SUMMARY
Dendritic cells (DCs) in mucosal surfaces are early targets for human immunodeficiency virus-1 (HIV-1). DCs mount rapid and robust immune responses upon pathogen encounter. However, immune response in the early events of HIV-1 transmission appears limited, suggesting that HIV-1 evade early immune control by DCs. We report that HIV-1 induces a rapid shutdown of autophagy and immunoamphisomes in DCs. HIV-1 envelope activated the mammalian target of rapamycin pathway in DCs, leading to autophagy exhaustion. HIV-1-induced inhibition of autophagy in DC increased cell-associated HIV-1 and transfer of HIV-1 infection to CD4+ T cells. HIV-1-mediated downregulation of autophagy in DCs impaired innate and adaptive immune responses. Immunoamphisomes in DCs engulf incoming pathogens and appear to amplify pathogen degradation as well as Toll-like receptor responses and antigen presentation. The findings that HIV-1 downregulates autophagy and impedes immune functions of DCs represent a pathogenesis mechanism that can be pharmacologically countered with therapeutic and prophylactic implications.
INTRODUCTION
Dendritic cells (DCs) are sentinels of the immune system because of their privileged location at mucosal sites and of their faculty to capture and present antigens to other immune cells such as Ag-specific T cells (Iwasaki, 2007; Trombetta and Mellman, 2005). DC-mediated immune responses during the early events of Simian immunodeficiency virus (SIV) and human immunodeficiency virus-1 (HIV-1) infection occur rapidly but are insufficient to control subsequent propagation of the virus (Davenport et al., 2004; Li et al., 2009b; Reynolds et al., 2005; reviewed in Pope and Haase, 2003).
Sexual transmission, via mucosal tissues, is by far the most common route of HIV-1 infection. DCs present in mucosal surfaces are likely to be early reservoir targets for the virus (reviewed in Piguet and Steinman, 2007; Wu and KewalRamani, 2006). HIV-1 has evolved strategies to evade, at least in part, the first line of defense of the immune system in mucosal tissues. HIV-1 degradation occurs early upon entry in DCs (Garcia et al., 2005; Moris et al., 2004; Turville et al., 2004), but ~5%–10% of initial viral input evades destruction and remains in a tetraspanin-rich compartment, at least in part, connected to the DC surface (Cavrois et al., 2007; Garcia et al., 2008; Yu et al., 2008). Upon DC-T cell contact, HIV-1 rapidly polarizes to the DC-T cell infectious synapse (Arrighi et al., 2004; McDonald et al., 2003) via a trafficking route that might share similarities to the exosome release pathway (Izquierdo-Useros et al., 2009; Wiley and Gummuluru, 2006). The inability of DC to fully degrade HIV-1, upon viral entry, is paralleled with a relative incapacity of DCs to mature, with the exception of infection by very high doses of virus (Harman et al., 2006); Granelli-Piperno et al., 2004; Hodges et al., 2007; Patterson et al., 2005; as reviewed in Piguet and Steinman, 2007).
DCs are able to process and present HIV-derived antigens to established CD4+ and CD8+ T cell clones in vitro. (Jones et al., 2007; Moris et al., 2004; Moris et al., 2006; Sabado et al., 2007). However, HIV-1-infected DCs exhibit a limited capacity to mature and to induce appropriate adaptive immune responses (Granelli-Piperno et al., 2004; Kawamura et al., 2003; Macatonia et al., 1990). The dysfunction of DCs could contribute to early suppression of immune control of HIV and SIV in mucosal tissues.
Autophagy, a specialized lysosomal degradation pathway, is a cellular self-digestion process involved in protein and organelle degradation (Klionsky, 2005; Levine and Kroemer, 2008). Dysregulation of autophagy is associated with cancer, neurodegenerative disorders, and aging because of its involvement in cell survival and general cellular homeostasis and function (Levine and Kroemer, 2008; Mizushima et al., 2008). More recently, autophagy has emerged as a cell-autonomous defense mechanism against a range of intracellular microbes including pathogens such as Mycobacterium tuberculosis or herpes simplex virus-1 (HSV-1) (Gutierrez et al., 2004; Orvedahl et al., 2007). Autophagy regulates innate and adaptive immune responses against intracellular pathogens (Deretic, 2005; Levine and Deretic, 2007; Virgin and Levine, 2009). There is now growing evidence that autophagic organelles called autophagosomes are able to engulf cytosolic components and allow this material to be delivered to the lumen of endosomal compartments, thus feeding MHC-II protein complexes with antigens (Schmid et al., 2007) and perhaps enhancing late-stage MHC-I antigen loading (English et al., 2009). This phenomenon, referred to as endogenous antigen presentation when self-antigens are in question, extends to foreign cytosolic or cytoplasmic antigens produced by viruses (Paludan et al., 2005). The underlying process of autophagic capture and delivery of cytosolic components to antigen-presenting compartments extends to cytosolic pathogen associated molecular patterns and their delivery to endosomal pattern recognition receptors and has been collectively termed autophagic topological inversion (Deretic, 2009). Given the variety of ways that autophagy contributes to elimination of intracellular microbes (Deretic and Levine, 2009), it is not unexpected that intracellular and extracellular pathogens have evolved strategies to modulate autophagy (Deretic and Levine, 2009).
In the case of HIV-1, the relationship between autophagy and its effects on immune responses has not been explored. Autophagic processes have been implicated in bystander T cell death, induced by interaction of the HIV-1 Env associated on the infected cell with CXCR4 in the contacting noninfected CD4+ T cell, thus potentially amplifying CD4+ T cell depletion (Denizot et al., 2008; Espert et al., 2006). There have been reports describing autophagy inhibition in myeloid, lymphoid, and neuronal cells upon HIV-1 or SIV infection (Alirezaei et al., 2008; Zhou and Spector, 2008), although autophagy has been implicated in resulting in higher viral yields in other cell types (Brass et al., 2008; Kyei et al., 2009).
In this study, we found that HIV-1 infection impacts autophagy in DCs. We observed that upon HIV-1 capture, the amount of autophagosome-associated protein LC-3 was dramatically downregulated after a few hours. Disappearance of autophagosomal proteins in DCs was virus dose and time course dependent. In DCs, we uncovered that HIV-1 Env induced a signaling program leading to activation of mTOR and S6K, negative regulators of autophagy. Upon inhibition of autophagosomal proteins by RNA interference or drugs in DCs, we observed that the amount of cell-associated virus was increased and that viral transfer from DCs to CD4+ T cells was enhanced. Treatment with rapamycin, a well-known mTOR inhibitor and autophagy inducer, lead to a reduction in HIV-1 content in DCs and a decrease in transfer of HIV-1 infection to T cells. Furthermore, we show that HIV-1-exposed DCs were poorly responsive to Toll-like receptor (TLR) agonists given that autophagy is required in DCs for optimal TLR signaling. Finally, inhibition of autophagy in DCs led to a decrease of MHC-II-dependent HIV-1 antigen presentation to HIV-specific CD4+ T cell clones. These studies highlight the presence of immunoamphisomes in DCs that enhance HIV degradation upon capture and amplify innate and adaptive immune responses.
In conclusion, we unravel a mechanism of immune escape developed by HIV-1. The presented data show that HIV-1 can counteract an autophagosome-mediated degradation in DCs via mTOR pathway activation, leading to enhanced virus transfer to T cells and resulting in impaired activation of both innate and adaptive immune responses.
RESULTS
HIV-1 Inhibits Autophagy in DCs
Pathogen engulfment within autophagosomes can restrict pathogen invasion. Autophagosomes are characterized by membrane incorporation of lipidated LC3 protein. We first evaluated whether HIV-1 had an influence on autophagosomes in DC looking at LC3+ puncta in cells upon HIV-1 capture.
Confocal analysis of immature monocyte-derived DCs (iDCs) exposed for 20 hr with wild-type HIV-1 showed 60% (p = 0.0216) less LC3+ puncta compared to nonexposed DC (NI) (Figure 1A). In contrast, LPS-treated mature DCs (mDCs) had a 3-fold increase in LC3+ puncta compared to nontreated iDCs (data not shown). In side-by-side comparisons of cells, the diminishment of LC3 signal was pronounced in HIV-1-containing iDCs revealed by cytoplasmic Gag (data not shown). To extend our analysis to primary blood-derived myeloid DCs (MyDCs), we treated purified MyDC with HIV-1 and observed, as for monocyte-derived DCs, a decrease in LC3+ puncta (Figure 1B). Autophagosomes are mainly characterized by the membrane incorporation of a phosphatidylethanolamine-lipidated form of LC3 (LC3-II) upon modification of cytosolic LC3 (LC3-I). The lipid modification of LC3 alters its electrophoretic mobility and thus allows monitoring conversion of LC3-I to LC3-II.
Figure 1. Loss of LC3-II in HIV-1-Infected DCs and MyDCs Is Due to a Block in the Initiation Step of Autophagic Flux.
(A) Confocal immunofluorescence analysis of LC3 (red) in unexposed iDC (NI) or 20 hr HIV-1-exposed iDCs (HIV-1). DAPI (nucleus) is shown in gray. Quantification of absolute number of LC3+ puncta (lower graph). Data are means of 30 acquired fields (±SD) and representative of three experiments. Scale bars represent 5 μm.
(B) Confocal immunofluorescence of HIVGag (green) and LC3 (red) in unexposed or HIV-1 pulsed MyDCs for 20 hr. Data are representative of two independent experiments. Scale bars represent 5 μm.
(C) The left panel shows immunoblot analysis of LC3 in lysates of DC exposed to HIV-1 for indicated times. The right panel shows quantification of LC3-I and LC3-II levels normalized to actin. Actin expression at time 0 was considered 100%. Data are means (±SD) of four independent experiments.
(D) Graph representing quantification of LC3-II normalized to actin in DCs (see representative experiment in Figure S1). Error bars represent the mean ± SD of three independent experiments.
(E) Lysates of iDCs, preincubated or not for 2 hr with rapamycin (50 μg/ml) and/or 30 min with chloroquine (10 μM) when indicated and then left untreated or treated with HIV-1 for 12 hr were immunoblotted with anti-LC3 (upper panels). Arrows indicate migration of LC3-I and LC3-II forms. Equal sample loading was controlled with anti-actin (lower panels). Data are representative of three experiments.
(F) Immunoblot analysis of p62 in lysates of DCs nonexposed (NI) or pulsed with HIV-1 for indicated times. Experiments were done in two donors.
(G) Immunoblot analysis of p62 in lysates of MyDC nonexposed or pulsed with HIV-1 for indicated times. Experiments were done in two donors.
(H) Confocal analysis of DC pulsed or not pulsed with HIV-1 for 20 hr. Cells were fixed, permeabilized, and stained with anti-HIV-1Gag (green), anti-poly-Ub (red), and anti-Lamp1 (blue). Quantification of poly-Ub fluorescence signal intensity was measured in unexposed DCs (NI) or DCs pulsed with increasing doses of HIV-1 (50–500 ng p24). Data are means of fluorescence intensity (±SD) in 30 cells and representative of two experiments. Scale bars represent 5 μm.
Time-course experiments of HIV-1-mediated decrease in LC3-II were performed with constant viral input (200 ng p24/105 cells). A decrease in LC3-II could be detected as early as 1 hr after viral exposure and was more pronounced at 10 hr (up to 62% downregulation) (Figure 1C). In contrast, cytosolic LC3 (LC3-I) remained unaffected at these time points (Figure 1C). Interestingly, decrease of autophagosomal LC3 upon 20 hr HIV-1 exposure was also observed in monocyte-derived macrophages (MDMs) but not in primary CD4+ T cells (Figure S1 available online). Hence, we observed by immunoblotting (Figure S1) up to a 60% decrease of LC3-II in iDCs exposed for 20 hr to increasing dose of the virus (Figure 1D). The use of rapamycin and/or chloroquine drugs, known to activate or inhibit autophagolysosomal-dependent degradation events, respectively, allows one to biochemically monitor autophagy flux. Accumulation of LC3-II form in lysates of 12 hr rapamycin-pulsed iDCs pretreated with chloroquine is representative of autophagy induction (Figure 1E). LC3-II accumulation was not observed in lysates of HIV-1-pulsed iDCs pretreated with chloroquine thus arguing that HIV capture by iDCs does not increase autophagy initiation (Figure 1E).
To ensure that HIV-1 alters autophagic flux, we analyzed the amounts of p62 (also known as SQSTM1) in DCs. p62 interacts with LC3 and links autophagy with degradation of polyubiquitinylated aggregates. Inhibition of autophagy is known to lead to p62 accumulation and inefficient polyubiquitinylated aggregates degradation (Ichimura et al., 2008; Korolchuk et al., 2009). We observed a decrease in p62 in iDCs treated for 1 hr with HIV-1, suggesting that initial autophagosome-mediated p62 degradation was not affected early upon HIV-1 exposure (Figure 1F). However, a strong increase in p62 expression was observed in iDC exposed with HIV-1 for 10 hr, suggesting a later block in p62 degradation (Figure 1F). Similar results were obtained in MyDCs (Figure 1G). Furthermore, confocal immunofluorescence analysis of HIV-1-pulsed iDCs showed an increased content of polyubiquitinylated proteins compared to nontreated cells (NI) (Figure 1H). Quantification of polyubiquitinylated proteins by confocal microscopy revealed a 2-fold increase upon low-viral-dose treatment (20–50 ng p24) and a 5.7-fold increase with higher viral doses (100–500 ng p24) (Figure 1H).
These data suggest that HIV-1, though not affecting autophagosome maturation during early events of iDC-mediated viral capture, alters autophagy initiation leading to progressive autophagy exhaustion and block in autophagosome-mediated degradation.
HIV Env-Mediated mTOR-and-S6K Activation Downregulates Autophagy in DCs
Mammalian target of rapamycin (mTOR) is a Ser-Thr kinase that functions as a central regulator of biomass growth or loss by affecting transcription, protein synthesis, mRNA stability, and autophagy initiation (Pattingre et al., 2008). When activated, rapamycin-sensitive mTOR complex 1 (mTORC1) upregulates S6-kinase (S6K) involved in substrate phosphorylation of a range of targets as ribosomal protein S6. Normally, mTOR inhibits autophagy at initiation step, but under nutrient deprivation, rapamycin treatment, or stress conditions, this inhibitory activity on autophagy is relieved. The link between the mTOR-S6K signaling pathway and autophagy regulation led us to analyze whether HIV-1 modulated this signaling pathway in DCs.
Lysates of iDCs exposed or not exposed with HIV-1 were immunoblotted with a cocktail of polyclonal antibodies recognizing phosphorylated forms of proteins involved in the mTOR signaling pathway. iDCs exposed for 15 min with HIV-1 showed increased phosphorylation of ribosomal protein S6 and Erk, the latter known to be upstream of mTOR activation (Figure 2A, left panel). Increased phosphorylation of S6 correlated with LC3-II loss (data not shown), strongly suggesting that HIV-1-mediated mTOR signaling induced a block of autophagy flux initiation leading to autophagosome exhaustion. HIV-1 envelope-expressing HeLa cells have been shown to induce syncitial apoptosis of CD4-expressing bystander HeLa cells via a signal cascade involving mTOR (Castedo et al., 2001; Castedo et al., 2002). Hence, interregulation between mTOR and autophagy is often connected to apoptosis or cell death (Edinger and Thompson, 2004). Therefore, autophagy impairment could occur via a noncontrolled side effect of mTOR activation on other signaling pathways involved in cell growth or death. To rule out this possibility, we analyzed global protein expression, cellular viability, and apoptosis in iDCs treated for 20 hr with HIV-1 in presence or absence of mTOR and autophagy modulators. At that time point in HIV-1-exposed iDCs, we observed no substantial changes in global mRNA content, cell size, cellular viability, or caspase-3 activity even in presence of mTOR or autophagy modulators (rapamycin and 3-MA respectively) (Figure S2 and data not shown). Because HIV-1-mediated S6 phosphorylation could be observed as early as 15 min after HIV exposure, we hypothesized that HIV-1 induced the mTOR-S6K pathway activation via HIV-1 Env-mediated signaling. By comparing HIV-1 wild-type (WT) and HIV-1-DEnv, we observed that HIV-1-DEnv viruses were unable to increase S6 phosphorylation (Figure 2B). Of note, both CXCR4 and CCR5-tropic HIV-1 could induce S6 phosphorylation (data not shown). HIV-1-F522Y, a fusion-defective mutant virus (Sourisseau et al., 2007), showed an increased phosphorylation of S6 and Erk (Figure 2C). Thus, viral fusion is not required to induce mTOR-S6K signaling in iDCs and subsequent inhibition of autophagy in DCs (data not shown).
Figure 2. HIV-1 Envelope Induces mTOR Signaling Pathway Activation.
(A) The upper panel shows an immunoblot of DC lysates with PathScan I cocktail recognizing phosphorylated mTOR pathway proteins after 15 min of HIV-1 exposure. Arrows indicate bands corresponding to Erk and S6 phosphorylated proteins. Experiment shown is representative of five independent experiments.
(B) Lysates of DC untreated or treated with HIV-1 or with HIV-1-ΔEnv (500 ng p24 each) for 15 min and immunoblotted with PathScan I cocktail. Arrow indicates S6 phosphorylated protein. Data are representative of three independent experiments.
(C) Immunoblot with PathScan I cocktail of lysates of DCs untreated or treated for 15 min with HIV-1 or HIV-1-F522Y. Arrows indicate bands corresponding to Akt, Erk, and S6 phosphorylated proteins. Loading was controlled with anti-actin (lower panel). Data are representative of two experiments.
(D) PathScan I immunoblotting on lysates of 5 × 105 DCs untreated or treated for 15 min with mouse Ig (10 μg/ml), rabbit Ig (10 μg/ml), mouse anti-CD4 (10 μg/ml), rabbit anti-DC-Sign (H-200 at 10 μg/ml), rgp120 (5 μg/ml), rantes (10 nM), and HIV-1 (1 μg p24). Phosphorylated Erk and S6 migrating bands are indicated by arrows. Similar results were obtained in three independent experiments.
(E) Lysates of DCs untreated or treated with HIV-1, HIV-1-ΔEnv, or HIV-1-F522Y (500 ng p24 each) for 15 min and immunoblotted with anti-phospho2448-mTOR. Arrow indicates phosphorylated mTOR protein. Equivalent loading was controlled by immunoblotting with anti-mTOR (lower panel). Data are representative of three independent experiments.
(F) Lysates of iDCs untreated or incubated for 12 hr with HIV-1 (1 μg p24), Ig isotype (10 μg/ml), rgp120 (5 μg/ml), and anti-CD4 (10 μg/ml) were immunoblotted with anti-LC3 (upper panels). Loading was controlled with anti-actin (lower panels). Data are representative of two experiments.
Next, we treated iDCs for 15 min with recombinant HIV-1 GP120 (rGP120), anti-CD4 and anti-DC-SIGN. HIV-1 GP120 and anti-CD4 could trigger S6 and Erk phosphorylation as efficiently as wild-type virus (Figure 2D). In contrast, H-200, a DC-SIGN agonist antibody, and Rantes and SDF-1α, ligands for CCR5 and CXCR4, respectively, did not show any effect on S6 phosphorylation (Figure 2D and data not shown). Activation of mTOR was confirmed to be envelope dependent, given that HIV-1-WT, nonfusogenic HIV-1-F522Y, and rGP120 were able to induce mTOR phosphorylation at serine residue 2448, whereas this was not observed with HIV-ΔEnv (Figure 2E). Engagement of iDC cellular receptors by HIV-1 and rGP120 was consistently correlated with a profound loss of autophagosomal LC3 upon 12 hr of treatment (Figure 2F). However, CD4 engagement alone on iDCs by agonist antibodies, although inducing signaling via the mTOR pathway, showed a slight decrease of autophagosomal LC3 upon 12 hr of stimulation (Figure 2F), suggestive of the possible need of synergic engagement of other cellular receptors on iDCs to recapitulate the LC3-II loss observed with HIV-1 envelope.
Together, these results indicate that HIV-1 Env triggers signaling events, in part through the CD4 receptor, that lead to mTOR activation, thereby downregulating autophagy initiation. Therefore, viral fusion upon endocytosis is required for productive infection (Miyauchi et al., 2009) but not for HIV-1 Env-mediated mTOR pathway activation.
HIV-1 Traffics to Lysosomes via Amphisomes
We hypothesized that HIV-1-mediated autophagy inhibition in DCs could be a viral defense mechanism against autophagosome-mediated viral degradation. This suggests that autophagosomes could intersect HIV-1 upon DC-mediated viral capture. Confocal immunofluorescence experiments showed colocalization between HIV-Gag and LC3+ vesicles early after incubation of HIV-1 with iDCs (Figure 3A). Upon 15 min of HIV-1 internalization by iDCs, LC3+ structures were colocalizing with HIV-1 (Figures 3A and 3B). After 1 hr of virus contact, intracellular colocalization between HIVGag and LC3+ profiles increased and were still detected after 2 hr of iDC-mediated HIV-1 capture (Figures 3A and 3B). At the 1 hr time point of HIV-1 incubation with iDCs, we could identify autophagolysosomes costaining with anti-Gag, anti-LC3, and anti-Lamp1 (Figure 3B), suggesting that a proportion of HIV-1-containing autophagosomes fused with lysosomes. Lysosomal inhibition by chloroquine allowed us to readily detect LC3, Lamp1, and Gag at 15, 60, and 120 min after viral internalization by iDCs (Figures 3A and 3B). Quantification of viral trafficking to autophagosomes and lysosomes was performed in the presence or absence of chloroquine. In as early as 15 min, we could observe a peak amount of virus present in autophagosomes (Figure 3C). Chloroquine treatment increased HIV-1 accumulation in LC3+ structures (Figure 3C). Of note, whereas DC-SIGN agonist antibody H-200 did not show any substantial effect on mTOR pathway activation (see Figure 2), we observed DC-SIGN colocalizing with LC3+ structures after 10 min stimulation with HIV-1, gp120, and antagonist antibody AZN-D1 (Figure S3). This suggests a potential link between DC-SIGN binding and trafficking to autophagosomes, but rules out a major role for DC-SIGN engagement in HIV-1-mediated mTOR activation.
Figure 3. HIV-1 Traffics via Autophagosomes Prior to Lysosomal Targeting.
(A) Confocal immunofluorescence analysis of HIV-1Gag (green), LC3 (red), and Lamp1 (blue) of DCs pretreated or not with chloroquine (10 μM) and exposed to HIV-1 for indicated times. White arrows indicated colocalization between HIV-1Gag (green) and LC3 (red). Data are representative of three independent experiments.
(B) Magnification of areas of HIV-1Gag colocalizing with LC3+ positive structures indicated by white arrows in (A).
(C) Quantification of autophagosomes (LC3+) colocalizing with HIV-1Gag at each indicated time in presence or absence of chloroquine (Chlq). Data are means of at least 20 cells per condition (±SD) at each time and representative of three independent experiments.
(D) Quantification of lysosomes (Lamp1+) colocalizing with HIV-1Gag at each indicated time in presence or absence of chloroquine (Chlq). Data are means of at least 20 cells (±SD) per condition at each time and representative of three independent experiments.
(E) Quantification of lysosomes (Lamp1+) colocalizing with autophagosomes (LC3+) at each indicated time in presence or absence of chloroquine (Chlq). Data are means of at least 20 cells (±SD) per condition at each time and representative of three independent experiments.
(F–H) The same as in (C)–(E) respectively but during longer time points. Data are means of at least 15 cells and representative of two experiments.
(I) DC exposed to HIV-1 for 120 min and processed as in (A). Z stack confocal analysis was done with slices of 1 μm. The image is from one field out of 20 and representative of three independent experiments.
At later time points, the virus accumulated in lysosomes in the presence of chloroquine (Figure 3D). Chloroquine treatment led to the accumulation of LC3+Lamp1+ structures, suggestive of increased autophagolysosomes in iDCs (Figure 3E). This effect could be observed up to 20 hr (Figure 3H). We also analyzed HIV-1 trafficking at later time points going from 2 hr to 20 hr (Figure S3) and quantified colocalization events of HIV-1 Gag with LC3+Lamp1+ structures. Colocalization of HIV-1 with LC3+ Lamp1+ structures peaked at 6 hr (Figures 3F and 3G) and decreased after 12 hr, correlating with the loss of autophagosomal LC3 at later time points. As observed for earlier time points, chloroquine pretreatment of iDCs led to an increased HIV-1 colocalization, suggestive of a marked viral degradation mediated by autophagolysosomal organelles (Figures 3F and 3G). Together, these results indicate that upon endocytosis, HIV-1 is first routed via LC3+ autophagosomes, namely amphisomes, and subsequently targeted to lysosomes for degradation.
To further define the LC3+ structures that colocalized with HIV-1 at early time points after internalization, we estimated the diameter of the HIV-1+LC3+ compartments. Z stacks of confocal images revealed that HIV-1-containing amphisomes had a diameter of 1.2 μm(Figure 3I) compatible with the average autophagosomal size (Klionsky, 2005). Next, we performed electron microscopy studies in iDCs unexposed or exposed for 2 hr with HIV-1 to allow virus capture and internalization. In these conditions, we could identify in DCs double-membrane autophagosome-like structures (Figure S3, black arrows). A proportion of these structures contained HIV-1 (Figure S3, right, black asterisk). Of note, the diameter of HIV-1-containing amphisomes measured on electron microscopy images was comparable to those in untreated DCs ranging from 0.6 to 1.2 μm (data not shown).
Taken together, these results indicate that intracellular LC3+ structures in DCs can intercept engulfed virions and target them toward lysosomal degradation. These events are reminiscent of structures called amphisomes formed upon fusion between endosomes and autophagosomes.
Autophagy Downregulation Leads to Increased HIV-1 Content in DCs
LC3, apart from being an autophagosomal marker, is also a regulator of autophagosome formation during membrane expansion and the final closing step of the vesicle (Geng and Klionsky, 2008; Nakatogawa et al., 2007). To explore the impact of autophagosomal machinery on HIV-1 capture by DCs, we transfected DCs with control siRNA (Ctrl) or siRNA directed against LC3 isoforms α and β. An efficient downregulation of LC3 expression of 60%–95% with siRNA directed against LC3 was routinely observed when compared to nontransfected DCs (NT) and siRNA Ctrl-treated DCs (Figure 4A). Downregulation of LC3 expression in siLC3-treated DCs was confirmed by confocal immunofluorescence and compared to siCtrl-DC (Figure 4B). HIV-1 exposure during 20 hr on siCtrl-DCs recapitulated LC3-II loss as previously described (Figure 4B). Strikingly, siLC3-treated DCs showed increased HIVGag+ cells with a more scattered HIVGag staining per cell (Figure 4B). Increased HIV-1 content in autophagy-deficient iDCs was also confirmed by intracellular HIVGag staining and FACS analysis (Figure 4C). Intracellular HIVGag staining in siLC3-treated DCs showed on average a 2.2 fold (±0.74) increase in HIV-1 content compared to NT and siCtrl DCs (Figure 4D). This effect was virus replication independent as demonstrated with a reverse transcriptase inhibitor (AZT) treatment (Figure 4D). To rule out nonspecific effects with siLC3, we repeated similar experiments but targeting another autophagosomal protein, ATG5, a main regulatory component of autophagosome formation (Mizushima et al., 1998). siATG5-DCs had a similar increase of HIV-1 content when compared to control cells (Figure S4). In order to define whether HIV-1 activation of mTOR occurs upstream of inhibition of autophagy by the virus, we analyzed mTOR activation by HIV-1 in autophagy-deficient or drug-treated DCs. Strikingly, we observed a defect in activation of phospho-mTOR (on S2448) and mTOR signaling upon HIV-1 treatment in siATG5 and siLC3 DCs compared to siCtrl DCs (Figure S4). In fact, mTOR signaling in autophagy-impaired cells was also decreased when cells were stimulated with PMA, a well-known activator of mTOR signaling (Figure S4). Therefore, our results indicate that HIV-1-mediated mTOR activation occurs upstream of inhibition of autophagy (see Figure 2).
Figure 4. Autophagy Inhibition in DCs Leads to Increased Cell-Associated HIV-1 Gag.
(A) Lysates of DCs nontransfected (NT), transfected with siCtrl, or transfected with siLC3 were immunoblotted with anti-LC3. LC3-I and LC3-II are indicated by arrows. Typical downregulation of LC3 expression in DCs with siLC3 ranged from 60% to 95%.
(B) siCtrl DCs or siLC3 DCs unexposed or exposed for 20 hr with HIV-1 were processed for confocal immunofluorescence analysis and stained with anti-HIV-1Gag (green) and anti-LC3 (red). Scale bars represent 5 μm.
(C) FACS analysis of HIV-1Gag intracellular staining in unexposed or DC exposed for 20 hr with HIV-1. DCs preincubated with AZT served as control to prevent viral replication.
(D) Mean fold of increase of HIV-1Gag+ cells determined by FACS data from five different experiments. Nontransfected DC exposed with HIV-1 were set to an arbitrary value of 1. Error bars represent the mean ± SD of the five independent experiments.
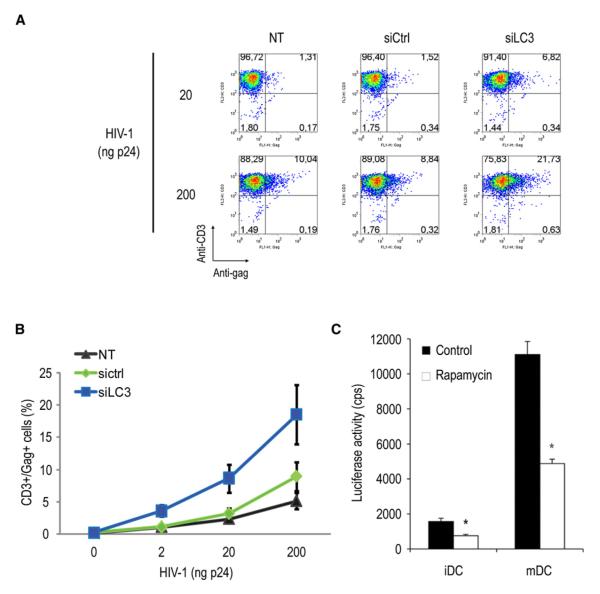
Autophagy Downregulation Enhances in trans DC-Mediated HIV Transfer to CD4+ T Cells
We next wondered whether DC-mediated HIV-1 transfer in trans to CD4+ T cells could also be affected in autophagy-deficient DCs. NT, siCtrl, and siLC3 DCs were exposed with HIV-1 for 8 hr before coincubation with autologous CD4+ T cells pretreated with the viral protease inhibitor indinavir, precluding any productive infection in target cells. With siLC3-DCs, HIV-1 transfer to CD4+ T cells was increased compared to siCtrl DCs (3.3 and 2.5 times greater with low and high viral dose, respectively) and to NT DCs (Figures 5A and 5B). Of note, siATG5 DCs showed an even more pronounced stimulation of transfer of HIV-1 infection to autologous CD4+ T cells, confirming that this effect was due to inhibition of the autophagosomal machinery (Figure S5). Rapamycin is a macrolide, known to induce autophagy via mTOR inhibition, and is considered a promising drug in multiple application ranging from cancer, aging, and neurodegenerative diseases (Rubinsztein et al., 2007). To investigate whether autophagy can block DC-mediated HIV-1 trans-infection, we compared viral transmission to CD4+ T cells between control and rapamycin-treated DCs. HIV-1 pseudotyped with the R5-tropic HIV-1 Env was used (Wang et al., 2007). As a background control, no detectable infection was found in HIV-pulsed DCs without T cell cocultures. Mature DCs (mDCs) were already shown to more efficiently transfer infection toward target cells and thus served as positive control. When DCs were cocultured with CD4+ T cells, mDC-enhanced HIV-1 trans-infection of T cells was 7-fold more efficient (p < 0.0001) compared with iDCs as expected (Figure 5C). Interestingly, rapamycin treatment of HIV-1-pulsed DCs significantly reduced iDC- and mDC-mediated HIV-1 transmission by 52% and 56% (p < 0.001), respectively. In addition, rapamycin treatment with DCs did not result in cytotoxic effects (see Figure S2). These data suggest that rapamycin-induced autophagy in DCs impairs DC-mediated HIV-1 trans-infection.
Figure 5. Autophagy Inhibition in DCs Leads to Increased Viral Cell-to-Cell Transfer.
(A) HIV-1 transfer (stained with anti-HIVGag) from nontransfected (NT), siCtrl, and siLC3 DCs to autologous CD4+ T cells (stained with anti-CD3) previously treated with indinavir (2 μg/ml).
(B) Mean values (±SD) of NT, siCtrl, and siLC3 DC-mediated HIV-1 transfer to CD3+/CD4+ autologous T cells from four independent experiments with increasing amounts of viral input (2–200ng p24).
(C) Trans-infection mediated by iDCs or mDCs pulsed with HIV-Luc/JRFL and treated or untreated with rapamycin (50 μg/ml) and then cocultured with CD4+ T cells (Hut-CCR5). Luciferase activities were quantified in cell lysates. Cps, counts per second. All data show the mean ± SD of duplicate or triplicate samples. One representative experiment out of three is shown.
HIV-1 Induced Autophagy Downregulation Alters DC Responses to TLR Ligands
Autophagy has been involved in positive regulation of some TLR-mediated innate immune response upon pathogen invasion (Delgado et al., 2009; Levine and Deretic, 2007). In addition, TLR responses alterations in DCs and macrophages from HIV-positive patients have been reported (Martinson et al., 2007; Nicol et al., 2008). We thus wondered whether HIV-1-induced autophagy downregulation could explain altered DC responses to specific TLR ligands. We first screened which TLR ligands could mediate a TNF-α response in DCs. Efficient TNF-α responses upon TLR3, TLR4, TLR6, and TLR8 stimulation were observed in iDCs, as expected (Figure S6). DCs were transfected with siCtrl, siLC3, and siATG5 and protein expression was controlled by immunoblotting (Figure 6A). When stimulated with LPS via TLR4, siLC3-treated-DC and siATG5-DC intracellular TNF-α responses were significantly reduced by more than 60% and 80%, respectively, compared to those of siCtrl DCs or untreated DCs (Figures 6B and 6C), and TLR8-mediated responses were also significantly reduced by more than 50% in both conditions (Figure 6D). HIV-1 exposure also reduced significantly TLR4- and TLR8-mediated intracellular TNF-α production to 50% and 64% respectively (Figures 6C and 6D), in agreement with the loss of LC3-II observed in these conditions (Figures 1 and 2). HIV-1 capture, however, only slightly decreased siLC3-DC and siATG5-DC TLR signaling (Figures 6C and 6D), in accordance with the expectation that most of HIV-1 impact on TLR4- and TLR8-mediated responses is autophagy dependent. Importantly, recombinant HIV-1 envelope could also decrease cytokine response upon LPS stimulation (Figure 6E). Of note, CD4 engagement alone was not able to fully recapitulate the inhibition mediated by HIV-1 envelope of LPS-dependent responses (data not shown). An efficient TLR-mediated response mostly relies on a well-coordinated receptor-ligand localization and/or well-regulated signaling cascades leading to critical transcription factors activation. In order to elucidate how HIV-1 and autophagy impairment could interfere with TLR-mediated responses, we investigated whether TLR4-mediated NF-κB signaling pathway was altered in HIV-1-treated and autophagy-deficient DCs. When compared with untreated DCs (NI), HIV-1-treated DCs showed a reduced phospho-IKK-α and -β pattern upon 15 min of LPS stimulation (Figure 6F). This correlated with less NF-κB phosphorylation because of decreased phosphorylation-dependent IκB-α degradation via the proteasome (Figure 6F). Interestingly, DCs exposed for 20 hr with HIV-1-ΔEnv were not altered in TLR4-mediated NF-kB pathway activation (Figure 6F), Defective NF-kB pathway activation was even more pronounced in autophagy-deficient cells when comparing siLC3 and siATG5 DCs to siCtrl DCs upon LPS stimulation (Figure 6G). Similar results were obtained with siBeclin1 DCs (data not shown). These results suggest that autophagy could regulate TLR-mediated signaling upstream of IKK complex activation.
Figure 6. RNA Interference or HIV-1-Mediated Autophagy Inhibition Dampen TLR4- and TLR8-Mediated Responses in DCs.
(A) Immunoblot analysis of LC3 and ATG5 expression in lysates from siCtrl, siLC3, and siATG5 DCs. Arrows denote LC3-I and LC3-II migrating forms.
(B) FACS analysis of TLR4-mediated intracellular TNF-α response upon 20 hr LPS stimulation of siCtrl, siLC3, or siATG5 DCs previously unexposed (NI) or pulsed with HIV-1 for 8 hr. BrefeldinA (10 μg/ml) was added 1 hr after adding LPS.
(C) TNF-α responses upon 20 hr TLR4 stimulation in non-transfected (NT), siCtrl, siLC3, and siATG5 DCs. Response was normalized to nonstimulated NT-DCs, and depicted as fold increase over control. Data represented means (±SD) of fold increase in TNF-α responses from five independent experiments.
(D) TNF-α responses in DCs exposed or not with HIV-1 for 8 hr and then stimulated with TLR8 agonist for 20 hr. Response was normalized to nonstimulated NT DCs and depicted as fold increase over control. Data represented means (±SD) of fold increase in TNF-α responses from five independent experiments.
(E) FACS analysis of TLR4-mediated intracellular TNF-α response upon 20 hr LPS stimulation of nontransfected (NT), siCtrl, or siLC3 DCs previously unexposed (NI) or pulsed with HIV-1 or rgp120 (5 mg/ml) for 8 hr. BrefeldinA (10 mg/ml) was added 1 hr after adding LPS. Intracellular TNF-α response was normalized to unstimulated NT DCs and represented on a graph (right) as fold increase over control. One representative experiment out of three is shown.
(F) DCs untreated (NI), or treated with HIV-1 or HIV-1-ΔEnv for 20 hr, were kept unstimulated or stimulated with LPS (1 μg/ml) for 15 min, in presence or absence of proteasome inhibitor Mg132 pretreatment when indicated (30 min at 1 μM). Lysates were then immunoblotted with anti-p176/180-IKKα/β, anti-IKK-α, anti-p32 IκB-α, anti-IκB-α, anti-p536 NF-κB, and anti-NF-κB. Sample loading was controlled with anti-actin. One representative experiment out of three is shown.
(G) The same experiment as in 6F was done on siCtrl, siATG5, and siLC3 DCs. One representative experiment out of three is shown.
Autophagy Is Required for HIV-1-Antigen Processing and Presentation to HIV-Specific CD4+ T Cells
Autophagy has recently been reported to be a key regulator of adaptive immune responses (reviewed in Deretic, 2009; Münz, 2006) by controlling intracellular antigen targeting to MHC-II compartments (Dengjel et al., 2005; Paludan et al., 2005; Schmid et al., 2007). Despite growing evidence of an intersection between autophagy and endocytosis (Liang et al., 2008; Razi et al., 2009), few reports demonstrate a clear role for autophagy-mediated extracellular antigen presentation after pathogen entry. We took advantage of our previously described model of HIV-1-derived antigen presentation to HIVGag-specific (HS) CD4+ T cell clones (Moris et al., 2006) to study the role of autophagy on exogenous HIV-1 antigen presentation on MHC II. Intracellular FACS revealed a striking reduction in HS CD4+ T cell clone TNF-α responses when we used HIV-1-pulsed siLC3 DC compared to HIV-1 pulsed NT DCs or siCtrl DCs (Figure 7A). The decrease of siLC3-DC-mediated HS CD4+ T cell responses was of 3-fold and 4.5-fold (Figure 7B, upper panel).
Figure 7. Autophagy-Deficient DCs Are Altered in HIV-1-Derived Antigen Processing and MHC Class II-Mediated Presentation to HIV-Specific T Cells.
(A) FACS analysis of intracellular TNF-α response in HIV-specific CD4+ T cells (CD3+) upon 8 hr coculture with nontransfected (NT), siCtrl, and siLC3 DCs previously pulsed for 20 hr with 100 ng p24 HIV-Mn/AT2. A positive control was done in parallel with DC pulsed for 20 hr with cognate Gag peptide (0.5 μg/ml). BrefeldinA (10 μg/ml) was added 1 hr upon coculture.
(B) Mean values of n-fold of increase of intracellular TNF-α responses from three independent experiments after pulsing DC with HIV-1 (upper) or Gag peptides (lower). Basal HIV-specific CD4+ T cells’ intracellular TNF-α expression was used as an arbitrary value of 1.
(C) ELISPOT assays measuring HIV-specific CD4+ T cells’ IFN-γ responses upon coincubation for 36 hr with nontransfected (NT), siCtrl, and siLC3 DCs previously pulsed for 20 hr with 100 ng p24 HIV-Mn/AT2 (left) or Gag peptide (right). Mean values (±SD) from one representative experiment is shown. Results are representative of three experiments performed in triplicates.
(D) Immunoblot analysis of LC3 expression in siCtrl and siLC3 DCs. Arrows indicate LC3-I and LC3-II.
(E) HIV-specific CD4+ T cells IFN-γ responses measured by ELISPOT upon 36 hr coincubation with HIV-1 pulsed DCs previously transfected with siCtrl or siLC3 or pretreated for 2 hr with rapamycin (10 μM), 3-MA (1 mM), or chloroquine (20 μM). The right panel shows HIV-specific CD4+ T cells’ IFN-γ responses upon stimulation with Gag-peptide pulsed DCs. Experiments were done in triplicates (±SD).
As positive controls, NT DCs, siCtrl DCs, and siLC3 DCs incubated with cognate Gag peptide (Moris et al., 2006) showed efficient and similar HS clone responses (Figure 7A and the lower panel of Figure 7B), thus ruling out a defective MHC class II expression at the cell surface in autophagy-deficient cells, as controlled by HLA-DR cell surface staining (Figure S7). Quantitative HIVGag-specific (HS) IFN-γ responses by ELISPOT assays of CD4+ T cell clones confirmed defective DC-mediated exogenous MHC II antigen presentation when autophagy was inhibited (Figure 7C, left panel). In ELISPOT assays, NT DCs, siCtrl DCs and siLC3 DCs incubated with cognate Gag peptide (Moris et al., 2006) showed efficient and similar HS clone responses as expected. Inefficient exogenous MHC-II antigen presentation was apparently not due to a global maturation defect in siLC3 DCs given that these cells were capable of maturation (upregulation of cell-surface CD83 and HLA-DR molecules compared to NT DCs and siCtrl DCs) upon IL-1β, TNF-α, and CD40L stimulation (Figure S7). Exogenous MHC-II-mediated antigen presentation toward the HS clone was also affected when siATG5-DC were used (Figure S7). Of note, while our study was under revision and in agreement with our results, a recent article showed that Atg5−/− DCs in a murine system had altered HSV-1-derived exogenous antigen processing and presentation thus leading to impaired CD4+ T cell priming (Lee et al., 2010). In addition, we did not find a deficit in DC maturation of autophagy-deficient DCs (see Figure S7), and we also ruled out a major role of autophagy in MHC-I cross-presentation. Indeed, MHC-I cross-presentation to HS CD8+ T cell clones was not altered when autophagy was inhibited by 3-MA in DCs loaded with HIV-1 (Figure S7).
We next analyzed by ELISPOT assays whether pharmacological modulation of autophagy induction could affect DC-mediated HIV-1 processing and antigen presentation to the HS clones. DC pretreatment with 3-methyladenine (3-MA), a well-known autophagy inhibitor, reproduced the decreased antigen-mediated HS clone response observed with siLC3 DCs (Figure 7E) in which LC3 expression was monitored by immunoblotting (Figure 7D). As expected, chloroquine, a lysosomal inhibitor, impaired HIV-1 antigen processing and presentation (Figure 7E). Strikingly, when DCs were pretreated with rapamycin, we observed a strong stimulation of HS clone IFN-γ responses, up to ranges that could only be obtained with a cognate Gag peptide (Figure 7E). In contrast, rapamycin had no effect on HS clone IFN-γ responses to DCs loaded with cognate Gag peptide, as expected (Figure 7E). This demonstrates that HIV-1, by downregulating autophagy, can reduce DC-mediated antigen processing and exogenous MHC II presentation. However, the inhibition by the virus could be bypassed by rapamycin-mediated stimulation of autophagy.
DISCUSSION
Although a large fraction of HIV-1 is degraded by DCs, a substantial amount of virus can escape this degradation and is efficiently transferred to CD4+ T cells. We provide evidence that HIV-1 shuts down autophagy in DCs. The two key consequences of the inhibition of autophagy in DCs, which likely contribute to the subsequent immunological events and inability to control the virus, are the downregulation of TLR responsiveness in DCs and inhibition of HIV-derived antigen presentation by DCs to CD4+ T cells. The effects of HIV-1 on autophagy are dependent on Env interaction with DC surface receptors, most likely CD4, and involve induction of mTOR signaling. In agreement with the latter, rapamycin could overcome HIV effects on autophagy. Furthermore, rapamycin diminished HIV-1 transfer from DCs to target T cells. Thus, autophagy plays a multitude of roles in the early events during HIV-1 infection with immediate consequences on infection of T cells and TLR signaling by DCs, as well as in antigen presentation events that set up adaptive immune responses to HIV.
DCs have a specialized endo-phagocytic machinery that links protein-pathogen capture and degradation to broad and potent innate and adaptive immune response (Iwasaki, 2007; Mellman and Steinman, 2001). Autophagy is an essential cellular process that leads to pathogen digestion and importantly an enhancement mechanism implicated at several steps in the activation of innate and adaptive immune responses (Levine and Deretic, 2007; Münz, 2007; Orvedahl and Levine, 2008). Despite some reports of RNA viruses able to co-opt autophagy for their own replication (Jackson et al., 2005), autophagy has been mainly described as a defense mechanism against other DNA virus infections such as HSV-1, CMV, or EBV.
As demonstrated in our study, HIV-1 capture in DCs leads to a rapid loss of autophagosomes. Upon HIV-1 exposure of monocyte-derived DCs or MyDCs, we observed a strong decrease of autophagosomal LC3 (LC3-II) by confocal immunofluorescence and by immunoblotting experiments. HIV-1-mediated loss of LC3-II could be observed as early as 1 hr of HIV-1 internalization by DCs and reached a maximum effect after ~10–20 hr. Intriguingly, after 20 hr of HIV-1 exposure, we also observed a disappearance of total LC3 and other ATG proteins (ATG5, ATG7, and Beclin-1) (data not shown); such a result could be explained by additional mechanisms such as an HIV-1-mediated effect on transcription or translation or effects related possibly to de novo virus replication. Our findings are in agreement with an inhibition of autophagy observed in HIV-1-infected CD4+ T cells, including a decrease of LC-3 at the protein and mRNA level (Zhou and Spector, 2008).
A recent report from Harman et al. (2009) showed that HIV-1 de novo replication in DCs after 48 hr markedly diminished cathepsin activity as a result of decreased expression of cathepsins B, C, S, and Z. The authors proposed that decreased lysosomal cathepsin activity could probably result in enhanced HIV-1 survival and transfer to contacting T lymphocytes but decreased HIV-1 antigen processing and presentation to these T cells. Thus it seems that HIV-1, by impairing autophagy induction upon early events of viral capture as well as lysosome proteases expression at later times during de novo replication, has developed means to counteract lysosome-mediated degradation during the two temporal phases of HIV-1 interaction with DCs.
The early impact of HIV-1 on LC3-II loss in DCs was clearly linked to a block in the autophagic flux, as demonstrated by p62 and polyubiquitinylated aggregates accumulation in DCs. We could also demonstrate the presence of HIV-1 in LC3+ amphisomes as early as 15 min after virus endocytosis. The block in autophagy in DCs induced by HIV-1 could be explained by the fact that HIV-1 envelope induced activation of the mTOR signaling pathway, the main regulator of autophagosome formation. By downregulating expression of critical autophagy regulators (LC3 and ATG5), we observed that HIV-1 content was increased in autophagy-deficient DCs, correlating with enhanced DC-mediated HIV-1 transfer in-trans to autologous CD4+ T cells. Rapamycin pretreatment could strongly decrease both HIV-1 content (data not shown) and DC-mediated viral transmission, thus revealing a possible tool to enhance autophagy-mediated HIV-1 restriction.
Autophagy, in addition to providing some defense against specific pathogens, is emerging as a potential regulator of TLR-mediated innate immune responses (Delgado et al., 2009; Sanjuan et al., 2007). Therefore, we analyzed whether HIV-1-mediated inhibition of autophagy could impact TLR-dependent innate immune responses. We clearly demonstrated that both autophagy-deficient cells (with RNAi targeting LC-3 and ATG5) and DC-mediated HIV-1 capture led to alteration of both TLR4- and TLR8-mediated responses at least partly because of a negative impact on NF-κB signaling. Our data in si-ATG5 or siLC-3 DCs suggest that immunoamphisomes could increase TLR signaling possibly by regulating TLR-ligand delivery. Similar observations were reported previously in Atg5−/− murine pDCs (Lee et al., 2007). In contrast, mice deficient in Atg16L did not show defects in TLR signaling (Saitoh et al., 2008). It is therefore possible that TLR signaling modulation by autophagy may be cell type specific or alternatively that only certain components of the autophagosomal machinery are critical for TLR signaling. Of note, we also observed autophagosome accumulation in LPS-stimulated DCs (data not shown), confirming that TLR4 engagement could upregulate autophagy in agreement with results reported previously in macrophages (Xu et al., 2007). Decreased TLR-4 response was also observed after treatment of DCs with recombinant HIV-1 envelope. The fact that HIV-1 could modulate autophagy-mediated innate immune responses in DC provides a potential explanation for several observations indicating that DC infected with HIV-1 are less able to mount proper immune responses (Granelli-Piperno et al., 2004; Kawamura et al., 2003).
Autophagy has mainly been involved in MHC-II-mediated antigen presentation from intracellular sources despite some evidences of involvement of autophagosomes upon pathogen endocytosis (Amer et al., 2005; Razi et al., 2009). Here, we provide evidence that autophagy is also crucial for exogenous antigen presentation on MHC II in the context of HIV-1 infection. Although global maturation processes seemed unaffected, LC3-deficient DCs were strongly altered in antigen processing upon HIV-1 exposure, thus leading to greatly decreased HIV-specific T cell responses. This defect in autophagy-mediated exogenous HIV-1 antigen presentation on MHC II was confirmed in DCs gene-targeted for ATG5 and beclin-1. Finally we demonstrate in this study, using the drug rapamycin, that stimulating autophagy in DCs prior to HIV-1 exposure could powerfully boost DC-mediated adaptive immune responses. Importantly, HIV-specific T cell responses obtained with rapamycin-treated DCs appear to be optimal when observed with DC-mediated presentation of cognate peptide. Enhancing immune responses by autophagy activation is now gaining more attention (Münz, 2009). Jagannath et al. (2009) demonstrated that autophagy activation in DCs by rapamycin led to enhanced in vitro and in vivo mycobacterial antigen Ag85B presentation, and additional data are now available on the role of autophagy in antigen presentation to CD8+ cells (Uhl et al., 2009). Related to the above, English et al. (2009) revealed a class of autophagosomes induced upon hyperthermia or IL-1b stimulation able to enhance viral peptides processing and loading on MHC I molecules upon HSV-1 infection. A recent report from Sanjuan et al., (2007) described autophagy requirement for TLR-dependent phagosomal maturation in macrophages. The authors observed the TLR-dependent recruitment of autophagosomal component to phagosome probably required for efficient phagosomal maturation and enhanced killing of ingested organism (Sanjuan et al., 2007). In contrast to the impact of HIV-1 inhibition of autophagy in DCs on MHC II presentation, inhibition of autophagy in DCs with 3-MA did not substantially alter HIV-1 cross-presentation on MHC I. This indicates that autophagy increases processing and presentation of HIV antigens on MHC II but not cross-presentation on MHC I at early times points after virus capture by DCs.
In our study, we describe LC3+ autophagosomal-like structures that are able to intersect with HIV-1 endocytosis and that probably regulate DC-mediated innate and adaptive immune responses. We propose thus to term such structures “immunoamphisomes” because of their link between autophagy and endocytosis and their function to amplify innate and adaptive immune responses.
Our data indicate that mTOR modulation could be a promising target to restrict early events of HIV-1 infection, while at the same time increasing MHC II immune responses against the virus. One potential problem of these approaches is that rapamycin and analogs have immunosuppressive capacities in addition to their potential immunostimulatory properties. In addition, rapamycin is not extremely efficient at simulating autophagy in human cells (Thoreen and Sabatini, 2009). New generations of inhibitors of mTOR might address these problems (reviewed in Guertin and Sabatini, 2009).
Animal models of mucosal transmission upon SIV challenges in vivo have now clearly established that immune responses against SIV (and by extension HIV) in mucosal tissues occur “too little, too late” and are insufficient to control subsequent propagation of the virus (Li et al., 2009b). Part of the problem appears to be unwarranted inflammation in tissues that increases number of potential immune targets at sites of virus infection. In support of this concept is the fact that glycerol monolaurate, which is anti-inflammatory, appears to have microbicidal properties against SIV infection (Li et al., 2009a). Derived from our studies of HIV-1 infection of DCs, we propose that mTOR inhibitors such as rapamycin and potentially newer more potent inhibitors would provide at least three benefits to the host during the early stages of HIV-1 infection. First, mTOR inhibitors, by activating autophagy, would lead to more efficient virus degradation by DCs and thereby offer less capacity of viral transmission to targets cells including CD4+ T cells and macrophages. Second, we showed that intact autophagy is required to mount proper TLR response in HIV-1 infected DCs. Third, stimulation of autophagy in DCs leads to more rapid and robust adaptive immune responses. Therefore stimulation of autophagy in the early events of HIV-1 infection could represent a novel method to “robustly and rapidly” mount efficient immune responses in mucosal tissues, thereby containing viral propagation. Our study, by demonstrating a critical role for autophagy in the early events of HIV-1 infection, yields important insights into the development of “rational” anti-HIV vaccines and current approaches aiming to prevent HIV-1 transmission.
EXPERIMENTAL PROCEDURES
Cells
Human monocytes from buffy coats were obtained in accordance with institutional guidelines of the ethical committee of the University of Geneva. Monocytes were purified after Ficoll gradient separation with CD14 MicroBeads (Miltenyi Biotec). Usual purity was >95% CD14+. Human MDDCs were generated by incubation of purified monocytes in IMDM supplemented with 10% FCS, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, 1% nonessential amino acids, 1 mM sodium pyruvate, 500 IU/ml GM-CSF, and 500 IU/ml IL-4 (both from Strathman Biotec, Germany). On days 2 and 4, a third of the culture medium was replaced by fresh medium containing GM-CSF and IL-4. iDCs were harvested at day 6 and analyzed by flow cytometry. Autologous CD4+ T lymphocytes and myeloid DCs (MyDCs) were purified with CD4+ T Cell Isolation kit II and CD1c Dendritic Cell Isolation Kit (Miltenyi Biotec), respectively, in accordance with the manufacturer’s instructions. Hut-CCR5 cell line (Wang et al., 2007) and autologous CD4+ T lymphocytes were maintained in supplemented RPMI1640. 293T human embryonic kidney cells and HeLa P4-R5 (NIH AIDS Research & Reference Reagent Program) cells were maintained in supplemented DMEM.
Virus Production
Virus stocks were produced as previously described (Arrighi et al., 2004). In brief, 293T cells were transiently transfected with calcium-phosphate coprecipitated proviral plasmid pR9 or pR8-Bal, encoding for full-length HIV-1 X4 and HIV-1 R5 strain provirus, respectively. Infectious titers of viral stocks were evaluated by limiting dilution on HeLa P4-R5 cells. These cells derive from HeLa P-4.2 cells stably expressing coreceptor CCR5 and containing the β-gal gene under the control of HIV long terminal repeat (LTR). Viral titers were expressed as infectious units (IU) per ml. Approximately 500 ng of p24gag on 2.5 × 105 CD4+ HeLa P4-R5 corresponds to a MOI of 1 (multiplicities of infection). Physical titers were evaluated by quantification of an HIV-1 p24gag by ELISA kit (Beckman Coulter, Paris, France).
Drugs
All chemicals were obtained from Sigma unless stated otherwise. For transfer and capture assays, 2 μg/ml indinavir (Merck) and 50 μg/ml zidovudine (AZT) (GlaxoSmithKline) were used. High-grade rapamycin was a kind gift from J. Curran.
Antibodies and Reagents
Antibodies against LC3 (F14), ATG5 (N18), p62 (SQSTM1) (H-290), and DC-SIGN (H-200) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-active Caspase-3, anti-CD4, anti-hTNF-α-APC, and FACS antibodies were obtained from BD Transduction Laboratories (Franklin Lakes, NJ). Anti-LC3 (PM036) used for immunoblotting was from MBL International (Woburn, MA). Anti-poly-Ub (FK2) was from BIOMOL (Exeter, UK). Anti-Lamp1 was from Abcam (Cambridge, UK). Anti-phospho2448-mTOR, anti-mTOR, NF-κB pathway kit, and PathScan Multiplex cocktails I and III were from Cell Signaling Technology (Danvers, MA). Anti-actin was from Sigma (St-Louis, MO). Monoclonal anti-HIVgag (KC57-FITC, from Beckman Coulter, Miami, FL) was used for immunofluorescence-confocal analysis as well as for flow cytometry analysis. DAPI (4′,6′ di amidino-2-phényl indole) was obtained from Molecular Probes. Recombinant HIV-1 gp120 (rgp120 HIV-1 IIIB) was from Immunodiagnostics (Woburn, MA). Soluble recombinant human CD40L and Enhancer were from Apotech (Epalinges, Switzerland). Recombinant human IL-1β and TNF-α were from Peprotech (London, UK).
siRNA Transfections
siCtrl ([5′-AAATGAACGTGAATTGCTCAA-3′]; siRNA sequence specific for Luciferase), siBecn1 ([5′-AAGATCCTGGACCGTGTCACC-3]; siRNA sequence specific for Beclin-1) were synthesized from QIAGEN (Hilden, Germany). Alternatively, the following siRNA mixes were purchased from Santa Cruz Biotechnology (Santa Cruz, CA): siLC3-α (sc-106197), siLC3-β (sc-43390), and siATG5 (sc-41445). HiPerFect Transfection Reagent (QIAGEN, Hilden, Germany) was used for transfection in accordance with the manufacturer’s recommendations. In brief, 4 × 105 iDCs were usually transfected with 100 nM siRNA in 500 μl of IMDM/1% SVF medium in 12-well plates. A second round of transfection was performed 24 hr later. Specific gene knockdowns were assessed by immunoblotting followed by densitometry analysis (Quantity One; Bio-Rad Laboratories, Hercules, CA) comparing with nontransfected and siCtrl conditions.
Infections
For biochemical experiments, 106 of iDCs or 5 × 105 of MyDCs were plated in 96-round well plates in 100 μl IMDM for challenge with R9-HIV-1 or R8Bal-HIV-1 at MOI ranging between 0.2 and 2 (corresponding to ~1 μg of p24gag per 4 × 105 DCs). Cells were left in contact with virus during indicated times at 37°C. Cells were then resuspended in IMDM and processed for experiments. For cellular assays, when not stated, HIV-1 treatment was done at 500 ng p24 HIV for 2.5 × 105 cells (corresponding to a MOI of 1) and adapted depending on cell number.
Immunoblotting
Cells were lysed for 20 min at 4°C in lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCL, 1% NP40, 1 mM NaVO4, 50 mM NaF, 10 mM Na4P2O7, and protease inhibitor cocktail [Roche]) and centrifuged at 26,000 g for 30 min. Postnuclear supernatants were submitted to electrophoresis on 4%–12% gradient SDS-PAGE gels, transferred to a nitrocellulose membrane, and immunoblotted. Membranes were stripped in 0.1 M Glycine buffer (pH 2.3) in case of reblotting for actin level control, Membranes were revealed with SuperSignal West Pico Solution (Thermos Scientific; Rockford; IL).
Transmission Electron Microscopy
Cells were harvested and fixed in 2% formaldehyde and 1.5% glutaraldehyde in 0.2 M sodium phosphate buffer (pH 7.4) at room temperature for 2 hr. Cells were then washed once in sodium phosphate buffer and prepared for epon sectioning. After ultramicrotome sectioning, sections were analyzed with a Philips CM10 transmission electron microscope (Philips, Eindhoven, Netherlands).
Flow Cytometry and Confocal Immunofluorescence
After isolation or differentiation, surface staining of primary cells (monocytes, MDDCs, MyDCs and autologous CD4+ T cells) were performed at 4°C for 30 min with mAbs from BD and directed against the following molecules: CD1a, CD3, CD4, CD8, CD14, CD19, CD45Ro, CD69, CD83, CD86, DC-SIGN, and HLA-DR. The following other mAbs from Miltenyi Biotec were used for MyDC cell surface staining: anti-CD1c, anti-CD11c, anti-CD123, and anti-CD303. Gag p24 expression was measured on permeabilized cells with anti-Gagp24 (FITC-coupled) mAb (KC57, Coulter). Isotype-matched mAbs were used as negative controls. Samples were analyzed by flow cytometry with a FacsCalibur (Becton Dickinson) and data processed with FlowJo software. For immunofluorescence, cells were left to adhere on poly-L-lysine-treated glass coverslips for 60 min at 37°C. Cells were then fixed, permeabilized, and stained with indicated antibodies and then stained with fluorescently labeled secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA). Gag staining was done with anti-Gagp24 KC57-FITC mAb. Confocal microscopy analysis was carried out on a Zeiss LSM510Meta with a 63× objective. Z series of optical sections were performed at 0.5–1 μm increments for qualitative analysis. Green, red, and far-red fluorescences were acquired sequentially. Quantifications of LC3+ puncta and colocalization events were performed with ImageJ Software (NIH) and analyzed with Excel software (Microsoft).
Transfer Assay
After 8 hr of HIV-1 treatment, 105 of iDCs were replated in 96-round well plates (Falcon; BD) in 100 μl final volume of IMDM and mixed with 105 autologous CD4+ T cells previously treated with 2 μg/ml indinavir (Merck). Cells were left in contact for coculture for 24–36 hr and then processed for FACS analysis of intracellular HIV-1 Gag content in CD3+ T cells. Transfer of infection with HIV-Luc/JRFL was performed as described previously (Wang et al., 2007).
TLR Stimulation
A total of 105 iDCs transfected with siRNA oligos or challenged with 200 ng p24 HIV-1 for 8 hr were stimulated with the Human TLR1-9 Agonist kit (InvivoGen, San Diego, CA). TLR ligands:Pam3CSK4 (1 μg/ml), HKLM (108 cells/ml), Poly(I:C) (2.5 μg/ml), LPS (200 ng/ml), Flagellin (1 μg/ml), FSL1 (1 μg/ml), Imiquimod (1 μg/ml), ssRNA40 (1 μg/ml), and ODN2006 (5 μM) were incubated with cells for 20 hr. BrefeldinA (10 mg/ml) was added upon 1 hr of TLR stimulation. Cells were then fixed in 2% paraformaldehyde and intracellular cytokine FACS was done.
Antigen Presentation
ELISPOT assays and Intracellular cytokine FACS on HS clone responses were adapted from Moris et al. (2006). In brief, for ELISPOT assays, iDCs were exposed for 4 hr to HIV-Mn/AT2 (100 ng/mL). Stimulator cells were then cocultured for at least 24–36 hr with CD4+ T cell clones. IFN-γ production was measured in an enzyme-linked immunosorbent spot (ELISPOT) assay as described (Buseyne et al., 2001; Moris et al., 2006) with a MABTECH IFN-γ ELISPOT assay kit (MABTECH, Sweden). As a positive control, stimulators were incubated with cognate peptides (0.5 mg/ml) before addition of CD4+ clones. When stated, rapamycin (10 μg/ml), 3-methyladenine (1 mM), or chloroquine (20 μg/ml) was added to stimulator cells 2 hr prior to viral exposure.
Statistical Analysis
Significant differences between groups were calculated with the Student’s t test. p values < 0.05 were considered significant and marked with an asterisk.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by amfAR grant 107160-44-RGRL and Bill and Melinda Gates Grand Challenge Explorations grant to V.D. and in part by grants from the NIH (R01-AI068493) to L.W. This work was supported by Swiss National Science Foundation grants PP00A3-114755 to V.P. and E.M.B.O long-term fellowship to F.P.B. This work was also supported by The Human Science Frontier Program to V.P. We thank J. Villard and the HLA-genotyping plateform for HLA-DR screening of PBMCs. We also thank F. Guivel-Benhassine, P. Carraux, G. Kyei, and C. Dong for excellent technical help. We are grateful to J. Curran for the kind gift of rapamycin. We also thank B. Mangeat, S. Anghel, and R. Correa for discussions and critical review of the text and figures. Electron microscopy samples preparation was carried out with the help of the “Pôle Facultaire de Microscopie Ultrastructurale (PFMU)” at the Faculty of Medicine of the University of Geneva. Flow cytometry analysis was performed with the support of the Flow Cytometry Core Facility of the Faculty of Medicine of the University of Geneva. Confocal immunofluorescence was done with the help of the Bioimaging core facility of the faculty of Medicine. F.P.B. and V.P. conceived the study. F.P.B., L.W., O.S., A.M, V.D., and V.P. helped in experimental design; F.P.B., D.S.N., M.L., E.G., R.S, and F.L. carried out experiments; F.P.B., V.D., and V.P. wrote the manuscript.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes seven figures and can be found with this article online at doi:10.1016/j.immuni.2010.04.011.
REFERENCES
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer AO, Byrne BG, Swanson MS. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy. 2005;1:53–58. doi: 10.4161/auto.1.1.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Buseyne F, Le Gall S, Boccaccio C, Abastado JP, Lifson JD, Arthur LO, Rivière Y, Heard JM, Schwartz O. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 2001;7:344–349. doi: 10.1038/85493. [DOI] [PubMed] [Google Scholar]
- Castedo M, Ferri KF, Blanco J, Roumier T, Larochette N, Barretina J, Amendola A, Nardacci R, Métivier D, Este JA, et al. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J. Exp. Med. 2001;194:1097–1110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, et al. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 2002;21:4070–4080. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Kreisberg JF, Greene WC. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 2007;3:e4. doi: 10.1371/journal.ppat.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MP, Ribeiro RM, Perelson AS. Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J. Virol. 2004;78:10096–10103. doi: 10.1128/JVI.78.18.10096-10103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol. Rev. 2009;227:189–202. doi: 10.1111/j.1600-065X.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot M, Varbanov M, Espert L, Robert-Hebmann V, Sagnier S, Garcia E, Curriu M, Mamoun R, Blanco J, Biard-Piechaczyk M. HIV-1 gp41 fusogenic function triggers autophagy in uninfected cells. Autophagy. 2008;4:998–1008. doi: 10.4161/auto.6880. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26:523–528. doi: 10.1016/j.it.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr. Opin. Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippé R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M, Piguet V. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Garcia E, Nikolic DS, Piguet V. HIV-1 replication in dendritic cells occurs through a tetraspanin-containing compartment enriched in AP-3. Traffic. 2008;9:200–214. doi: 10.1111/j.1600-0854.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: Beyond the usual suspects’ review series. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci. Signal. 2009;2:e24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Harman AN, Wilkinson J, Bye CR, Bosnjak L, Stern JL, Nicholle M, Lai J, Cunningham AL. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J. Immunol. 2006;177:7103–7113. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- Harman AN, Kraus M, Bye CR, Byth K, Turville SG, Tang O, Mercier SK, Nasr N, Stern JL, Slobedman B, et al. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood. 2009;114:85–94. doi: 10.1182/blood-2008-12-194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kominami E, Tanaka K, Komatsu M. Selective turnover of p62/A170/SQSTM1 by autophagy. Autophagy. 2008;4:1063–1066. doi: 10.4161/auto.6826. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borràs FE, Puertas MC, Connor JH, Fernández-Figueras MT, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WT, Giddings TH, Jr., Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr., Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat. Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- Jones L, McDonald D, Canaday DH. Rapid MHC-II antigen presentation of HIV type 1 by human dendritic cells. AIDS Res. Hum. Retroviruses. 2007;23:812–816. doi: 10.1089/aid.2006.0280. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Gatanaga H, Borris DL, Connors M, Mitsuya H, Blauvelt A. Decreased stimulation of CD4+ T cell proliferation and IL-2 production by highly enriched populations of HIV-infected dendritic cells. J. Immunol. 2003;170:4260–4266. doi: 10.4049/jimmunol.170.8.4260. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy. Curr. Biol. 2005;15:R282–R283. doi: 10.1016/j.cub.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol. Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009;186:255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009a;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O’Mara L, Southern PJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009b;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat. Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, Tomai M, Krieg AM, Ghanekar S, Baum LL, Landay AL. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell. Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood. 2004;103:2648–2654. doi: 10.1182/blood-2003-07-2532. [DOI] [PubMed] [Google Scholar]
- Moris A, Pajot A, Blanchet F, Guivel-Benhassine F, Salcedo M, Schwartz O. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood. 2006;108:1643–1651. doi: 10.1182/blood-2006-02-006361. [DOI] [PubMed] [Google Scholar]
- Münz C. Autophagy and antigen presentation. Cell. Microbiol. 2006;8:891–898. doi: 10.1111/j.1462-5822.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- Münz C. Viral evasion of autophagy. Cell Host Microbe. 2007;1:9–11. doi: 10.1016/j.chom.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Münz C. Enhancing immunity through autophagy. Annu. Rev. Immunol. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Nicol MQ, Mathys JM, Pereira A, Ollington K, Ieong MH, Skolnik PR. Human immunodeficiency virus infection alters tumor necrosis factor alpha production via Toll-like receptor-dependent pathways in alveolar macrophages and U1 cells. J. Virol. 2008;82:7790–7798. doi: 10.1128/JVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Viral evasion of autophagy. Autophagy. 2008;4:280–285. doi: 10.4161/auto.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- Patterson S, Donaghy H, Amjadi P, Gazzard B, Gotch F, Kelleher P. Human BDCA-1-positive blood dendritic cells differentiate into phenotypically distinct immature and mature populations in the absence of exogenous maturational stimuli: differentiation failure in HIV infection. J Immunol. 2005;174:8200–8209. doi: 10.4049/jimmunol.174.12.8200. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Piguet V, Steinman RM. The interaction of HIV with dendritic cells: Outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J. Cell Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoé G, Wilson N, Miller CJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: Too late and too little. J. Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Sabado RL, Babcock E, Kavanagh DG, Tjomsland V, Walker BD, Lifson JD, Bhardwaj N, Larsson M. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur. J. Immunol. 2007;37:1752–1763. doi: 10.1002/eji.200636981. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stössel H, Romani N, Piatak M, Jr., et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Levine B. Autophagy genes in immunity. Nat. Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J. Virol. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat. Rev. Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4:e1000134. doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.