Abstract
Oral cancer is a significant health problem in the USA and throughout the world. Most oral cancer patients are diagnosed at a late stage, when treatment is less successful and treatment-associated morbidity is more severe. A number of new diagnostic aids to conventional oral examination have recently been introduced to assist in the early detection of oral neoplasia. In particular, autofluorescence imaging has emerged as a promising adjunctive technique to improve early identification of oral premalignant lesions. Direct visual inspection of tissue autofluorescence has shown encouraging results in high-prevalence populations, but the technique requires subjective interpretation and depends on the visual recognition skills of the examiner. Capturing and analyzing digital fluorescence images can reduce subjectivity and potentially improve sensitivity of detection of precancerous changes. Recent studies of wide-field autofluorescence imaging in low-prevalence populations suggest that benign lesions such as inflammation may give rise to false-positive results. High-resolution fluorescence imaging is a new modality that can be used in conjunction with wide-field imaging to improve specificity by imaging subcellular detail of neoplastic tissues. The combination of wide-field and high-resolution fluorescence imaging systems with automated image analysis should be investigated to maximize overall diagnostic performance for early detection of oral neoplasia.
Keywords: diagnosis, image processing, optical fluorescence imaging, oral cancer
Oral cancer is the eighth most common cancer worldwide, with approximately 274,000 new cases reported annually [1]. In the USA alone, it was estimated that 35,000 new cases were reported in 2009, and 7600 deaths were expected [2]. The majority of patients diagnosed with oral cancer live in developing countries [3]. In India, for example, oral cancer ranks number one in prevalence among all cancers in male patients and number three among cancers in female patients. In south central Asia, oral cancer ranks among the three most common types of cancer. Recent epidemiologic data show sharp increases in the incidence of oral cancer reported in European countries and, to a lesser extent, the USA [4].
Oral cancer survival rates are strongly dependent on the stage at diagnosis. Patients diagnosed with oral cancer at a localized stage have a substantially greater chance of successful treatment and less treatment-associated morbidity [2] than those diagnosed at a late stage. Improving early detection represents one of the best ways to improve survival and quality of life for oral cancer patients worldwide. In the USA, there has been only marginal improvement in the relative 5-year survival rates for oral cancer since 1975. The modest improvement in survival is due to a combination of earlier diagnosis and improved treatment [4]. In developing countries, oral cancer patients tend to be diagnosed at a later stage than in developed countries [5]. Thus, there remains an important need to improve early detection of oral cancer and its precursors.
Challenges in oral cancer diagnosis & treatment
The current goal of the International Agency for Research on Cancer, American Cancer Society and the WHO is to reduce the predicted 15 million cancer cases by a third by diagnosing and treating these cancers at their pre-neoplastic levels. Oral cancer is an ideal choice for this strategy because the oral cavity provides easy access for clinical inspection, and oral cancer development is preceded by visible mucosal changes [6]. However, only 40% of oral cancers are currently diagnosed as localized disease, which is the same rate as that of colon cancers [6].
Despite recent diagnostic and therapeutic advances, the 5-year survival rate for oral cancer has remained less than 50% over the last 50 years owing to the following reasons:
The majority of oral cancer cases (60%) present with advanced stages (III and IV) at diagnosis;
Oral cancer has the highest risk for the development of second primary tumors (‘field cancerization phenomenon’) of any cancer.
The 5-year recurrence-free survival rate is 80% for stage I oral cancer patients, whereas only 20% of patients with stage IV oral cancer survive after 5 years [7–9]. Moreover, early diagnosis of oral cancer significantly reduces treatment-related morbidity and improve overall long-term survival [10,11]. Patients with a history of oral cancers are at risk of developing second primary tumors at a rate of 3.7% per year because of ‘field cancerization’, and a quarter of all oral cancer-related deaths are caused by second primary tumors [12]. Hence, patients who are successfully treated for oral cancer should be closely monitored, preferably using a noninvasive diagnostic test.
Diagnosis of oral cancer at an early stage or at the pre-neoplastic level is critical to improve survival in oral squamous cell carcinoma patients. However, screening via clinical examination alone by general dentists during the patients' routine dental examination has resulted in poor detection rates. Dentists frequently detect white or red patches during routine screening of an asymptomatic patient. Based on analysis of biopsies of potentially malignant oral mucosal lesions (n = 926) submitted to an UT-Dental Branch Houston oral pathology biopsy service in 2009, more than 75% of these lesions are confounding lesions, which will be microscopically diagnosed as benign, except for a small proportion (<25%) being diagnosed as oral cancer or its precursor.
General dental practitioners do not have the clinical training and experience to distinguish potentially malignant lesions from confounding lesions; hence, many of these patients need to be referred to a specialist clinic for scalpel biopsy for a definitive diagnosis.
Referring patients with potentially malignant oral lesions to specialist centers is plagued by a long waiting time leading to significant diagnostic delays [13]. A recent study conducted in the USA reported that the mean time from the initial detection of a potentially malignant lesion by a primary healthcare provider and referral to a specialist for evaluation was 35.9 days [14]. In some cases, this delay exceeded 10 months [14]. For patients with newly diagnosed oral cancer, the median delay in initial diagnosis in Canada was 4.5 weeks, which is significantly shorter than in the USA, which is reported to be 18.4 weeks [14,15]. This longer delay is attributed to the disparity in healthcare systems and health insurance-related issues in the USA [14]. It should be noted that for patients with oral cancer, delays in diagnosis by even 1 month may contribute to a diagnosis of a later stage disease [16]. Moreover, treatment delays of more than 40 days in early-stage oral cancer were associated with an increased risk of locoregional failure impacting their survival [17]. In addition, scalpel biopsy is time consuming, uncomfortable and stressful for the patient and is a relatively expensive procedure.
Therefore, developing and validating an acceptable noninvasive diagnostic test that can discriminate benign oral mucosal lesions from oral cancers and its precursors with minimal false-positive and false-negative results would be beneficial not only for the patient but also to society by reducing heathcare costs through avoiding unnecessary scalpel biopsies.
Current oral cancer screening & diagnostic methods
The standard method for oral cancer screening has long been conventional oral examination and palpation, usually performed by dentists or physicians. Visual inspection of the oral cavity is performed under normal white light illumination, followed by palpation of suspicious lesions. Downer et al. has systematically reviewed the performance of visual examination for oral cancer detection [18]. Across the eight studies reviewed by Downer et al., sensitivity values ranged from 60 to 97% and specificity values from 75 to 99% [18]. Oral cancer specialists can often recognize subtle visual changes associated with early lesions, but community practitioners or general dentists may lack the experience to identify early lesion development.
Several visualization adjuncts to standard oral examination are now commercially available. Toluidine blue is a vital dye that has been used in the oral cavity for decades to improve the visibility of lesions during visual exam. In a recent review of the performance of visual exam with toluidine blue, sensitivity ranged from 38 to 98%, while specificity varied from 9 to 93% [19]. In general, examination with toluidine blue is associated with low specificity, and this has prevented toluidine blue from becoming a standard component of early oral cancer detection efforts in the USA.
The ViziLite® (Zila Pharmaceuticals, Inc., AZ, USA) system offers an alternative to white light illumination for visual examination; a disposable chemiluminescent light source illuminates tissue with blue light. Providers view reflected blue light to detect abnormal changes in oral cavity. Initial studies conducted by Epstein et al. [20] and Kerr et al. [21] indicated that the ViziLite could potentially aid in the detection of oral premalignant lesions by improving brightness and sharpness. Epstein et al. examined 134 patients who had identified oral lesions using conventional white light and ViziLite illumination [20]. The study showed that two lesions became clinically visible only after ViziLite examination. Kerr et al. examined 501 patients who had a positive tobacco history using conventional white light, followed by ViziLite illumination [21]. The study reported that six lesions not previously seen by conventional examination were identified by ViziLite examination. However, other studies have reported that the ViziLite does not aid in the identification of oral lesions [22–24]. In a study conducted by Ram and Siar, 40 patients in a high prevalence population were examined with the ViziLite, following conventional examination of the oral cavity [22]. Farah and McCullough examined 55 patients referred for assessment of an oral white lesion with the ViziLite, following conventional oral examination [23]. Both studies concluded that examination with the ViziLite did not change the diagnosis. The authors noted that ViziLite examination could not discriminate between benign or inflammatory and premalignant or malignant oral lesions. In a study conducted by Oh and Laskin [24], 100 patients who presented for dental screening were examined with the ViziLite, following conventional examination. Results demonstrated that all of the lesions were detected during standard oral examination, and no additional lesions were detected by the ViziLite. Thus, there remains an important need for alternative diagnostic methods that can enhance the visualization of oral lesions and particularly help discriminate benign and premalignant lesions.
Emerging technologies
Wide-field fluorescence imaging
In the previously described approaches, clinicians illuminate tissue with white or blue light and observe light that is reflected from the mucosal surface. However, there are a range of light–tissue interactions that can be exploited to improve the visualization of neoplastic lesions. In particular, tissue autofluorescence has recently shown promise as an adjunctive diagnostic tool. Fluorophores within the oral epithelium and stroma absorb UV and visible light and can re-emit some of this light at longer wavelengths in the form of fluorescence. When the reflected illumination light is blocked with an absorbing filter, it is possible to visualize the longer wavelength fluorescence even with the naked eye. Autofluorescence originates from a variety of fluorophores in the oral cavity, and is sensitive to alterations in both tissue morphology and biochemistry associated with neoplasia [25,26]. Oral cancer and precancer display a loss of autofluorescence across a broad range of UV and visible excitation wavelengths; as described later, this loss of fluorescence is largely attributed to a decrease in fluorescent crosslinks associated with stromal collagen that underlies the neoplastic lesion. The VELscope® (LED Dental, Inc., White Rock, BC, Canada) is a commercially available device to visualize tissue autofluorescence in the oral cavity. The VELscope has been approved by the US FDA as an adjunct to traditional oral examination to enhance the visualization of oral mucosal abnormalities, and its clinical use appears to be growing. Lane et al. described an initial version of this device for the direct visualization of oral cavity tissue fluorescence [27]. Visual identification of oral neoplastic lesions using the VELscope is based on the premise that abnormal tissue appears dark brown to black owing to decreased levels of autofluorescence, while normal healthy tissue emits pale green autofluorescence (Figure 1). The device achieved a sensitivity of 98% and specificity of 100% using histology as the ‘gold standard’ when discriminating normal mucosa from severe dysplasia/carcinoma in situ or invasive carcinoma in 50 biopsy sites from 44 patients. Two case study reports suggested that the VELscope can assist clinicians in detecting oral lesions that are occult under white light examination and in more effectively identifying which regions to biopsy [28,29].
Figure 1. Wide-field autofluorescence imaging.
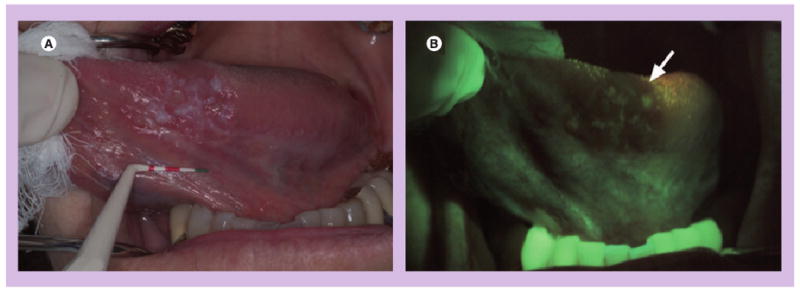
(A) A white light image of the ventral tongue of a patient with an oral premalignant lesion, which, when biopsied, was confirmed to be severe dysplasia and (B) a corresponding autofluorescence image obtained with the VELscope® (LED Dental Inc., BC, Canada). The arrow indicates the region of fluorescence visualization loss and biopsy location.
Reprinted from with permission from [26] © SPIE 2006.
A number of recent studies have suggested that the VELscope can be used as an adjunct to visual examination to improve the detection of oral neoplasia [30–35]. For example, Paulis suggests that the VELscope provides dental professionals with guidance in detecting oral cancer, noting that while it may provide some false-positive findings, it is a screening device not a definitive diagnostic tool [36]. However, although promising results have been reported, most of these studies have been limited to small numbers of patients in high-prevalence populations; several recent reviews [19,37–43] have highlighted the need for additional research to evaluate VELscope performance in general practice. Balevi noted that the potential for false positives may cause unnecessary stress and fear among patients, and increase morbidity and costs for patients when it is used in general practice as a routine screening tool [44].
A recent study reporting VELscope performance in a low-prevalence population highlighted this need. Huber examined 130 subjects who smoked at least one packet of cigarettes per day with the VELscope, following conventional oral examination [45]. The author found that ten suspicious lesions were detected by conventional examination, and no occult lesions were identified by the VELscope. Moreover, 72% (80 out of 111) of lesions or conditions clinically characterized as inflammation, ulceration or pigmentation demonstrated a loss of fluorescence on VELscope examination, potentially making it difficult to distinguish them from neoplastic lesions, which also exhibit decreased autofluorescence (Figure 2). The author concluded that the VELscope may be a useful adjunct for experienced practitioners, especially to monitor recognized lesions, but questioned its value as a general screening adjunct.
Figure 2. Limitations of wide-field autofluorescence imaging.
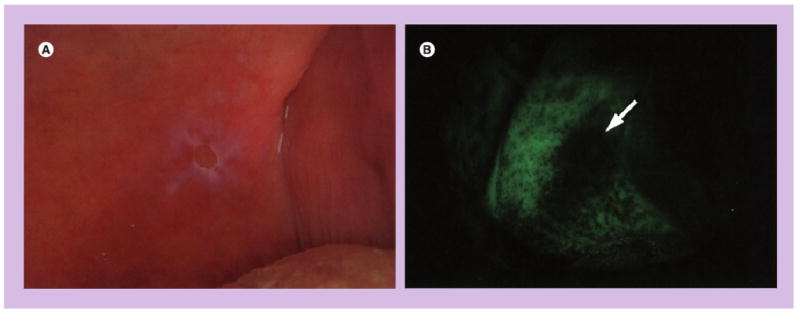
(A) A white light image of the soft palate of a patient with ulceration and (B) corresponding autofluorescence image obtained with VELscope® (LED Dental Inc., BC, Canada). The arrow indicates a region of fluorescence visualization loss.
Reprinted with permission from [32].
In a recently published study, Mehrotra et al. examined the diagnostic accuracy of the ViziLite Plus and VELscope in detecting oral dysplasia and carcinomas in 256 oral mucosal lesions deemed to be clinically innocuous based on conventional white light oral examination by an expert clinician [46]. The authors determined the specificity and sensitivity of the ViziLite Plus and VELscope in detecting oral dysplasia and carcinomas by comparing their respective finding with the gold standard scalpel biopsy results. They reported a disappointing 0% sensitivity and 75.5% specificity for the ViziLite Plus and 50% sensitivity and 38.9% specificity for the VELscope. The use of toluidine blue has been assessed as an adjunct to determine resection margins for oral cancer. In a case study of a patient with oral squamous cell carcinoma conducted by Missmann et al., small suspect lesions away from the main tumor were detected upon toluidine blue staining, and the resection area was enlarged to include these areas [47]. Resulting margins were clear, and the patient was disease-free for more than 3 years with follow-up. Kerawala et al. examined 14 oral squamous cell carcinomas from 11 patients to preoperatively determine resection margins with toluidine blue [48]. Invasive carcinoma stained with toluidine blue, which enabled complete removal in all cases. However, dysplasia or carcinoma in situ, which did not stain with toluidine blue, was identified in the margin of eight of the 14 samples (57%). The authors suggest that toluidine blue staining may be of value in identifying invasive cancers at margins, but may be of little value in delineating positive margins of dysplasia or carcinoma in situ and may not reduce incidence of local recurrences.
Direct visualization of loss of fluorescence also has potential to identify high-risk fields of precancer and cancer in order to better delineate surgical margins of oral cancer. Poh et al. showed that VELscope imaging could identify oral neoplasia in the operating room setting with a sensitivity of 97% and specificity of 94% in a study of 122 oral mucosa biopsies from 20 patients [49]. Poh et al. recently examined the rate of recurrence for oral cancer patients whose lesions were resected with standard visual guidance versus autofluorescence image guidance [50]. With a minimum 12-month follow-up, 32% of the 22 control-group patients experienced recurrence, while none of the 38 fluorescence-guided patients experienced recurrence.
The VELscope relies on the ability of the examiner to recognize loss of fluorescence, and performance depends on subjective visual recognition skills. As an alternative, digital images of tissue fluorescence can be captured and analyzed using quantitative image interpretation methods. To test this approach, Roblyer et al. developed a multispectral digital microscope, capable of collecting narrow-band reflectance and fluorescence images at a variety of illumination and emission conditions [26]. Roblyer et al. used the multispectral digital microscope to select optimal wavelengths to distinguish neoplastic from non-neoplastic oral mucosa [51]. Results showed that the normalized red-to-green fluorescence intensity ratio at 405 nm excitation provided the best discrimination between neoplastic and non-neoplastic areas. A quantitative algorithm, based on the red-to-green fluorescence intensity ratio from regions of interest, could discriminate normal tissue from dysplasia and cancer in a high-prevalence population, with a sensitivity of 95.9% and specificity of 96.2% in a training set of 46 subjects, and with a sensitivity of 100% and specificity of 91.4% in a validation set of 21 subjects.
This same approach was used to analyze the signal from each pixel in a fluorescence image to create a disease-probability map, indicating the likelihood that tissue underlying each pixel in the image contained neoplasia. Figure 3 shows an example of such a disease-probability map, superimposed atop a white light image. As shown in Figure 3, the results of the disease-probability map agree with histology. This type of image analysis approach may provide a more objective way to detect and delineate oral neoplasia in primary healthcare settings.
Figure 3. Automated analysis of autofluorescence images.
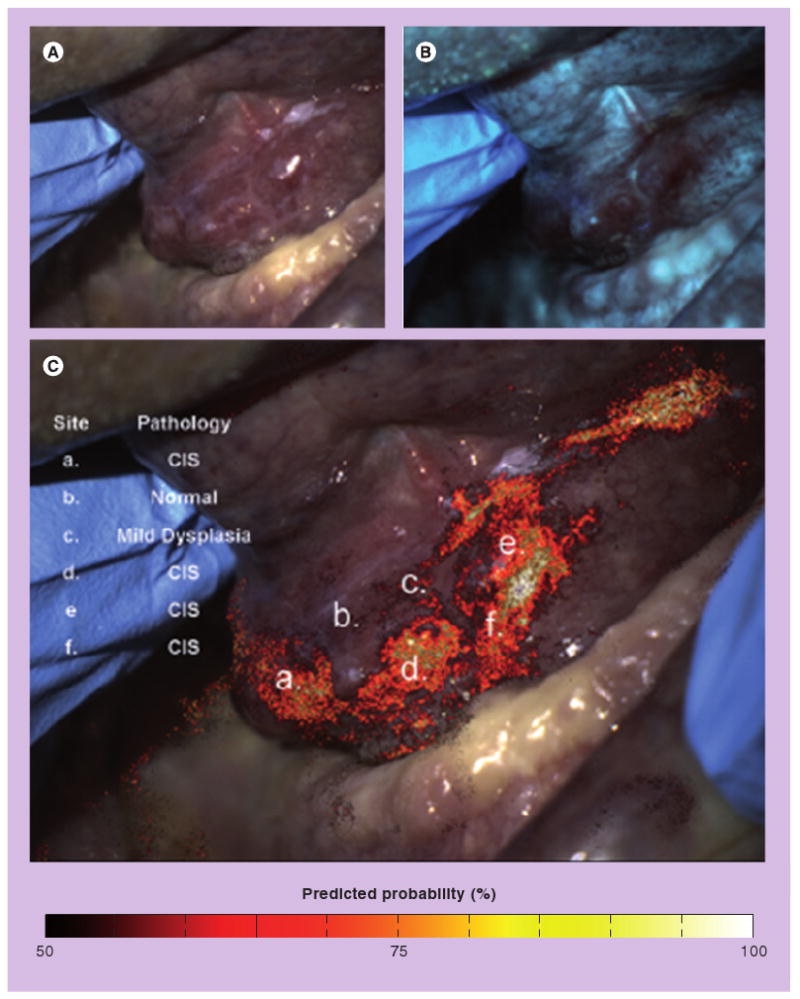
(A) A white light image of the floor of the mouth with a histopathologically confirmed dysplasia and carcinoma in situ. (B) 405 nm excitation fluorescence image showing areas with decreased autofluorescence. (C) White light image with disease-probability map showing the predictive probability of a neoplastic lesion superimposed. The disease-probability map indicates the probability that, based on the ratio of red:green fluorescence intensity, the underlying tissue is neoplastic. Letters indicate specific locations where pathology is known, and results of digital image analysis show good agreement with histology. Roblyer et al. provided a detailed explanation of how the map was developed and evaluated in a high-prevalence setting [51]. The high sensitivity and specificity obtained suggest that such disease-probability maps can be used to help objectively delineate the presence and extent of lesions, highlighting suspicious areas that may require further examination. However, further studies in low-prevalence populations are required to assess performance with potentially confounding lesions, such as benign inflammation.
Reprinted with permission from [51].
Optical properties of oral neoplastic tissue
Recent studies have characterized the biochemical and micro-anatomic origins of tissue fluorescence, as well as how tissue autofluorescence changes with neoplastic progression. The autofluorescence properties of oral tissues vary based on anatomic site and pathologic diagnosis [52–54]. In normal squamous oral mucosa, autofluorescence in the UV and visible region of the spectrum is predominantly associated with collagen in the stroma. The epithelium shows weak autofluorescence, primarily associated with mitochondrial NADH and FAD in basal epithelial cells based on studies with MitoTracker® (Molecular Probes, Inc., OR, USA) and mitochondrial poisons. In addition, superficial keratin contributes to epithelial fluorescence. Neoplasia is associated with a strong loss of stromal autofluorescence, which is likely to be responsible for the loss of autofluorescence observed in wide-field images. In addition, epithelial dysplasia is associated with increased mitochondrial fluorescence throughout the epithelium. Inflammatory lesions are associated with a loss of both epithelial and stromal autofluorescence (Figure 4). Thus, wide-field fluorescence imaging, a technique that captures autofluorescence generated primarily in the stroma, may give rise to a loss of fluorescence in both benign and precancerous lesions. One approach to improve diagnostic accuracy is to obtain images from more superficial layers using high-resolution imaging systems.
Figure 4. Confocal fluorescence images at 488 nm excitation of fresh organ cultures.
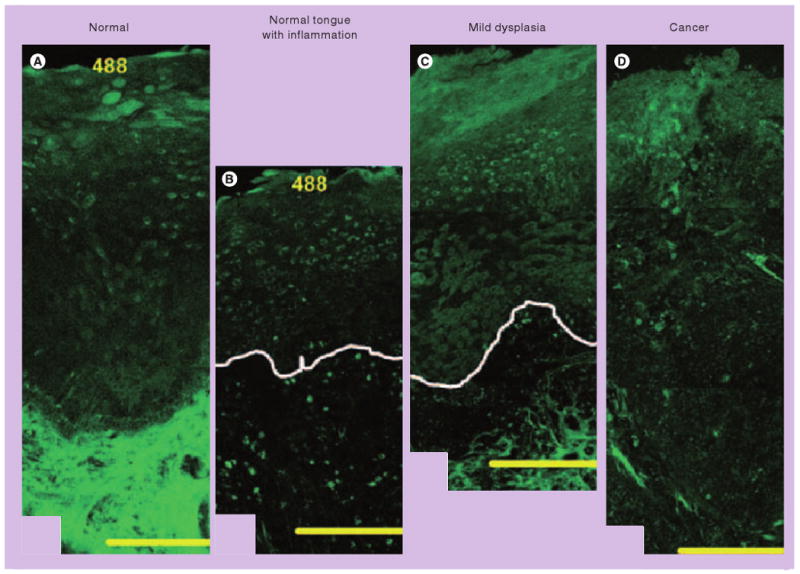
(A) The origins of epithelial and stromal fluorescence in the normal tongue. Adjacent images of lesions in the tongue illustrate the changes in epithelial and stromal fluorescence associated with severe inflammation (B), and mild dysplasia and mild-to-moderate inflammation (C). White lines indicate the approximate location of the basement membrane. (D) A fluorescence image of poorly differentiated carcinoma in the palate. Scale bars: 200 μm. Inflammatory lesions show decreased epithelial fluorescence, whereas dysplastic lesions display increased epithelial fluorescence compared with normal oral tissue. Stromal fluorescence in both inflammatory and dysplastic lesions drops significantly. Thus, wide-field fluorescence imaging, a technique that captures autofluorescence generated primarily in the stroma, may fail to distinguish inflammation from precancerous lesions. One approach to improve diagnostic accuracy is to obtain images from more superficial layers using high-resolution imaging systems.
Reprinted with permission from [52].
High-resolution optical techniques for oral cancer detection
An advantage of optical imaging of oral tissue is the ability to record images with subcellular resolution in vivo without performing biopsies. Several high-resolution imaging approaches have advanced to preclinical and clinical trials. Confocal reflectance microscopy is an optical technology that can provide detailed images of tissue architecture and cellular morphology throughout the epithelium of living tissue in near real time. Contrast is based on differences in refractive index, which can be enhanced using simple contrast agents such as acetic acid [55,56]. A miniaturized fiber optic confocal reflectance microscope has been developed to image oral neoplasia in vivo [57]. Distinct features indicative of oral precancer, such as nuclear enlargement, crowding and pleomorphism can be imaged and correlated well with histologic features observed in subsequent biopsies, as shown in Figure 5A.
Figure 5. High-resolution imaging.
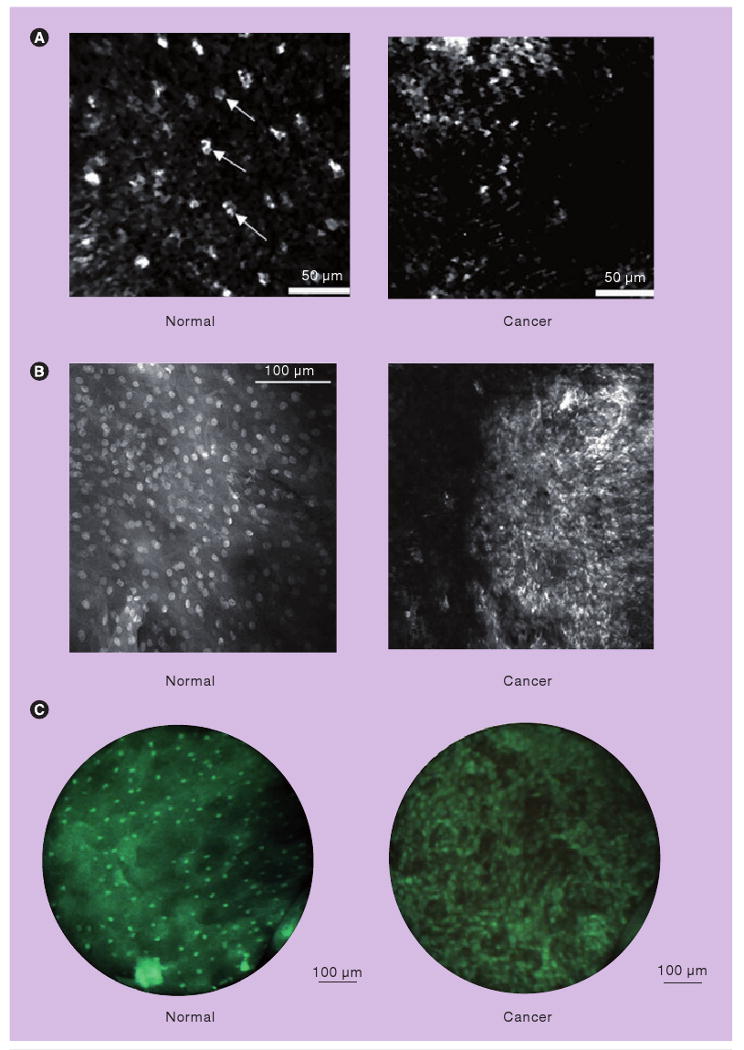
(A) High-resolution images obtained in vivo with a fiber optic confocal reflectance microscope. Small, regularly spaced epithelial cell nuclei are clearly visible in the intermediate squamous epithelium of the normal site (left). A confocal image of oral squamous cell carcinoma is characterized by disordered tissue structure (right). Scale bars: 50 μm. (B) High-resolution fluorescence images after topical application of acriflavine hydrochloride in ex vivo specimens. Normal mucosa with regular configuration of cell nuclei (left) and in an invasive carcinoma of the floor of the mouth showing different sizes of nuclei (right) (imaging plane depth: ∼50 μm). Scale bar: 100 μm. (C) High-resolution fluorescence images of oral tissue after topical application of proflavine obtained with a fiber optic fluorescence microendoscope, demonstrating qualitative differences between normal and cancerous tissue. Scale bars: 100 μm. The high-resolution microendoscope used to obtain images in (C) is portable, battery-powered and has been used in vivo in a variety of clinical settings.
(A) Reprinted with permission from [57] © Elsevier (2008). (B) Reprinted with kind permission from [59] © Springer Science+Business Media (2009). (C) is reprinted with permission from [60] © Optical Society of America (2007).
Alternatively, fluorescent dyes can be applied to enhance image contrast and imaged using confocal fluorescence microscopy. Thong et al. investigated the capability of a confocal fluorescence microscope system to identify morphologic details in living tongue tissue [58]. Morphologic differences between normal and neoplastic lesions in tongue were distinguished using fluorescein and 5-aminolevulinic acid -induced protoporphyrin IX fluorescence. Recently, a pilot study of a confocal fluorescence microendoscope was performed to investigate the feasibility of high-resolution optical imaging for detection of oral neoplasia [59]. Confocal images obtained with exogenous contrast agents (topical acriflavine and intravenous fluorescein) showed architectural details of tissues, such as changes in nuclear size and spacing, and changes in capillary networks, providing the potential to distinguish carcinoma from normal mucosa as shown in Figure 5B. A fiber optic high-resolution fluorescence microendoscope was developed to visualize subcellular detail in living tissue [60]. The portable system uses light-emitting diode illumination to excite and collect fluorescence through a fiber bundle with no requirement for complex scanning mechanisms. Following topical application of proflavine, a surgically resected tissue specimen from the human oral cavity was imaged across the clinical margin, demonstrating qualitative and quantitative differences between normal and cancerous tissue based on subcellular image features such as nuclear-to-cytoplasmic (N/C) ratio, as shown in Figure 5C.
Carlson et al. developed a dual-mode reflectance and fluorescence confocal microscope (DCM) to image molecular properties of tissue as well as tissue architecture and cellular morphology using reflective and fluorescent molecular-specific contrast agents [61]. Images obtained with the combination of reflectance and fluorescence provide information about both the morphologic and molecular changes associated with cancer progression. A total of 33 biopsies of normal and abnormal oral mucosa obtained from 14 patients were imaged with DCM [62]. Information such as mean fluorescence labeling intensity from fluorescence confocal images may add complementary information to that such as N/C ratio from reflectance confocal images, improving the ability to distinguish oral neoplastic lesions from normal tissue.
Future perspective
Combination of wide-field & high-resolution systems
Wide-field imaging enables rapid inspection of large mucosal surfaces, to aid in the recognition of suspicious lesions. Neoplasia is associated with a loss of autofluorescence, and this approach can reveal lesions that are difficult to detect with standard white light examination. However, the presence of inflammation is also associated with loss of stromal autofluorescence, and may give rise to false-positive results with wide-field fluorescence imaging. By contrast, high-resolution imaging can probe epithelial changes with subcellular detail, comparable to that of conventional histopathology. Once suspicious regions are identified from wide-field images, these areas could be imaged with subcellular resolution using a high-resolution system potentially improving specificity. Wide-field and high-resolution images can both be analyzed objectively using image-processing algorithms to indicate disease probability, as demonstrated in previous studies [51]. The results of these algorithms can be used to flag suspicious regions on wide-field images in real time, designating target areas for high-resolution imaging. Similarly, high-resolution images can be analyzed to calculate morphologic features, such as average N/C ratio and nuclear separation. The combination of wide-field and high-resolution imaging may enhance the performance of optical imaging for real-time, objective detection of neoplastic changes. While multimodal optical imaging has shown interesting preliminary results, it has not yet been evaluated in a low-risk population. Moreover, the expertise requirement and cost–effectiveness of the technique needs to be evaluated before suggesting this for population-based low-risk screening. Finally, there is a critical need to engineer imaging systems that are appropriate for use not just in specialized clinics but for first-line practice settings, such as dental offices.
Executive summary.
Oral cancer detection
Early detection and diagnosis of oral neoplastic changes is the best way to improve patient outcomes.
Conventional oral examination is based on visual inspection under normal white light and palpation of suspicious lesions, usually performed by dentists or physicians.
Diagnostic aids & adjunctive techniques
A variety of diagnostic aids and adjunctive techniques are commercially available, such as toluidine blue and the ViziLite®.
Data indicate that alternative diagnostic techniques can improve diagnostic performance in high-risk populations, but there is little evidence to support their effectiveness in low-risk populations.
Wide-field autofluorescence imaging
The VELscope® is a commercially available device to visualize loss of tissue autofluorescence associated with precancer and cancer in the oral cavity.
Digital image processing of wide-field autofluorescence images can be used to outline suspicious regions in real time.
The autofluorescence observed in wide-field images of the normal oral mucosa originates primarily from stromal collagen. Oral neoplasia is associated with a loss of stromal autofluorescence.
Benign lesions, such as inflammation, are also associated with loss of stromal autofluorescence, which may limit diagnostic specificity especially in low-risk populations.
High-resolution imaging
High-resolution imaging of oral tissue can visualize morphologic and architectural features of the epithelium in vivo with subcellular resolution, including the characteristic changes in nuclear size, shape and density associated with oral precancer.
High-resolution imaging may provide a tool to discriminate benign changes, such as inflammation, from neoplasia with better specificity than wide-field imaging.
Combination of wide-field & high-resolution imaging
Multimodal optical imaging – a combination of wide-field autofluorescence and high-resolution imaging – may yield the best sensitivity and specificity for detection of oral neoplasia.
Particular emphasis should be given to evaluating multimodal optical imaging in a low-risk population.
Acknowledgments
Rebecca Richards-Kortum serves as an unpaid scientific advisor to Remicalm LLC, holds patents related to optical diagnostic technologies that have been licensed to Remicalm LLC and holds minority ownership in Remicalm LLC. Ann Gillenwater has served as a paid consultant to Sanofi-Adventis, US LLC, has a minority equity interest in Onconome, Inc. and serves as an unpaid scientific advisor to Remicalm LLC. This work was funded through NIH grants R01 CA103830, RO1 EB007594 and R01 CA124319.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Garcia M, Jemal A, Ward EM, et al. Global Cancer Facts & Figures 2007. American Cancer Society; GA, USA: 2007. [Google Scholar]
- 4.Peterson PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st Century – the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl. 1):3–24. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Scott SE, Grunfeld EA, McGurk M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005;41(4):396–403. doi: 10.1016/j.oraloncology.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Evans SJ, Langdon JD, Rapidis AD, Johnson NW. Prognostic significance of STNMP and velocity of tumor growth in oral cancer. Cancer. 1982;49(4):773–776. doi: 10.1002/1097-0142(19820215)49:4<773::aid-cncr2820490428>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Mashberg A, Samit AM. Early detection, diagnosis, and management of oral and oropharyngeal cancer. CA Cancer J Clin. 1989;39(2):67–88. doi: 10.3322/canjclin.39.2.67. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH. Squamous cancer of the tongue. CA Cancer J Clin. 1985;35(4):252–256. doi: 10.3322/canjclin.35.4.252. [DOI] [PubMed] [Google Scholar]
- 10.Dolan RW, Vaughan CW, Fuleihan N. Symptoms in early head and neck cancer: an inadequate indicator. Otolaryngol Head Neck Surg. 1998;119(5):463–467. doi: 10.1016/S0194-5998(98)70102-0. [DOI] [PubMed] [Google Scholar]
- 11.Vernham GA, Crowther JA. Head and neck carcinoma – stage at presentation. Clin Otolaryngol Allied Sci. 1994;19(2):120–124. doi: 10.1111/j.1365-2273.1994.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 12.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70(1):14–19. doi: 10.1002/1097-0142(19920701)70:1<14::aid-cncr2820700103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JB, Sciubba JJ, Banasek TE, Hay LJ. Failure to diagnose and delayed diagnosis of cancer: medicolegal issues. J Am Dent Assoc. 2009;140(12):1494–1503. doi: 10.14219/jada.archive.2009.0100. [DOI] [PubMed] [Google Scholar]
- 14.Peacock ZS, Pogrel MA, Schmidt BL. Exploring the reasons for delay in treatment of oral cancer. J Am Dent Assoc. 2008;139(10):1346–1352. doi: 10.14219/jada.archive.2008.0046. [DOI] [PubMed] [Google Scholar]
- 15.Yu T, Wood RE, Tenenbaum HC. Delays in diagnosis of head and neck cancers. J Can Dent Assoc. 2008;74(1):61. [PubMed] [Google Scholar]
- 16.Allison P, Locker D, Feine JS. The role of diagnostic delays in the prognosis of oral cancer: a review of the literature. Oral Oncol. 1998;34(3):161–170. doi: 10.1016/s1368-8375(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 17.Fortin A, Bairati I, Albert M, Moore L, Allard J, Couture C. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929–936. doi: 10.1016/s0360-3016(01)02606-2. [DOI] [PubMed] [Google Scholar]
- 18.Downer MC, Moles DR, Palmer S, Speight PM. A systemic review of test performance in screening for oral cancer and precancer. Oral Oncol. 2004;40:264–273. doi: 10.1016/j.oraloncology.2003.08.013. [DOI] [PubMed] [Google Scholar]; ■ Reviews eight prospective investigations of the performance of visual examination for populations screening for oral cancer and precancer.
- 19.Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis. J Am Dent Assoc. 2008;139:896–905. doi: 10.14219/jada.archive.2008.0276. [DOI] [PubMed] [Google Scholar]
- 20.Epstein JB, Gorsky M, Lonky S, Silverman S, Jr, Epstein JD, Bride M. The efficacy of oral lumenoscopy™ (ViziLite®) in visualizing oral mucosal lesions. Spec Care Dentist. 2006;26(4):171–174. doi: 10.1111/j.1754-4505.2006.tb01720.x. [DOI] [PubMed] [Google Scholar]; ■ Multicenter study describing the use of visual examination with chemiluminescent illumination as an adjunct to conventional oral examination. Chemiluminescent examination did not improve visualization of red lesions, but improved the brightness and sharpness of the margins of white lesions and red and white lesions.
- 21.Kerr AR, Sirois DA, Epstein JB. Clinical evaluation of chemiluminescent lighting: an adjunct for oral mucosal examinations. J Clin Dent. 2006;17(3):59–63. [PubMed] [Google Scholar]
- 22.Ram S, Siar CH. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int J Oral Maxillofac Surg. 2005;34(5):521–527. doi: 10.1016/j.ijom.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Farah CS, McCullough MJ. A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite™) in the visualisation of oral mucosal white lesions. Oral Oncol. 2007;43:820–824. doi: 10.1016/j.oraloncology.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Oh ES, Laskin DM. Efficacy of the ViziLite system in the identification of oral lesions. J Oral Maxillofac Surg. 2007;65(3):424–426. doi: 10.1016/j.joms.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 25.De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–131. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Roblyer D, Richards-Kortum R, Sokolov K, et al. Multispectral optical imaging device for in vivo detection of oral neoplasia. J Biomed Opt. 2008;13(2):024019. doi: 10.1117/1.2904658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11(2):024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]; ■■ Pilot study describing the performance of an initial version of the VELscope® to discriminate normal oral mucosa from neoplastic lesions based on loss of autofluorescence.
- 28.Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29(1):71–76. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 29.Kois JC, Truelove E. Detecting oral cancer: A new technique and case reports. Dent Today. 2006;25(10):94, 96–97. [PubMed] [Google Scholar]
- 30.Poh CF, Williams PM, Zhang L, Rosin MP. Heads up! – A call for dentists to screen for oral cancer. J Can Dent Assoc. 2006;72(5):413–416. [PubMed] [Google Scholar]
- 31.Westra WH, Sidransky D. Fluorescence visualization in oral neoplasia: shedding light on an old problem. Clin Cancer Res. 2006;12(22):6594–6597. doi: 10.1158/1078-0432.CCR-06-2253. [DOI] [PubMed] [Google Scholar]
- 32.Laronde DM, Poh CF, Williams PM, et al. A magic wand for the community dental office? Observations from the British Columbia Oral Cancer Prevention Program. J Can Dent Assoc. 2007;73(7):607–609. [PubMed] [Google Scholar]
- 33.Rosin MP, Poh CF, Guillard M, Williams PM, Zhang L, MacAulay C. Visualization and other emerging technologies as change makers for oral cancer prevention. Ann NY Acad Sci. 2007;1098:167–183. doi: 10.1196/annals.1384.039. [DOI] [PubMed] [Google Scholar]
- 34.Williams PM, Poh CF, Hovan AJ, Ng S, Rosin MP. Evaluation of a suspicious oral mucosal lesion. J Can Dent Assoc. 2008;74(3):275–280. [PubMed] [Google Scholar]
- 35.Rosin MP, Poh CF, Elwood JM, et al. New hope for an oral cancer solution: together we can make a difference. J Can Dent Assoc. 2008;74(3):261–266. [PMC free article] [PubMed] [Google Scholar]
- 36.Paulis M. The influence of patient education by the dental hygienist: acceptance of the fluorescence oral cancer exam. J Dent Hygiene. 2009;83(3):134–140. [PubMed] [Google Scholar]
- 37.Kalmar JR. Advances in the detection and diagnosis of oral precancerous and cancerous lesions. Oral Maxillofac Surg Clin N Am. 2006;18:465–482. doi: 10.1016/j.coms.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Kelloff GJ, Sullivan DC, Baker H, et al. Workshop on imaging science development for cancer prevention and preemption. Cancer Biomark. 2007;3:1–33. doi: 10.3233/cbm-2007-3101. [DOI] [PubMed] [Google Scholar]
- 39.Lingen MW. Oral cancer screening aids: where is the science? Oral Surg Oral Med Oral Path Oral Rad Oral Endo. 2007;103(2):153–154. doi: 10.1016/j.tripleo.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2007;44(1):10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Review of the literature describing the performance of oral cancer screening adjuncts including toluidine blue, brush cytology, tissue reflectance and autofluorescence, concluding that well-designed clinical studies are needed to assess the utility of these diagnostic aids to detect oral lesions unseen by conventional examination.
- 41.Trullenque-Eriksson A, Muñoz-Corcuera M, Campo-Trapero J, Cano-Sánchez J, Bascones-Martínez A. Analysis of new diagnostic methods in suspicious lesions of the oral mucosa. Med Oral Patol Oral Cir Bucal. 2009;14(5):E210–E216. [PubMed] [Google Scholar]
- 42.Rhodus NL. Oral cancer and precancer: improving outcomes. Compend Contin Educ Dent. 2009;30(8):486–488. 490–494, 496–498. [PubMed] [Google Scholar]
- 43.Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 2009;1(1):5. doi: 10.1186/1758-3284-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balevi B. Evidence-based decision-making: should the general dentist adopt the use of the VELscope for routine screening for oral cancer? J Can Dent Assoc. 2007;73(7):603–606. [PubMed] [Google Scholar]
- 45.Huber MA. Assessment of the VELscope as an adjunctive examination tool. Tex Dent J. 2009;126(6):528–535. [PubMed] [Google Scholar]
- 46.Mehrotra R, Singh M, Thomas S, et al. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. J Am Dent Assoc. 2010;141(2):151–156. doi: 10.14219/jada.archive.2010.0132. [DOI] [PubMed] [Google Scholar]
- 47.Missmann M, Jank S, Laimer K, Gassner R. A reason for the use of toluidine blue staining in the presurgical management of patients with oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:741–743. doi: 10.1016/j.tripleo.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Kerawala CJ, Beale V, Reed M, Martin IC. The role of vital tissue staining in the marginal control of oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2000;29:32–35. [PubMed] [Google Scholar]
- 49.Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12(22):6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]; ■ Exploratory study using the VELscope to assess whether alterations in autofluorescence around oral cancers correspond to subclinical field changes. Loss of fluorescence was found to extend beyond the clinical margin and was associated with dysplasia and loss of heterozygosity at 3p and/or 9p.
- 50.Poh CF, MacAulay CE, Zhang L, Rosin MP. Tracing the “at-risk” oral mucosa field with autofluorescence: steps toward clinical impact. Cancer Prev Res. 2009;2(5):401–404. doi: 10.1158/1940-6207.CAPR-09-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Examines the rate of recurrence for oral cancer patients whose lesions were resected with standard visual guidance versus autofluorescence image guidance. With a minimum 12-month follow-up, 32% of the 22 control-group patients experienced recurrence, while none of the 38 fluorescence-guided patients experienced recurrence.
- 51.Roblyer D, Kurachi C, Stepanek V, et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev Res. 2009;2(5):423–431. doi: 10.1158/1940-6207.CAPR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Describes the use of digital fluorescence imaging to identify the presence and boundaries of oral neoplastic lesions in a training and validation data set; digital fluorescence images are analyzed to produce disease-probability maps indicating the likelihood of neoplasia.
- 52.Pavlova I, Williams M, El-Naggar A, Richards-Kortum R, Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Clin Cancer Res. 2008;14(8):2396–2404. doi: 10.1158/1078-0432.CCR-07-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Confocal fluorescence microscopy of fresh organ cultures of oral mucosa is used to document the biological basis for differences in autofluorescence of normal, inflammatory and neoplastic oral tissue.
- 53.Pavlova I, Weber CR, Schwarz RA, et al. Monte Carlo model to describe depth selective fluorescence spectra of epithelial tissue: applications for diagnosis of oral precancer. J Biomed Opt. 2008;13(6):064012. doi: 10.1117/1.3006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavlova I, Weber CR, Schwarz RA, Williams MD, Gillenwater AM, Richards-Kortum R. Fluorescence spectroscopy of oral tissue: Monte Carlo modeling with site-specific tissue properties. J Biomed Opt. 2009;14(1):014009. doi: 10.1117/1.3065544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dwyer PJ, DiMarzio CA, Rajadhyaksha M. Confocal θ line-scanning microscope for imaging human tissues. Appl Optics. 2007;46(10):1843–1851. doi: 10.1364/ao.46.001843. [DOI] [PubMed] [Google Scholar]
- 56.Shin HJ, Pierce MC, Lee D, Ra H, Solgaard O, Richards-Kortum R. Fiber-optic confocal microscope using a MEMS scanner and miniature objective lens. Opt Express. 2007;15(15):9113–9122. doi: 10.1364/oe.15.009113. [DOI] [PubMed] [Google Scholar]
- 57.Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44(11):1059–1066. doi: 10.1016/j.oraloncology.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thong PS, Olivo M, Kho KW, et al. Laser confocal endomicroscopy as a novel technique for fluorescence diagnostic imaging of the oral cavity. J Biomed Opt. 2007;12(1):014007. doi: 10.1117/1.2710193. [DOI] [PubMed] [Google Scholar]
- 59.Haxel BR, Goetz M, Kiesslich R, Gosepath J. Confocal endomicroscopy: a novel application for imaging of oral and oropharyngeal mucosa in human. Eur Arch Otorhinolaryngol. 2010;267(3):443–448. doi: 10.1007/s00405-009-1035-3. [DOI] [PubMed] [Google Scholar]
- 60.Muldoon TJ, Pierce MC, Nida DL, Williams MD, Gillenwater A, Richards-Kortum R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15(25):16413–16423. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Describes a novel high-resolution fluorescence imaging system to image changes in nuclear size and density in oral mucosa associated with neoplasia.
- 61.Carlson AL, Coghlan LG, Gillenwater AM, Richards-Kortum RR. Dual-mode reflectance and fluorescence near-video-rate confocal microscope for architectural, morphological and molecular imaging of tissue. J Microsc. 2007;228(1):11–24. doi: 10.1111/j.1365-2818.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 62.Carlson AL, Gillenwater AM, Williams MD, El-Naggar AK, Richards-Kortum RR. Confocal microscopy and molecular-specific optical contrast agents for the detection of oral neoplasia. Tech Canc Res Treat. 2007;6(5):361–374. doi: 10.1177/153303460700600501. [DOI] [PMC free article] [PubMed] [Google Scholar]


