Abstract
The halobacterial phototaxis receptors sensory rhodopsin I and II (SRI, SRII) enable the bacteria to seek optimal light conditions for ion pumping by bacteriorhodopsin and/or halorhodopsin. The incoming signal is transferred across the plasma membrane by means of receptor-specific transducer proteins that bind tightly to their corresponding photoreceptors. To investigate the receptor/transducer interaction, advantage is taken of the observation that both SRI and SRII can function as proton pumps. SRI from Halobacterium salinarum, which triggers the positive phototaxis, the photophobic receptor SRII from Natronobacterium pharaonis (pSRII), as well as the mutant pSRII-F86D were expressed in Xenopus oocytes. Voltage-clamp studies confirm that SRI and pSRII function as light-driven, outwardly directed proton pumps with a much stronger voltage dependence than the ion pumps bacteriorhodopsin and halorhodopsin. Coexpression of SRI and pSRII-F86D with their corresponding transducers suppresses the proton transport, revealing a tight binding and specific interaction of the two proteins. These latter results may be exploited to further analyze the binding interaction of the photoreceptors with their downstream effectors.
The electrical properties of eukaryotic membrane proteins can easily be analyzed by employing the oocyte expression system from Xenopus laevis. In previous work, it has been demonstrated that bacteriorhodopsin (BR) from Halobacterium salinarum could also be functionally expressed into the plasma membrane of oocytes, which allowed the elucidation of the electrogenic characteristics of this proton pump under well-defined voltage–clamp conditions (1, 2). This work indicated that the other members of the bacterial rhodopsin family, the chloride pump halorhodopsin, and the sensory rhodopsins can also be analyzed by this approach. The sensory rhodopsins (SRI and SRII) are, as their names already point to, phototaxis receptors. Although SRII solely transmits the photophobic reaction of the bacteria, SRI displays a dual function. In a one-photon process, a photoattractant response is triggered; however, another blue photon absorbed by an intermediate of the cyclic photoreaction elicits a photophobic response. The excitation of both SRI and SRII leads to an activation of receptor-specific transducers HtrI and HtrII, respectively, which subsequently trigger the bacterial signal transduction chain (reviewed in refs. 3 and 4).
Because the sequence homology between the four retinylidene proteins is relatively high, it appeared likely that the sensory rhodopsins could also function as light-driven proton pumps. Indeed, the electrogenic properties of SRI have been extensively studied, and recently data have also been obtained for SRII. However, the picture emerging from these investigations is still controversial.
Olson & Spudich (5) and Bogomolni et al. (6) investigated pH changes by using envelope vesicles and reported that SRI is an outwardly directed proton pump that is driven by orange light in a one photon process. Haupts et al. (7, 8) performed similar experiments with intact cells and, additionally, analyzed photocurrents of SRI containing membranes attached to the black-lipid membrane. From their results, the authors concluded that the proton transfer is based on a two-photon process in which the S373-intermediate absorbs a “blue” photon to short-cut the photocycle. Moreover, an inversion of the pump direction was observed under certain light- and pH-conditions and attributed the altered vectoriality to the two spectral distinct species in the SRI ground state (8). The absorption spectrum of SRI consists of two superimposed bands with maxima at around 550 nm and 580 nm, representing the protonated and deprotonated Schiff base counterion Asp76, respectively (9). Like Bogomolni et al. (6), Haupts et al. (8) assign the outwardly directed pumping to SRI550, but, in addition, they postulate that SRI580 generates an inverted pump.
Also, in the case of SRII, different experimental results were obtained. Spudich and coworkers (10), by using pH measurements in suspensions of envelope vesicles containing sSRII (the pigment from H. salinarum), described experiments which showed that proton uptake and proton release occurs at the same (extracellular) side of the protein during the late steps of the photocycle. Clearly, these data yield a futile proton pump. Kamo and his group (11) analyzed proton movements to and from a surface of pSRII (the protein from N. pharaonis) with an SnO2 electrode. These experiments are in line with the results from Spudich and coworkers (10) concerning the time scale of proton uptake and release. However, the authors suggest that the vectoriality of the proton transfer is not to be disturbed (11). The latter conclusions were also drawn from experiments using black lipid membranes, in which it was demonstrated that pSRII functions as a proton pump (12).
Although the signal is small, pSRII generates a distinct stationary photocurrent at pH 5, demonstrating an outwardly directed proton transport across the membrane. The introduction of Asp86 in the mutant pSRII-F86D, which corresponds to the SB proton donor group Asp96 in BR, increases the pump efficiency significantly, and a stationary photocurrent is visible already at physiological proton concentrations. In either case—wild-type pSRII or pSRII-F86D—small amounts of azide catalyze the proton transport to the effect that the stationary photocurrents are enhanced to the same level as was observed for BR (13).
An interesting observation was made by Bogomolni et al. (6), who showed that the binding of the transducer sHtrI to sSRI inhibits the proton pump. Haupts et al. (8) contradicted this data because they still could detect a photocurrent of SRI in the complex with the transducer HtrI. For sSRII, Spudich and coworkers (10) could not observe an effect of the transducer on the proton transfer steps.
The oocyte system is an ideal tool to study the electrogenic properties of the two sensory rhodopsins and those of the receptor/transducer complexes because the incorporation into the plasma membrane occurs side specific. Under voltage–clamp conditions, the potential dependent transport properties can be analyzed, which provides insight into the potency of the proton pumps. The electrical properties of receptor-transducer complexes can provide valuable information about the specificity of protein/protein interaction. In the present work, SRI and pSRII as well as those of the corresponding receptor–transducer complexes are expressed into the plasma membrane of oocytes, and their electrogenic properties are investigated under defined voltage clamp conditions.
Materials and Methods
Plasmids and mRNA Synthesis.
As a template for the in vitro synthesis of the mRNA, the vector pNK4, a derivative of pNKS2-myc that lacks the myc-tag, was used (14). All genes except htrI were cloned into the pNK4 by NcoI and XhoI digestion of the plasmids pet27bmodpsopII-His, pet27bmodF86DpsopII-His (12), pet27bmodSRI-His (15), and pet27bmodpHtrII-His. htrI was amplified from the genomic DNA (H. salinarum strain L33) introducing a NdeI site at the start codon ATG and a XhoI site at the stop codon. The PCR product was ligated into the vector pNK4mod in which the NcoI site was exchanged against a NdeI site. The pNK4/pNK4mod vectors allow the synthesis of mRNA, which contains a 3′ poly(A) tail and a 5′ sequence encoding the ribosomal binding site of the β-subunit of the X. laevis Na/K ATPase.
For the mRNA synthesis (SP6-mMessagemMachine kit; Ambion, Austin, TX), the XbaI-linearized DNA was used.
Oocyte Microinjection and Voltage–Clamp Measurements.
Oocytes have been isolated and prepared according to ref. 16. For the expression of pSRII, PSRII-F86D and SRI oocytes were injected with 15 nl (15 ng) mRNA. For the coexpression of the SR/Htr complexes, an additional 50 nl (50 ng) mRNA pHtrII or HtrI was injected. After the injections, the oocytes were incubated for 3–5 days at 18°C in the presence of 1 μM all-trans retinal.
The voltage–clamp setup was essentially that of Nagel et al. (1, 2). If not mentioned otherwise, the membrane potential was clamped at −40 mV (50 Hz signal filtering) in the case of pSRII, pSRII-F86D, and pSRII(F86D)/pHtrII and at −20 mV (20 Hz signal filtering) for SRI and SRI/HtrI. The bath solution was 90 mM NaCl, 5 mM BaCl2, 20 mM TEA⋅Cl, 10 mM Mops, pH 7.6.
Results
Expression of pSRII and pHtrII.
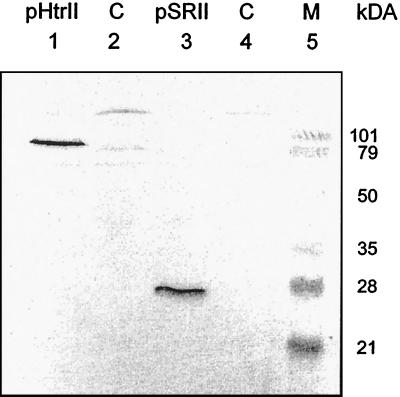
The expression of pSRII and pHtrII in oocytes was analyzed by Western blot analysis of oocyte plasma membranes. For this experiment, 50 oocytes each were injected with 30 ng of pSRII mRNA or pHtrII mRNA. A total of 2 × 50 oocytes were used as a control. The plasma membranes of the four samples were isolated by subsequent sucrose and nycodenz density gradient centrifugation, applied to an SDS/PAGE, and subsequently treated with an antibody against the C-terminal histidine tag of the proteins. As can be seen in Fig. 1, the transducer (lane 1) and the photoreceptor (lane 3) are expressed at almost the same expression level. The apparent molecular masses of about 85,000 Da and 25,000 Da correspond quite well to those expected for pHtrII and pSRII, respectively. The experiment also indicates that pSRII and pHtrII are incorporated into the plasma membrane of the oocytes, although contaminations from the membrane of the endoplasmatic reticulum cannot be excluded.
Figure 1.
Western blot of oocyte plasma membranes. Membranes of 50 oocytes, independently injected with either pSRIImRNA or pHtrIImRNA, have been isolated by subsequent centrifugation over a sucrose and nycodenz density gradient. The blotted membranes have been incubated with an antibody (Qiagen, Hilden, Germany) against the C-terminal 6× histidine-tag of the proteins. The two prominent bands in lanes 1 and 3 at ≈85 kDa and ≈25 kDa can be assigned to pHtrII and pSRII, respectively. Lanes 2 and 4 show control membranes of not injected oocytes.
Photocurrent Measurements.
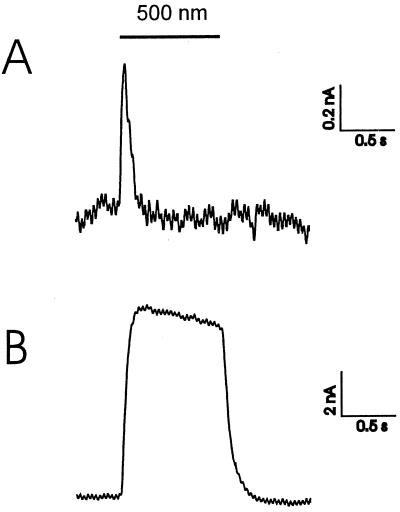
Green illumination (K50 broadband filter, Schott, Mainz, Germany; 480–520 nm) of oocytes containing pSRII in their plasma membrane resulted in a positive transient signal of 1–2 nA (Fig. 2A), which reflects an outwardly directed movement of positive charges. From the time course of the rise of the photocurrent, the charge (probably proton) translocations occur during the L to M transition. In the presence of azide, a photostationary current of about 10 nA is observed (Fig. 2B). However, contrary to Sasaki et al. (10) sSRII acts at pH 7.0 as an outwardly directed proton pump. In oocytes, a photostationary current of around 3 nA was measured (data not shown).
Figure 2.
Voltage–clamp signals of pSRII at −40 mV (50 Hz filtering). (A) The photocurrent of pSRII at pH 7.6 (90 mM NaCl, 5 mM BaCl, 20 mM TEA⋅Cl, 10 mM Mops), whereas the lower trace given in B was recorded with the same oocyte at pH 5.6 and the presence of 50 mM sodiumazide (40 mM NaCl, 5 mM BaCl, 20 mM TEA⋅Cl, 10 mM Mes).
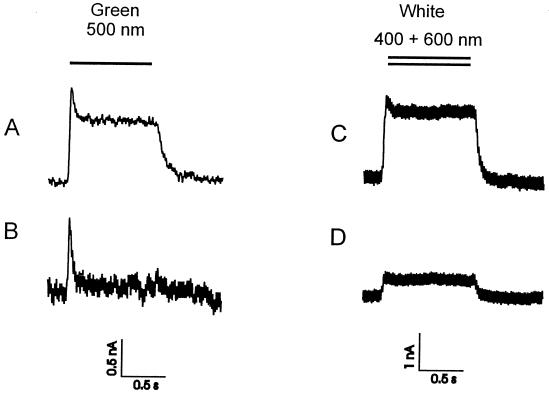
In contrast to the wild type, the mutant pSRII-F86D clearly displays a distinct stationary photocurrent of about 2 nA (Fig. 3A), which can be increased by the addition of azide of up to 10 nA (data not shown). Consistent with the insensitivity of the photocycle to different proton concentrations (17), the size of the photocurrent is nearly pH-independent (Fig. 4B). It should be mentioned that under voltage clamp conditions only the external pH (bath solution) is under experimental control. After switching off the light, the signal decreases with a half-life of 50 ms to the initial level. These results are consistent with data obtained from experiments using black lipid membranes (BLM; ref. 12).
Figure 3.
Photocurrent of pSRII-F86D (A and B) and SRI (C and D) in the absence (A and C) and presence of their corresponding transducers pHtrII and pHtrI (B and D). The voltage–clamp signals have been recorded 4 days after the injection of the SR's mRNA as well as the SR's/Htr's mRNA at a membrane potential of −20 mV (20 Hz filtering) for SRI and SRI/HtrI and −40 mV (50 Hz filtering) for pSRII-F86D and pSRII-F86D/pHtrII. The bath solution consisted of 90 mM NaCl, 5 mM BaCl, 20 mM TEA⋅Cl, 10 mM Mops, pH 7.6.
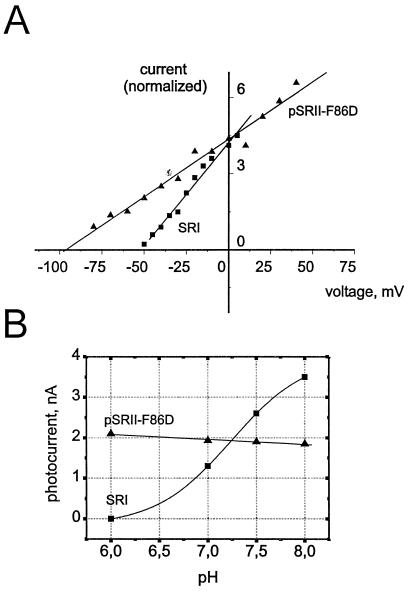
Figure 4.
Voltage- (A) and pH-dependence (B) of the SRI and pSRII-F86D photocurrents. Triangles represent data points of pSRII-F86D (normalized to SRI current at 0 mV), squares are indicative for SRI. Both pigments show a steep linear current/voltage-dependence and the signals do not invert at any observed potential (A). The pH-dependence (B) was recorded at a constant membrane potential of −20 mV (SRI) or −40 mV (pSRII-F86D). In the case of pSRII-F86D, only a slight linear increase of the photocurrent with increasing proton concentration is visible, whereas SRI shows a sigmoidal pH dependence. The deflection point corresponds to the pKa 7.2 (Boltzmann fit; Imin = −0.16 ± 0.2, Imax = 4.0 ± 0.3, pH (Imax/2) = 7.2 ± 0.1).
The photoactive pigment SRI exhibits a stationary outwardly directed photocurrent only in the presence of blue light (unfiltered white light or K40 broadband filter, Schott; 380–420 nm) (Fig. 3C). The size of the signal is 2–3 nA, which is in the same order of magnitude as that of pSRII-F86D; however, the off-response is slowed down to a half-life of approximately 100 ms. Orange light (λ > 500 nm cutoff) only induces a transient positive peak current (data not shown) similar to that of pSRII under green light, which demonstrates that both orange and blue light are required for efficient proton pumping. The results can be interpreted by the assumption of a two-photon process.
The amplitude of the SRI photocurrent is highly dependent on the external pH. The size of the signal drops systematically with decreasing pH (Fig. 4B). At pH 6.0, a photocurrent cannot be detected. A sigmoidal fit of the data yields an inflection point at pH 7.2 which is exactly the pKa of Asp76 (6, 9), indicating that SR550 rather than SR580 is responsible for the proton transport.
The Transducers Inhibit the Photocurrent.
The signals of pSRII/F86D and SRI are changed considerably when their corresponding transducers, pHtrII and HtrI, are coexpressed. As can be seen in Fig. 3 B and D, the stationary photocurrent substantially decreases with the binding of the transducer, although the transient photocurrent is only minimally affected. The inhibition of the photocurrent varies from oocyte to oocyte. There are examples in which the stationary current is undetectable, whereas, in other cases, a considerable current remains. Also, in wild-type pSRII, the transient peak is still visible in the presence of the transducer. From these observations, it can be concluded that, although the amount of the coinjected mRNA for receptors (15 ng) and transducers (50 ng) has not been varied, the yield of functional complexes differ from oocyte to oocyte. However, these results demonstrate clearly that the binding of the transducer to the receptors inhibits the capability of SRI and pSRII-F86D to pump protons.
The receptor/transducer interaction is quite specific, which is shown in a hybrid experiment. When SRI and pHtrII or pSRII-F86D and HtrI are coinjected, the stationary photocurrent has not been affected in any case, even if the mRNA of the transducer is injected at a 3-fold molar excess. These data strongly indicate that a crossreaction between the different photoreceptor systems does not occur.
Current/Voltage Behavior of the Sensory Rhodopsins.
Because cells can be polarized up to −200 mV, the investigation of the voltage dependence of the proton pumping is important to analyze the physiological relevance of this process. Indeed, the size of the currents is highly dependent on the membrane potential. From +40 mV to −100 mV, the amplitude of the pSR-II-F86D signal is reduced with hyperpolarization (Fig. 4A). Measurements beyond this range are hampered by endogenous currents across the oocyte plasma membrane, which increase the background noise. Similar results were also obtained for SRI. The data plotted in Fig. 4 show a linear current-voltage dependence as it has been described for BR (1, 2). Extrapolation to 0 nA provides maximal membrane potentials, which can be achieved by the photoreceptors. The potentials are with −100 mV (pSRII-F86D) and −50 mV (SRI) much lower than those observed for BR or pHR. In these cases, potentials of about −250 mV have been determined (1, 2, 7, 18). The value of −50 mV for SRI is quite close to the −80 mV published by Sasaki & Spudich (6, 10). It should be noted that at no potential a reversed (negative) signal was observed, which indicates a quantitative right side out orientation of the pigments in the oocyte membrane.
Discussion
The expression of bacterial retinal proteins and insertion into the plasma membrane of Xenopus oocytes opened the opportunity to study with electrophysiological methods the pumping mechanism of the sensory rhodopsins and the interaction of these proteins with their corresponding transducers. Indeed, as has been shown in this work, the two photoreceptors SRI and pSRII as well as the functional signaling complexes with their corresponding transducers HtrI and pHtrII can also be expressed into the plasma membrane of Xenopus oocytes (Fig. 1). Compared with earlier investigations of pH changes to elucidate sensory rhodopsin transport properties, the voltage–clamp method is much more sensitive and allows the time resolved analysis of electrogenic events.
Photoexcitation of these oocytes clearly reveals that the sensory rhodopsins display a transient photocurrent, whereas only for SRI and the pSRII mutant F86D a stationary photocurrent could be observed (Figs. 2 and 3).
The findings obtained for pSRII and pSRII-F86D confirm earlier work using the BLM technique. Schmies et al. (12) have shown that pSRII is capable of an outwardly directed—although weak—proton transport and that the pumping efficiency is considerably enhanced in the mutant pSRII-F86D or by the addition of azide, as has been suggested by Subramaniam et al. (19) for the photoreactivity of the L93A mutant of BR. The results were explained by assuming a two-photon process in the case of the mutant as well as in the presence of azide. For wild-type pSRII, it was rationalized that the reduced ability to pump protons originates from the slow photocycle turnover in conjunction with a single photon excitation. Consistent with this interpretation, Kamo and coworkers (11) reported from investigations of light-induced potential changes in a photoelectrical SnO2 cell that pSRII is electrogenic.
The capability of SRI to pump protons has been studied in greater detail (5–8). Olson et al. (5) and Bogomolni et al. (6) demonstrated that the proton transfer is driven by orange light in a single photon process. On the other hand, Haupts et al. (7) described the process as a two-photon driven reaction with blue background illumination. From the voltage–clamp data presented here, it is evident that the photocurrent is indeed induced by a two-photon process (Fig. 3). However, it should be noted, that the data presented here are not in disagreement to those described earlier (6, 10). A possible stationary photocurrent resulting from the slow cycling SRI (≈1 s), as observed in the latter references would be in the order of 0.5 nA, which is close to the detection limit of the method used. On the other hand, the pH measurements of SRI-containing vesicles integrates proton transfer over a long period (≈10 min).
This two-photon process is unlike the response of pSRII or BR toward additional blue light where an inhibition of the stationary photocurrent has been observed (20). In all three examples, the photocycle is triggered by the long wavelength laser to form an M-like intermediate with an absorption maximum at around 400 nm. To explain the differences between SRI on the one hand and BR or pSRII on the other hand, one has to assume that the second (blue) photon probes states of the proteins that have proceeded to different extent into the photo reaction cycle. In the case of SRI, the proton release and the switch that represents the change of the SB accessibility between the cytoplasmatic and the extracellular channels have already occurred. Thus, the second photon accelerates the photocycle as was described in ref. 21 and the efficiency of the proton pump is enhanced. On the other hand, for BR and pSRII this switch has not yet taken place at the moment blue light excites the chromophores. It follows that this short circuit of the photocycle reduces the concentration of actively pumping species and, therefore, the stationary photocurrent decreases. It is interesting to note that pSRII can adopt an SRI-like behavior by the addition of increasing amounts of azide. At a concentration above 50 mM azide (pH 4.8), the inhibitory effect of blue light turns into an amplification of the photocurrent (12). This shift in sign of the blue light effect by external parameters could also be a reason for the inhibitory influence of blue light on the proton pump of SRI observed by Bogomolni et al. (6).
It has been mentioned in the introduction that the absorption maximum of SRI can be shifted from 580 nm to 550 nm by increasing the pH. The pK of this transition is 7.2, and the responsible residue has been assigned to Asp76 (6, 9). The same pK has now been found for the pH dependency of the stationary photocurrent. The change of the amplitude of the signal corresponds exactly to the contribution of SR550 in the SR550/SR580 equilibrium, indicating that a deprotonated Asp76 is a necessary prerequisite for pumping activity, a conclusion which was also drawn by Bogomolni et al. (6). Consequently, SRI580 would correspond to the very inefficient proton pump D85NBR (22) or the inactive D75NpSRII (12). An inverted transient photocurrent, as was observed for these pigments, has not been observed for SRI. However, in the case of SRI, the transient current is not well resolved under voltage–clamp conditions because a low filter frequency of 20 Hz was applied to compensate the background noise. Because no M-intermediate is detected in the SRI photocycle at low pH 4.0 (15), as observed for D85NBR and D75NpSRII at any pH, this result substantiates the proposal of the two-photon mechanism to explain the pump activity of SRI observed in oocytes. It should be noted that, in the discussion of the pH dependence, the proton gradient between the interior of the oocyte and the bath solution must be taken into consideration. However, the almost pH-insensitive photocurrent of pSRII-F86D indicates that this effect can be neglected.
The analysis of proton transfer reactions under controlled voltage–clamp conditions allows to determine the current/voltage behavior of SRI and pSRII. Because a quantitative voltage dependency of the transient photocurrents is difficult to obtain, the experiments were performed by using SRI as well as the pSRII mutant F86D and measuring the stationary photocurrents. As can be seen in Fig. 4A, SRI shows a strong voltage dependence and closes to pump at about −50 mV, a value that quite well agrees with the published data of −80 mV (6). Similarly, for pSRII-F86D, −80 mV has been determined as a potential where pumping stops. Although the stationary photocurrent is negligible below −80 mV, a transient photocurrent can still be detected. This observation indicates that a reversal of the photocurrent does not take place. The data have to be compared with those from the ion pumps BR and HR, which are in the range of −250 mV (1, 2, 18). It is obvious that the photocurrent of the sensory rhodopsins is much more sensitive to changes of the membrane potential, which indicates that the conformational movements during the photocycle are considerably increased compared with the ion pumps. It is tempting to speculate whether these observations reflect the sensory function because the mechanism of signal transduction presumably involves a tilt of helix F to activate the transducers (3, 23).
The voltage–clamp signals of the coinjected oocytes demonstrate that the binding of the transducers to their receptors suppresses the pumping of the sensory rhodopsins. Because the expression level of the proteins cannot be directly correlated with the amount of injected mRNA, it is not possible to determine the stoichiometry of the complex by a titration. To achieve a complete inhibition of the photocurrent in respect to the receptor mRNA, a 2-fold molar amount of transducer mRNA was injected. The small stationary currents, which are still detected in some oocytes, can therefore be assigned to free SR molecules.
The inhibition of the photocurrents suggests similar interactions among the SR/Htr complexes. The recent model of the signal relay involves a light-induced tilt of the cytoplasmic region of the receptor helix F, which is detected by the second transmembrane helix (TM2) of the transducer (3, 23). Indeed, from mutant analysis in the SRI/HtrI complex, it is assumed that a physical contact of HtrI TM2 with SRI blocks the cytoplasmic part of the SRI proton pathway (24–26). Consistent with these models, our data point to interaction sites located in the cytoplasmic part of the receptors as well.
The strongest indication for this assumption is that the proton movements in the extracellular part of the proton channel are not influenced by the transducer as demonstrated by the presence of transient signal in pSRII/HtrII and F86D/pSRII. Sasaki et al. (10) also report that, in sSRII, the electrogenic events in the extracellular side are not altered if sSRII is complexed with sHtrII. It is important to note that the futile proton pump described by Sasaki et al. (10) could not be verified in the present work. As pointed out in Results, sSRII displays a small but significant stationary photocurrent, even at pH 7.0, proving an outward directed proton pump.
The interactions of SRI with HtrI and pSRII-F86D with pHtrII are very specific because the inhibition is not observed in the case that HtrI or pHtrII are coexpressed with pSRII-F86D or SRI, respectively. Similarly, Zhang et al. (26) observed that the receptors exclusively recognize their own transducers.
In the case of SRI/HtrI, an alternative explanation for the inhibition of the proton transport is also possible. The pH dependence of the SRI photocurrent indicates that SR550 is an active and SR580 an inactive pump. Because the pKa of the transition between these two forms is shifted in the SRI/HtrI complex to pKa = 8.5, the protonation of Asp76 could account for the inactivation of the pump. Thus, the SRI/HtrI complex would mimic the mutant D76NSRI, which is also not active (6, 8). This line of argumentation would imply different mechanisms to inhibit the proton transport among the two receptors because pSRII does not show any spectral transition on transducer binding.
Acknowledgments
We thank Sven Geibel for excellent technical support and valuable discussion. We thank Peter de Vries for technical help with the Western blot analysis and Johann Klare for the amplification of the sopI gene. Furthermore, we thank the Deutsche Forschungsgemeinschaft for financial support. G.S. gratefully acknowledges a fellowship of the Boehringer Ingelheim Fonds.
Abbreviations
- BR
bacteriorhodopsin
- SRI and SRII
sensory rhodopsin I and II
- Htr
receptor-specific transducer proteins
- BLM
black lipid membranes
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031562298.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031562298
References
- 1.Nagel G, Möckel B, Büldt G, Bamberg E. FEBS Lett. 1995;377:263–266. doi: 10.1016/0014-5793(95)01356-3. [DOI] [PubMed] [Google Scholar]
- 2.Nagel G, Kelety B, Möckel B, Büldt G, Bamberg E. Biophys J. 1998;74:403–412. doi: 10.1016/S0006-3495(98)77797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spudich J L. Mol Microbiol. 1998;28:1051–1058. doi: 10.1046/j.1365-2958.1998.00859.x. [DOI] [PubMed] [Google Scholar]
- 4.Marwan W, Oesterhelt D. Am Soc Microbiol News. 2000;66:83–89. [Google Scholar]
- 5.Olson K D, Spudich J L. Biophys J. 1993;65:2578–2585. doi: 10.1016/S0006-3495(93)81295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogomolni R A, Stoeckenius W, Szundi I, Perozo E, Olson K D, Spudich J L. Proc Natl Acad Sci USA. 1994;91:10188–10192. doi: 10.1073/pnas.91.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haupts U, Haupts C, Oesterhelt D. Proc Natl Acad Sci USA. 1995;92:3834–3838. doi: 10.1073/pnas.92.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haupts U, Bamberg E, Oesterhelt D. EMBO J. 1996;15:1834–1841. [PMC free article] [PubMed] [Google Scholar]
- 9.Rath P, Olson K D, Spudich J L, Rothschild K J. Biochemistry. 1994;33:5600–5606. doi: 10.1021/bi00184a032. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki J, Spudich J L. Biophys J. 1999;77:2145–2152. doi: 10.1016/S0006-3495(99)77055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto M, Shimono K, Sumi M, Koyama K, Kamo N. J Phys Chem. 1999;103:10311–10315. [Google Scholar]
- 12.Schmies G, Lüttenberg B, Chizhov I, Engelhard M, Becker A, Bamberg E. Biophys J. 2000;78:967–976. doi: 10.1016/S0006-3495(00)76654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamberg E, Apell H-J, Dencher N A, Sperling W, Stieve H, Läuger P. Biophys Struct Mech. 1979;5:277–292. [Google Scholar]
- 14.Gloor S, Pongs O, Schmalzing G. Gene. 1995;160:213–217. doi: 10.1016/0378-1119(95)00226-v. [DOI] [PubMed] [Google Scholar]
- 15.Schmies G, Chizhov I, Engelhard M. FEBS Lett. 2000;466:67–69. doi: 10.1016/s0014-5793(99)01760-3. [DOI] [PubMed] [Google Scholar]
- 16.Grygorczyk R, Hanke-Baier P S W, Passow H. Methods Enzymol. 1989;173:453–466. doi: 10.1016/s0076-6879(89)73032-9. [DOI] [PubMed] [Google Scholar]
- 17.Chizhov I, Schmies G, Seidel R, Sydor J R, Lüttenberg B, Engelhard M. Biophys J. 1998;75:999–1009. doi: 10.1016/S0006-3495(98)77588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel H, Oesterhelt D. Biochemistry. 1980;19:4615–4619. doi: 10.1021/bi00561a012. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam S, Greenhalgh D A, Rath P, Rothschild K J, Khorana H G. Proc Natl Acad Sci USA. 1991;88:6873–6877. doi: 10.1073/pnas.88.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancshazy Z, Drachev L A, Ormos P, Nagy K, Skulachev V P. FEBS Lett. 1978;96:59–63. doi: 10.1016/0014-5793(78)81062-x. [DOI] [PubMed] [Google Scholar]
- 21.Bogomolni R A, Spudich J L. Biophys J. 1987;52:1071–1075. doi: 10.1016/S0006-3495(87)83301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tittor J, Schweiger U, Oesterhelt D, Bamberg E. Biophys J. 1994;67:1682–1690. doi: 10.1016/S0006-3495(94)80642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegener A A, Chizhov I, Engelhard M, Steinhoff H J. J Mol Biol. 2000;301:881–891. doi: 10.1006/jmbi.2000.4008. [DOI] [PubMed] [Google Scholar]
- 24.Jung K H, Spudich J L. J Bacteriol. 1998;180:2033–2042. doi: 10.1128/jb.180.8.2033-2042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X-N, Spudich J L. J Biol Chem. 1998;273:19722–19728. doi: 10.1074/jbc.273.31.19722. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X-N, Zhu J, Spudich J L. Proc Natl Acad Sci USA. 1999;96:857–862. doi: 10.1073/pnas.96.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]