi) or exposure to N. meningitidis, K. pneumoniae, E. coli, N. lactamica, or S. pneumoniae, at an MOI of 10 n=6, ** p<0.01, *** p<0.001 vs. Mi, ANOVA with Dunnett's post-test. The percentage of monocytes with detectable intracellular reactive oxygen species (ROS) (B) 1-4h and (C) 12h after mock infection (Mi) or exposure to N. meningitidis, K. pneumoniae, E. coli, N. lactamica, or S. pneumoniae, MOI=10. ROS was measured by flow cytometry following incubation with 2′, 7′-dichloro-dihydrofluorescein diacetate (DCF). n=3* p<0.05, ANOVA with Dunnett's post-test. Bacterial colony forming units (CFU) in these experiments are shown in supplementary Table 1B
In keeping with the sustained viability of monocytes and the continued capacity to phagocytose opsonized particles we observed monocytes were still producing significant quantities of pro-inflammatory cytokines 12h after N. meningitidis exposure (Figure 8A-G). This was unlike the situation with most of the other infections investigated, which demonstrated minimal cytokine production, or in the case of E. coli exposure levels below the limit of detection (data not shown), by 12h post-infection. N. lactamica exposed monocytes retained functional capacity despite significant levels of apoptosis. These cells produced a different pattern of cytokines to N. meningitidis challenged monocytes. While TNF-α and IL-8 levels were similar, levels of IFN-γ, IL-1β, IL-6 and IL-12 were lower and those of IL-10 were greater. This demonstrated that prolonged survival of monocytes following bacterial challenge has a significant impact on the resulting pattern of cytokine release.
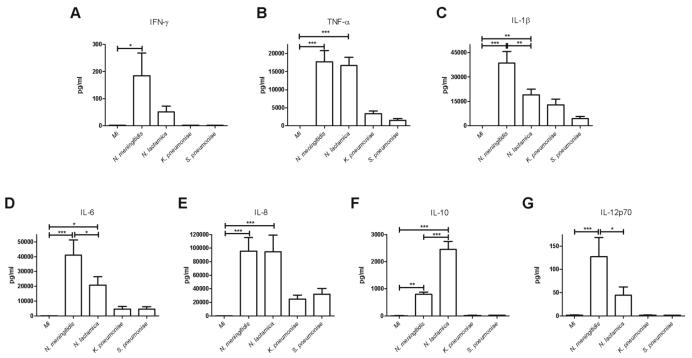
Figure 8. Sustained pro-inflammatory cytokine production by monocytes exposed to N. meningitidis.
Cytokine production by monocytes 12h after mock infection (Mi) or exposure to N. meningitidis, K. pneumoniae, N. lactamica, or S. pneumoniae at an MOI of 10. Cytokine levels in the culture media were determined by cytokine bead array (CBA); IFN-γ (A), TNF-α (B), IL-1β (C), IL-6 (D), IL-8 (E), IL-10 (F) and IL-12p70 (G). n=8, * p<0.05, ** p<0.01, *** p<0.001, ANOVA with Tukey's post-test.
Discussion
Investigation of cell death mechanisms in highly purified primary human monocytes challenged with bacteria has been a comparatively neglected area in comparison to other myeloid cells including macrophages (11). We demonstrate a rapid loss of cell viability over the first 12 hours of exposure to bacteria. Some infections (E. coli and K. pneumoniae) induce non-apoptotic death. Only monocytes exposed to N. meningitidis maintained significant viability by 12h post-infection. Loss of cell viability terminated anti-bacterial innate responses. The prolonged immune competence of monocytes exposed to N. meningitidis, however, comes at the cost of an extended pro-inflammatory immune response.
Monocytes are short-lived cells with a high-level of susceptibility to constitutive caspase-dependent apoptosis (19, 20, 22). During interaction with bacteria monocytes phagocytose bacteria briskly and kill these in their phagolysosomes, thus phagolysosomal escape is an important bacterial survival strategy (47-49). Monocytes phagocytose bacteria with slightly less efficiency than neutrophils but microbicidal killing appears comparable (5). The lysosomal system of monocytes is less developed than in tissue macrophages, lacking the capacity for more prolonged microbial killing (9, 50). Monocytes are therefore more reliant on acute microbicidal strategies, such as ROS-dependent killing, than are macrophages and are better equipped to control extracellular bacteria that can be rapidly killed in phagolysosomes than those that rely on prolonged intracellular killing in phagolysosomes (7). The bioenergetic demands of rapid bacterial internalization and intracellular killing stress phagocytes (6, 51) and monocytes have a lower density of mitochondria than differentiated macrophages (9). This places monocytes under considerable bioenergetic stress and we show that the fate of monocytes is more similar to that of neutrophils than the differentiated macrophage.
Our findings underline the variety of cell death processes that occur following the interaction of monocytes with bacteria, reinforcing the need for careful characterization (52). Trypan blue positivity did not correlate closely with specific cell death features suggesting subtle disruption of micromolecular transport may be observed without other features of loss of viability (33). By analysis of nuclear morphology and membrane integrity we identified infections predominantly associated with apoptotic cell death, as evidenced by specific features of apoptosis, such as cell shrinkage, nuclear fragmentation and preservation of cell surface membrane integrity (24, 39), while for E. coli or K. pneumoniae challenge DNA fragmentation was associated with cytolysis but not nuclear fragmentation. Caspase-1 activation, a feature of pyroptosis, but not usually of macrophage apoptosis, was also observed (11) and loss of lysosomal acidification occurred in a greater percentage of cells in these infections. Loss of lysosomal acidification can result from impaired fusion of phagosomes with lysosomes, impairment of hydrogen ion pumps or lysosomal membrane permeabilization (53-55), although we did not distinguish which of these mechanisms contributed in this case. More marked disruption of the lysosomal membrane, however, favours non-apoptotic death processes (56, 57) and caspase-1 activation by ‘inflammasomes’, containing nucleotide binding and oligomerization domain (NOD)-like receptor (NLR) family members, activated by the release of microbial components into the cytosol (58).
There was also evidence of extracellular DNA and histone release from monocytes and of association of bacteria with these extracellular structures following E. coli and K. pneumoniae infection. The release of DNA, to form extracellular traps containing histones and proteases, is observed from neutrophils and has also been described for basophils (13, 41, 42). To our knowledge a similar process has not been described for monocytes. The large disruption of the cellular membranes, as we observed with these infections, would facilitate the release of intracellular contents, including DNA, from these cells. This process increased in proportion to the infectious inoculum and was reduced by caspase 1 inhibition. Extracellular traps were not identified after S. pneumoniae exposure in keeping with the known ability of this organism to produce an endonuclease, which degrades extracellular DNA (59).
Individual microorganisms can mediate more than one death process (11, 52). Bacterial numbers, cell activation state and, as we suggest, energy state may be other inter-related factors. We have not addressed activation state in this study but Bergsbaken and Cookson made the important observation that activation of macrophages can convert apoptosis to caspase 1 dependent pyroptosis (14). Macrophage activation by LPS reduces apoptosis (60) and could have reduced apoptosis following E. coli and K. pneumoniae infection. The influence of bacterial numbers on the death process was complex. We have previously shown that apoptosis in differentiated macrophages is related to the intracellular burden of S. pneumoniae (61), but we did not specifically examine the influence of intracellular, as opposed to extracellular bacterial numbers in this study. The non-apoptotic death processes, observed after E. coli and K. pneumoniae exposure, were associated with higher numbers of extracellular bacteria but not higher numbers of intracellular bacteria 4h after infection. Nevertheless, reducing extracellular and intracellular bacterial numbers by antimicrobial treatment increased rates of apoptosis, relative to pyroptosis, while increasing the infectious dose increased rates of extracellular trap formation for these infections. Survival of monocytes following N. meningitidis exposure was associated with lower intracellular bacterial numbers but was not altered by increasing intracellular bacterial numbers. These conclusions emphasise that unique microbial factors interact with bacterial burden and cellular factors to determine the cells fate.
The concentration of intracellular ATP influences the mechanism of cell death. Apoptosis requires intracellular ATP but processes with a high affinity for ATP, such as many of the executors of apoptosis, are only compromised when cellular levels are depleted 100 fold (62). If there is not a source for this low level of ATP then necroptosis, a necrosis-like cell death, results in membrane disruption with the potential release of phagocytosed bacteria and inflammatory mediators (12, 39). Little is known of the exact energy requirements of pyroptosis but caspase 1 activation also requires ATP (63). Since the levels of ATP required for these death processes are so low, estimation of these differential energy requirements will require ultra-sensitive assays. Nevertheless our preliminary analysis suggests that by further depleting cellular ATP the death program shifts from apoptosis to pyroptosis. Monocytes must balance the large energy requirements of their rapid antibacterial response and their relatively limited capacity to generate ATP with the need to execute the most appropriate cell death program while there are still sufficient ATP reserves for its completion.
Execution of a program of apoptosis allows a monocyte, which has exhausted its functional capacity to kill ingested bacteria, to enhance intracellular bacterial killing (64, 65) and terminate pro-inflammatory responses (66). Increased cell activation, a fall in intracellular ATP below the levels required for apoptosis and cytosolic sensing of microbial products would favour pyroptosis (11, 14, 63). Pyroptosis is known to facilitate control of intracellular bacteria, which escape into the cytoplasm (67). Extracellular ATP release could activate the same process in bystander cells (68). This phlogistic death process could be viewed as a compromise in overwhelming infection in which the monocyte although terminating its own inflammatory response by cell death does not terminate the overall tissue inflammatory response, as would happen with apoptosis, but instead ensures its perpetuation with a further round of inflammatory cell recruitment. In contrast extracellular traps would ensure unphagocytosed extracellular bacteria could be contained (13, 41, 42). Linking extracellular bacterial numbers to intracellular bacterial numbers and sensing this through recognition of levels of cytosolic microbial products or intracellular ATP would provide the monocyte with an effective mechanism by which the apoptotic program could be over-ridden when the antibacterial capacity of the phagolysosomal compartment is over-run.
Infection with N. meningitidis, the meningococcus, was distinct since it resulted in sustained monocyte viability in association with maintenance of intracellular ATP. Preservation of intracellular ATP levels was the result of activation of both glycolytic metabolism and oxidative phosphorylation and associated with upregulation of HIF-1α (44). This response appeared to be intrinsic to N. meningitidis since intraphagolysosomal loading with an unencapsulated mutant (45), failed to reduce ATP or enhance apoptosis. Exposure to N. meningitidis resulted in sustained innate responses. This is noteworthy since marked pro-inflammatory cytokine release is central to the pathogenesis of meningococcal disease (69). A sub-population of ‘viable’ monocytes after N. lactamica infection maintained intracellular ATP, continued to phagocytose latex beads and showed distinct cytokine expression with reduced pro-inflammatory cytokine expression but enhanced IL-10 responses. If the cytokine expression was normalised to numbers of adherent ‘viable’ cells the levels of IL-1β, IL-6 and IL-12 become comparable between N. lactamica and N. meningitidis, the levels of TNF-α and IL-8 become greater for N. lactamica infection but the increase in IL-10 expression for N. lactamica infection was even more accentuated (data not shown). In addition to showing increased levels of trypan blue positivity, N. lactamica infection resulted in a loss of cell adherence, and decreased cell numbers, with evidence that apoptosis was the predominant mechanism of cell death. N. lactamica is more readily internalized than N. meningitidis but the simultaneous ingestion of apoptotic bodies would be predicted to de-activate pro-inflammatory cytokine responses and enhance IL-10 release (66). In contrast maintenance of intracellular ATP and failure to induce apoptosis in N. meningitidis exposed cultures would be anticipated to have both direct effects by sustaining cytokine production in the monocytes ingesting meningococci but also indirect effects by limiting the capacity of apoptotic bodies to downregulate pro-inflammatory cytokine responses. In addition the persistent generation of factors such as ROS would contribute to tissue injury (70). Failure to engage one of the regular death processes that result when monocytes ingest bacteria may underpin the pro-inflammatory features of meningococcal infection, providing an important illustration of the importance of cell death in limiting persistent high-level innate responses in monocytes.
In summary, we provide evidence that highly purified human monocytes engage an apoptotic program to down-regulate innate responses to bacteria. The host response to some infections may prevent this process and substitute an alternative death process, which aims to contain extracellular bacteria by ensuring recruitment of additional inflammatory cells. Failure to engage either of these programs is likely to result in persistent innate responses, which place the host at risk of sepsis and multi-organ failure as observed in meningococcal sepsis.
Supplementary Material
Acknowledgements
The authors acknowledge the help of Chris Hill in generating transmission electron micrographs and Professor Mumtaz Virji for sharing the unencapsulated (¢13) strain of MC58 N. meningitidis.
Funding: This work was supported by a Wellcome Trust Senior Clinical Fellowship to DHD, #076945. SRW is supported by a Wellcome Trust Clinician Scientist Fellowship #078244.
Abbreviations
- LDH
lactate dehydrogenase
- ROS
reactive oxygen species
- DCF
2′, 7′-dichloro-dihydrofluorescein diacetate
- ΔΨm
loss of inner mitochondrial trans-membrane potential
Footnotes
While in revision, we have become aware of the publication describing the production of extracellular traps by monocytes in response to nanoparticles.
Bartneck, M., H. A. Keul, G. Zwadlo-Klarwasser, and J. Groll. 2009. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett 10:59-63.
References
- 1.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North RJ. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970;132:521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steigbigel RT, Lambert LH, Jr., Remington JS. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974;53:131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen AB, Cline MJ. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971;50:1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plomp PJ, Gordon PB, Meijer AJ, Hoyvik H, Seglen PO. Energy dependence of different steps in the autophagic-lysosomal pathway. J Biol Chem. 1989;264:6699–6704. [PubMed] [Google Scholar]
- 9.Cohn ZA, Benson B. The Differentiation of Mononuclear Phagocytes. Morphology, Cytochemistry, and Biochemistry. J Exp Med. 1965;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008;180:7485–7496. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labbe K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coakley RJ, Taggart C, McElvaney NG, O'Neill SJ. Cytosolic pH and the inflammatory microenvironment modulate cell death in human neutrophils after phagocytosis. Blood. 2002;100:3383–3391. doi: 10.1182/blood.V100.9.3383. [DOI] [PubMed] [Google Scholar]
- 16.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss OH, Kim S, Wewers MD, Doseff AI. Regulation of monocyte apoptosis by the protein kinase Cdelta-dependent phosphorylation of caspase-3. J Biol Chem. 2005;280:17371–17379. doi: 10.1074/jbc.M412449200. [DOI] [PubMed] [Google Scholar]
- 19.Voss OH, Batra S, Kolattukudy SJ, Gonzalez-Mejia ME, Smith JB, Doseff AI. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J Biol Chem. 2007;282:25088–25099. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- 20.Cline MJ, Lehrer RI, Territo MC, Golde DW. UCLA Conference. Monocytes and macrophages: functions and diseases. Ann Intern Med. 1978;88:78–88. doi: 10.7326/0003-4819-88-1-78. [DOI] [PubMed] [Google Scholar]
- 21.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192:1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- 22.Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- 23.Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- 24.Dockrell DH. Apoptotic cell death in the pathogenesis of infectious diseases. J Infect. 2001;42:227–234. doi: 10.1053/jinf.2001.0836. [DOI] [PubMed] [Google Scholar]
- 25.Darton T, Guiver M, Naylor S, Jack DL, Kaczmarski EB, Borrow R, Read RC. Severity of meningococcal disease associated with genomic bacterial load. Clin Infect Dis. 2009;48:587–594. doi: 10.1086/596707. [DOI] [PubMed] [Google Scholar]
- 26.Russo TA, Singh G. An extraintestinal, pathogenic isolate of Escherichia coli (O4/K54/H5) can produce a group 1 capsule which is divergently regulated from its constitutively produced group 2, K54 capsular polysaccharide. J Bacteriol. 1993;175:7617–7623. doi: 10.1128/jb.175.23.7617-7623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil G, Virji M. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb Pathog. 1997;22:295–304. doi: 10.1006/mpat.1996.0126. [DOI] [PubMed] [Google Scholar]
- 28.Dockrell DH, Lee M, Lynch DH, Read RC. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J Infect Dis. 2001;184:713–722. doi: 10.1086/323084. [DOI] [PubMed] [Google Scholar]
- 29.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 30.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraoka W, Vazquez N, Nieves-Neira W, Chanock SJ, Pommier Y. Role of oxygen radicals generated by NADPH oxidase in apoptosis induced in human leukemia cells. J Clin Invest. 1998;102:1961–1968. doi: 10.1172/JCI3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman SA, Randers L, Rao G. Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol Prog. 1993;9:671–674. doi: 10.1021/bp00024a017. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Bano J, Lopez-Prieto MD, Portillo MM, Retamar P, Natera C, Nuno E, Herrero M, Del Arco A, Munoz A, Tellez F, Torres-Tortosa M, Martin-Aspas A, Arroyo A, Ruiz A, Moya R, Corzo JE, Leon L, Perez-Lopez JA. Epidemiology and Clinical Features of Community-Acquired, Healthcare Associated and Nosocomial Bloodstream Infections in Tertiary and Community Hospitals. Clin Microbiol Infect. 2009 doi: 10.1111/j.1469-0691.2009.03089.x. [DOI] [PubMed] [Google Scholar]
- 35.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–896. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan AT, Gorringe A, Davenport V, Williams NA, Heyderman RS. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J Immunol. 2009;182:2231–2240. doi: 10.4049/jimmunol.0802531. [DOI] [PubMed] [Google Scholar]
- 37.Liao PC, Lieu CH. Cell cycle specific induction of apoptosis and necrosis by paclitaxel in the leukemic U937 cells. Life Sci. 2005;76:1623–1639. doi: 10.1016/j.lfs.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriyama Y, Takano T, Ohkuma S. Acridine orange as a fluorescent probe for lysosomal proton pump. J Biochem. 1982;92:1333–1336. doi: 10.1093/oxfordjournals.jbchem.a134053. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 43.Krysko DV, Roels F, Leybaert L, D'Herde K. Mitochondrial transmembrane potential changes support the concept of mitochondrial heterogeneity during apoptosis. J Histochem Cytochem. 2001;49:1277–1284. doi: 10.1177/002215540104901010. [DOI] [PubMed] [Google Scholar]
- 44.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Read RC, Zimmerli S, Broaddus C, Sanan DA, Stephens DS, Ernst JD. The (alpha2-->8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pericone CD, Overweg K, Hermans PW, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000;68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leijh PC, van den Barselaar MT, van Zwet TL, Daha MR, van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979;63:772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson PK, Verhoef J, Schmeling D, Quie PG. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977;136:502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- 49.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohn ZA, Fedorko ME, Hirsch JG. The in vitro differentiation of mononuclear phagocytes. V. The formation of macrophage lysosomes. J Exp Med. 1966;123:757–766. doi: 10.1084/jem.123.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selvaraj RJ, Sbarra AJ. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966;211:1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- 52.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Shen X, Bryan A, Banga S, Swanson MS, Luo ZQ. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 6:e1000822. doi: 10.1371/journal.ppat.1000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prince LR, Bianchi SM, Vaughan KM, Bewley MA, Marriott HM, Walmsley SR, Taylor GW, Buttle DJ, Sabroe I, Dockrell DH, Whyte MK. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180:3502–3511. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, Yuan X, Nordgren G, Dalen H, Dubowchik GM, Firestone RA, Brunk UT. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000;470:35–39. doi: 10.1016/s0014-5793(00)01286-2. [DOI] [PubMed] [Google Scholar]
- 57.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 58.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 60.Ruckdeschel K, Richter K. Lipopolysaccharide desensitization of macrophages provides protection against Yersinia enterocolitica-induced apoptosis. Infect Immun. 2002;70:5259–5264. doi: 10.1128/IAI.70.9.5259-5264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali F, Lee ME, Iannelli F, Pozzi G, Mitchell TJ, Read RC, Dockrell DH. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J Infect Dis. 2003;188:1119–1131. doi: 10.1086/378675. [DOI] [PubMed] [Google Scholar]
- 62.Hand SC, Menze MA. Mitochondria in energy-limited states: mechanisms that blunt the signaling of cell death. J Exp Biol. 2008;211:1829–1840. doi: 10.1242/jeb.000299. [DOI] [PubMed] [Google Scholar]
- 63.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 65.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Deuren M, van der Ven-Jongekrijg J, Bartelink AK, van Dalen R, Sauerwein RW, van der Meer JW. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 70.Kolls JK. Oxidative stress in sepsis: a redox redux. J Clin Invest. 2006;116:860–863. doi: 10.1172/JCI28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.