Abstract
The usage of Humulus lupulus for brewing increased the demand for high-quality plant material. Simultaneously, hop has been used in traditional medicine and recently recognized with anticancer and anti-infective properties. Tissue culture techniques have been reported for a wide range of species, and open the prospect for propagation of disease-free, genetically uniform and massive amounts of plants in vitro. Moreover, the development of large-scale culture methods using bioreactors enables the industrial production of secondary metabolites. Reliable and efficient tissue culture protocol for shoot regeneration through organogenic nodule formation was established for hop. The present review describes the histological, and biochemical changes occurring during this morphogenic process, together with an analysis of transcriptional and metabolic profiles. We also discuss the existence of common molecular factors among three different morphogenic processes: organogenic nodules and somatic embryogenesis, which strictly speaking depend exclusively on intrinsic developmental reprogramming, and legume nitrogen-fixing root nodules, which arises in response to symbiosis. The review of the key factors that participate in hop nodule organogenesis and the comparison with other morphogenic processes may have merit as a study presenting recent advances in complex molecular networks occurring during morphogenesis and together, these provide a rich framework for biotechnology applications.
1. General Introduction
Humulus lupulus, belonging to the Cannabinaceae family, has a long traditional use as a bittering agent in brewing industry. Lupulin glands located behind the bracts of the cones of the female plant, known as hops, are covered with lupulin powder. Lupulin contains the hop acids, essential oils, and polyphenols that give the bitterness and flavour to the beer. Hop acids, humulones (α-acids), and lupulons (β-acids) contribute to bitter taste of beer and stability of the beer froth. Essential oils constitute the volatile fraction of the hop and include the so-called hydrocarbon-terpenes that give hop its pleasant aroma. Moreover, hop plants have a long and proven history of herbal use, being employed mainly for their soothing, sedative, tonic, and calming effect. In fact, hop was considered the medicinal plant of the year 2007 by the Journal of Medicinal & Spice plants. In order to identify the active principles and mechanism of action responsible for their phytochemical and pharmacological effects, many in vitro and in vivo studies have been conducted and reviewed elsewhere [1–3]. Hop is also rich in numerous important secondary metabolites: prenylated chalcones, desmethylxanthohumol, and xanthohumol. In recent years, some prenylated chalcones have received much attention for their biological effects. In particular, xanthohumol has been shown to exert cancer chemopreventive activity in in vitro experiments. In addition, 8prenylnaringenin has been characterized as one of the most potent phytoestrogens isolated until now. Nevertheless much additional work is needed to open up new biomedical application of these compounds.
In 2008, the world-wide total area cultivated with hops was approximately 50,000 Ha and total world production of hops harvested was approximately 104,000 T (International Hop Growers' Convention 2008). This report also expressed concern on the need for the best quality of hops for beer brewing and on the rising prices due to increasing demand and poor harvests. In an attempt to tackle these challenges, an international consortium (Australia, USA, UK, Slovenia) screened using Diversity Arrays Technology accessions of hop from Europe, North America, Asia, and Australia, including examples of Humulus lupulus var. lupuloides, Humulu lupulus var. pubescens and Humulu lupulus var. neomexicanus. The accessions included high and low α-acid, aroma, powdery and downy mildew resistant, and susceptible cultivars among others. Preliminary results anticipate that this technology will provide a platform for exploration of marker-trait associations to develop marker-assisted selection strategies in hop [4].
As in many crops, the availability and quality of planting material are major limiting factors for industrial exploitation. Rapid and efficient in vitro regeneration methods that minimise somaclonal variation are critical for assisting conventional breeding programs, mass propagation, and genetic engineering of commercial varieties. This is potentially helpful to supply clones with desirable traits and free of pathogens. For many plants, specialized techniques have been developed that allow for the multiplication of isolated cells, tissues, and organs. These techniques are based in the capacity of cultured plant tissues and cells to undergo morphogenesis, resulting in the formation of discrete organs or whole plants.
Stimulated mainly by the usage of hop in brewing, protocols for propagation of different cultivars have been established [5–7]. Starting from different explants, these protocols are based in the intermediary formation of a callus which makes the regeneration prone to undesirable somaclonal variations. More recently, it has been reported in vitro regeneration of hop through organogenic nodule culture [8, 9].
Organogenic nodule formation was proposed as an efficient system for plantlet regeneration parallel to somatic embryogenesis. Nodules are independent, spherical, and dense cell clusters, which form a cohesive unit and display a consistent internal cell/tissue differentiation [10] and their production has been described for several species [10–13]. Hop nodule culture constitutes an optimal tool for the engineering of agroeconomic traits such as maturity date, disease resistance, and aromatic oils content. A case of success was the introduction of resistance to V. album-atrum fungi in hop var. Eroica by particle bombardment of petioles [14]. The regeneration of the transgenic plants was achieved through organogenic nodule formation and plantlet regeneration. The impact of such achievements still needs to be determined but since several pathogen threaten commercial hop plantations yield every year, innovations in particular aspects of this technology grant the successful application of biotechnology to crop improvement.
The fundamental techniques to achieve in vitro morphogenesis have been long well-established. As a result, plant regeneration from cultured cell and tissues has been reported for a wide range of species. This has been achieved mostly based on empirical studies through manipulation of culture medium, especially growth regulators and selection of genotypes and explant source. However, many plant species and cultivars remain recalcitrant in regeneration or the rate of multiplication is either slow or limited. Relationships between the culture medium and explant leading to morphogenesis are complex and, despite extensive study, remain poorly understood. In recent years, galvanized by the advancement in functional genomics research, significant advances have been made in the identification of key regulatory elements that are involved in both in situ and in vitro plant morphogenesis.
Using differential gene-expression profiling, bioinformatics, in situ analysis, and functional characterization, a number of interesting candidate genes have been identified that are required for hop organogenic nodule formation. Here, we discuss how candidate genes have been coupled with targeted functional analysis to gain insight into the molecular mechanisms underlying morphogenesis. Moreover, we highlight common features between organogenic nodule formation in hop and other morphogenic processes such as somatic embryogenesis and the organogenesis of nitrogen-fixing root nodules.
2. Organogenic Nodules as a Biotechnological Tool for Production of Bioactive Compounds
Hop compounds have remarkable bioactive properties and for this reason hop is being regarded as a strong candidate to molecular farming. This can be achieved by using cell suspension culture or organ cultures such as organogenic nodules. It offers obvious advantages for the production of bioactive compounds over whole plant cultivation as it enables a strict control of products' availability, and safety/quality, with the production occurring under controlled and optimized conditions. Besides, it avoids problems related to storing and processing huge amounts of biomass [15] and it gives independence from external factors such as climate changes, agricultural policies, and pests. Moreover, production of pharmaceuticals and chemicals from plants would require available land to be effectively used. Recently, a high production of xanthohumol was obtained in cell suspension cultures established from leaf-derived calli [16]. However, many products of interest are synthesized in organized tissues, but are not formed in poorly differentiated cultures [17]. Plant production in bioreactor through regeneration from organogenic nodules constitutes an attractive alternative to the in nature whole plant cultivation for the production of high-value secondary metabolites. Two efficient methods with high potential for scale up and automation were described for mass propagation of Humulus lupulus L. in liquid medium [9]. This hop in vitro culture system enables a large-scale production of plantlets in batch cultures; these cultures remained proliferating for more than 5 years and presented genetic stability [9].
In an attempt to understand the mechanisms regulating the production of bioactive compounds such as prenylflavonoids, researchers have cloned a complete sequence of chalcone synthase gene which was highly expressed in glandular trichomes during cone maturation [18]. The promoter region of the valerophenone synthase gene was also isolated from hop; this enzyme is a member of the chalcone synthase superfamily [19]. A major effort in the sequencing and analysis of 10,581 ESTs of hop glandular trichomes led to the identification of an O-Methyltransferase that performs the final reaction of xanthohumol biosynthesis [20]. Recently, two genes coding for transcription factors (HlbZIP1 and HlbZIP2) that showed highly specific expression in lupulin glands were cloned and shown to interfere with the accumulation of flavonol glycosides, anthocyanins, and other phenolic compounds [21].
Taking together the knowledge gathered on some genes regulating the production of useful compounds and the reliable in vitro systems for regeneration and transformation of hop plants, we can consider that the hop in vitro system herein reviewed appear as a very powerful system not only for production of secondary metabolites normally produced by in-nature hop growing plants but also for production of secondary compounds from other plant origin and even for biotransformation.
3. Histological Analysis of the Morphogenic Process
In hop, wounding appears to play a pivotal role in the induction of morphogenesis from internode-derived nodules [8]. Abiotic factors such as temperature, humidity, and light also seem to be involved in induction of morphogenesis in hop. Small variations in these factors may have a major impact in organogenic nodule formation and plantlet regeneration. A high regeneration rate can only be obtained in growth chambers at specific conditions. Previously, several conditions of light, temperature, and humidity were tested and in certain cases morphogenesis was totally impaired (Fortes and Pais, unpublished data). Besides, growth regulators and their balance have a pivotal role in the induction of the process. Control explants cultured on basal medium without indole-3-acetic acid and benzylaminopurine addition never gave rise to neither nodule nor shoot bud, showing the appearance of incipient calluses after two-three weeks of culture [8].
The morphogenic process in Humulus lupulus var. Nugget started with divisions of cambial and cortical cells of internodes cultured for one week on induction medium with hormones (Figure 1(a)). After 15–19 days in culture, cambial cells were dividing tangentially and ultimately differentiating into vascular bundles. This gave rise to several prenodular structures [8]. At this stage, the entire explant was almost covered by masses of cells partially individualized from the original explant that were surrounded by calli cells.
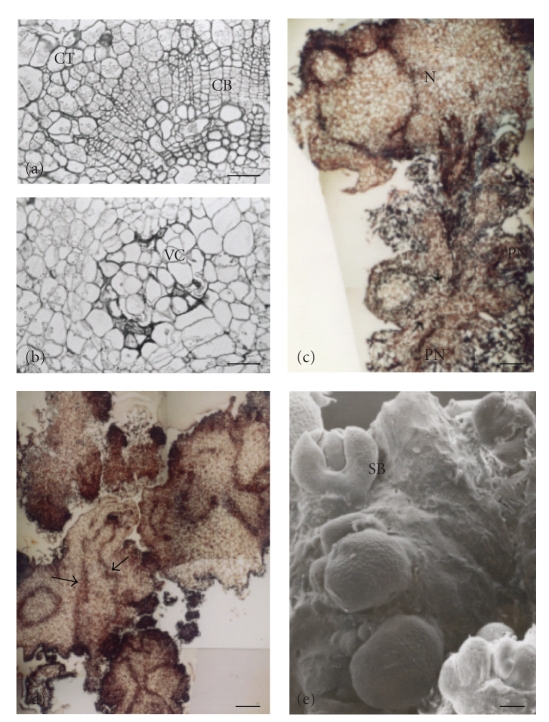
Figure 1.
(a)–(e) Histological and ultrastructural features during organogenic nodule formation and plantlet regeneration from internodal explants cultured on Murashige and Skoog medium (Murashige and Skoog [22]) supplemented with 2 mg/L benzylaminopurine and 0.05 mg/L indole-3-acetic acid. (a) Bright field image of a cross-section of an internode after 15 days in culture showing divisions in cambial and cortical cells. The material was previously embedded in resin. (b) Nodule arising after 28 days in culture and showing a vascular center surrounded by a cortical parenchyma area. (c) Section showing prenodular and nodular structures arising from a single internode after 28 days in culture. Material was previously embedded in paraffin wax. (d) Bright field image showing plantlet regeneration from nodules and separation of polycenter nodules into “daughter nodules.” Arrows point to vascular connections established between vascular bundles of nodule and growing plantlet. Material was previously embedded in paraffin wax. (e) Scanning Electron Microscopy imaging showing multiple shoot bud regeneration from an organogenic nodule. Bar in (a) = 100 μm; bar in (b) = 50 μm; bars in (c) and (e) = 200 μm; bar in (d) = 300 μm. cb: cambial cells, ct: cortical cells, n: nodule, pn: prenodule, sb: shoot bud, s-, vc: vascular center. (c,d,e) were reproduced from [8].
After 25–28 days in culture, prenodular structures surrounded by callus tissue form a cohesive unit able to undergo cell and tissue differentiation (Figures 1(b) and 1(c)), giving rise to nodules. Nodules obtained from Humulus lupulus var. Nugget internodal explants showed a central vascular area and a cortical parenchyma area surrounded by elongated cells (Figure 1(b); [8]). Nodules would increase then their volume and show multiple vascularization centers, the so-called “polycenter nodules” [10] around which nodulation could occur and form small “daughter nodules” (Figure 1(d)). Each of the “daughter nodules” grew further and then underwent organogenesis after 45 days in culture. This greatly increased the overall number of regenerated shoot buds obtained per internodal explant (Figures 1(d) and 1(e)). A high regeneration rate is greatly advantageous for mass propagation and genetic transformation.
4. Sugar Signaling and Secondary Metabolism
Plant cells cultured in vitro require the addition of exogenous sugar, in particular sucrose, since photosynthetic activity decreases. Transcriptomic and metabolomic studies showed an upregulation of gene coding for sucrose synthase and an increase in carbohydrate pool (glucose and sucrose) in prenodules and nodules [23] accounting for the importance of sucrose uptake from the culture medium and its later degradation. The glucose resulting from this degradation will most probably be used to the huge starch accumulation occurring during prenodule formation which will be later mobilized during development of nodules and plantlet regeneration [8].
The highest fold change obtained for upregulation of gene coding for sucrose synthase as well as downregulation of gene coding for Rubisco was obtained after 24 h upon culture initiation [23], which is in agreement with increased jasmonic acid (JA) levels, a growth regulator known to be involved in regulation of carbohydrate metabolism [24, 25].
Sugars can act as morphogens, providing positional information to the cell cycle machinery and organogenic programs [26]. Sugars can repress photosynthetic activity chlorophyll accumulation and development. They play a role in antioxidant balance [27] and regulate the expression of lipoxygenase (LOX) genes, pathogenesis-related genes, and other stress-inducible genes [26] as well as carbon and nitrogen metabolism. In fact, studies carried out with the model legume Medicago truncatula support that nodule-enhanced Sucrose synthase 1 is required for the establishment and maintenance of an efficient N-fixing symbiosis [28]. It is noteworthy that hop control samples cultured for 28 days in medium without growth regulators accumulated sucrose to the highest extent whereas glucose levels were low. In these samples, sucrose is not being degraded upon uptake from the medium leading to low levels of glucose which may be impairing growth and morphogenesis.
A crucial element of transcriptional and metabolic regulation in response to stress and energy status are the evolutionarily conserved energy sensor protein kinases, SNF1 (sucrose nonfermenting 1) in yeast, AMPK (AMP-activated protein kinase) in mammals, and SnRK1 (SNF1-related kinase 1) in plants [29]. Among the identified SnRK1 target genes are the ones coding for sucrose synthase and α-amylase (reviewed by [29]). Moreover, SnRK1 is involved in the posttranslational redox activation of ADP-Glucose pyrophosphorylase and thus in starch biosynthesis [30, 31]. To the best of our knowledge, these kinases have not been implicated in in vitro morphogenic processes; nevertheless they can be expected to play an important role in organogenic nodule formation.
Hop morphogenic structures produce energy mainly anaerobically, and according to the expression of genes coding for glycolytic enzymes, this process is more active during the prenodular stage (15 days of culture). Transcriptional profiling showed that several genes coding for proteins putatively related to photosynthesis such as Rubisco were downregulated in prenodules and, to lesser extent, in nodular explants [23]. An inverse correlation between photosynthesis- and defence-related gene regulation is probably due to an energy reallocation mechanism. Downregulation of proteins such as Rubisco may result from JA action as well as the induction of proteinase inhibitors and enzymes involved in the biosynthesis of secondary metabolites [24, 25]. In fact, jasmonates modulate the sucrose-induced expression of flavonoid biosynthetic genes in Arabidopsis [32].
The phosphoenolpyruvate generated in glycolysis provides carbon skeletons to the phenylpropanoid-flavonoid pathway. Since the increase in dihydrophenylpropanoids was observed after 15 days, it is most probably related to development of prenodules than to stress response [23]. The knowledge on the involvement of phenylpropanoids and flavonoids in morphogenesis is to date very incipient. The increase in dihydrophenylpropanoids and concomintant upregulation of genes coding for a cinnamic acid hydroxylase and a flavonol synthase during prenodule formation indicate a general role of flavonoid during organogenesis. In an analogous way to nitrogen-fixing root nodules [33], the flavonoid pathway might be crucial for nodule formation. Certain flavonoids are known to regulate polar auxin transport and formation of auxin gradients [34]. The leaf vascular strands depend on the accumulation of auxin in the procambial cells and its gradual canalization through polarization of auxin efflux carriers [35]. The formation of vascular centers in prenodules might similarly depend on strict regulation of auxin transport necessary for vascular tissue differentiation.
5. Role of Stress-Induced Enzymes and Derivative Products
5.1. Mitogen-Activated Protein Kinases and Oxidative Stress
Plant stress responses are a key factor in steering and reprogramming the development of cells, tissues, and organs. In hop, wounding appears to play an essential role on the induction of morphogenesis from internode [8].
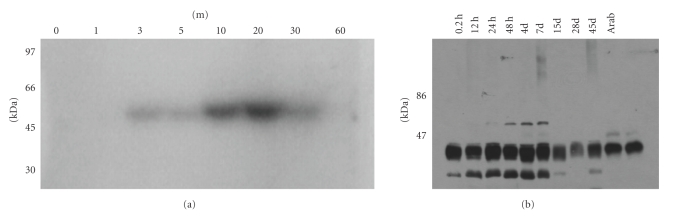
Mitogen-activated protein kinases (MAPK) are important signal transducers connecting the perception of external stimuli to cellular responses. MAPK cascades have been implicated in signaling various abiotic and biotic stresses, like wounding and pathogen infection, temperature stress, or drought, but are also involved in mediating the action of some plant hormones, such as ethylene and auxin [36]. MAPK cascades may link auxin signalling to oxidative stress responses and cell cycle regulation [36]. A systemic increase in transcripts encoding a wound-induced protein kinase, a MAPK homolog in tobacco, was observed in response to mechanical wounding [37]. The same was noticed for hop internodes 5 min following wounding [38]. This increase in wound-induced protein kinase transcripts was accompanied by increased kinase activity that peaked after 20 min (Figure 2(a)). The involvement of MAPK3, MAPK4, and MAPK6 in hop morphogenesis was also detected (Figure 2(b); Dr. Joana Costa, University of Lisbon, personal communication). Hydrogen peroxide, a reactive oxygen species (ROS) released by wounding, and JA activate in Arabidopsis a MAPK cascade, involving MAPK 6 (MPK6) [39, 40]. Mitogen-activated protein kinase signalling pathways have also been shown to regulate diverse aspects of plant growth and development. MAPK cascades can be involved in the restriction of asymmetric cell division frequency, in maintaining polarity, and in coordination of cell fate specification [41]. Genes encoding MPK3 and MPK6 were suggested to regulate anther cell division and differentiation and ovule development [42, 43].
Figure 2.
(a) Detection of MBP Kinase Activity in hop extracts at different time points (T0, T1, T3, T5, T10, T20, T30, and T60 min) immediately after wounding. In in-gel kinase assays MAPK phosphorylates MBP. A band of approximately 50 kDa was induced upon wounding of hop internodes. (b) MAPK 6 activity during hop organogenesis (T0, T2h, T12h, T24h, T48h, T4d, T7d, T15d, T28d, and T45d). Phosphorylation of MBP was analyzed by autoradiography following immunoprecipitation with MAPK6 antibody. In hop extracts and Arabidopsis (lanes Arab) subjected to in-gel kinase assay, a band of approximately 46-kDa was detected.
The extracellular signal-regulated kinase (ERK) pathway is a conserved MAPK module across the eukaryote evolution. In animal and yeast cells, the last members of this cascade, ERK1 and ERK2, have been involved in diverse cellular processes including control of cell growth, proliferation, and differentiation [44]. Depending on the duration of ERK activation, both proliferation and differentiation processes can be activated in the same cell [44]. Moreover, these MAPKs were shown to be activated by cytokines and hydrogen peroxide [45]. In plants, orthologues of these MAPKs have been shown to regulate fundamental processes such as the cell cycle and cell survival [46].
In hop organogenic nodules, two ERK isoforms have been implicated in the initiation of cell differentiation into nodules [47]. In prenodules, active ERKs were localized mostly in the nucleus of dividing cells. As in animals [48], the regulation of the MAPK accessibility to the nucleus might be a key-signaling event by which cells control the intensity and temporal activation of genes during cell growth and differentiation. Once nodular cells start proliferating to form shoot meristems, ERK1 and 2 were detected in the cytoplasm [47]. Thus, the presence of ERKs in the nucleus of prenodular cells seems to correlate with the initiation of cell differentiation into nodules. ERK2 was shown to be induced by ROS and act throughout the organogenic process. However ERK1 appears to be involved in the later stages, namely, daughter nodules and meristem formation (28 and 45 days after induction).
Recently, a gene coding for a MAP kinase-like protein was found upregulated in organogenic nodules comparing to control explants cultured on medium without growth regulators [23]. This observation further demonstrates the role of MAPK in the differentiation and proliferation of nodular cell upon morphogenic stimulus such as the application of growth regulators. Cell competence is associated with a particular metabolic cell-state most probably induced by growth regulators and stress conditions, which enables to switch on defense mechanisms in a way that triggers morphogenesis. Indeed, arrangement of cells into morphogenic structures such as somatic embryos might depend on a tight control between cell proliferation and cell death [49]. The cell mitotic cycle is also tightly regulated by the cell redox balance. Reactive oxygen species (ROS) are not only toxic by products of aerobic metabolism but also linked to the activation of several stress-responsive MAPK pathways in plants [40]. Both reactive oxygen and nitrogen species (ROS/RNS) production and detoxification are actively regulated in the response to environmental cues and developmental stage. ROS/RNS detoxification relies on the antioxidant defence, which involves enzymatic activities (catalases, superoxide dismutases, and peroxidases) and antioxidant molecules (glutathione and ascorbate). Recently, ROS and RNS species emerged as ubiquitous signalling molecules participating in the recognition of and the response to stress factors.
Transcriptomic analysis of hop organogenic nodules showed a clear upregulation of genes related to stress response [23]. Production of reactive oxygen species is likely to be counter-balanced by a set of antioxidant agents resulting in the tight control of oxidative status. In fact, explants cultured on medium without growth regulators showed increased expression of a Senescence-Associated Gene and a gene coding for a putative gamma-thionin. Moreover, the accumulation of glutamine and glutathione gives strong support that these tissues become extremely oxidized upon wounding and during in vitro culture. Concomitantly, instead of nodule formation taking place, cell death was widely occurring in these control samples. The role of oxidative stress in morphogenesis was also supported by the induction of genes coding for several peroxidases which can be involved in cell wall modification associated with growth and developmental processes [23].
Reactive oxygen species were shown to accumulate at a necrotic cell layer that marks the future region of nodule separation [50, 51]. Hydrogen peroxide which accumulates in nodular cells may act as a signalling molecule-regulating development and programmed cell death [52]. The occurrence of programmed cell death in certain nodular cells may also play a role in recycling nutrients for the growing structure. Moreover, ROS may have a direct influence on primary metabolism by modulating enzymes involved in starch synthesis/degradation [53].
5.2. Lipoxygenases Expression and Lipid Metabolism
Stress response and lipid metabolism involve expression of lineolate oxygen oxidoreductases (lipoxygenases, EC 1.13.11.12; LOXs [54, 55]). Lipoxygenases catalyze the conversion of (1Z, 4Z)-pentadiene polyunsaturated fatty acids into their corresponding hydroperoxy derivatives [54]. Several compounds in the linoleate cascade from LOX have physiological effects in plants, for example, jasmonic acid, methyljasmonate, and some volatile aldehydes [24, 25].
Lipoxygenases were shown to be developmentally regulated throughout the process of nodule formation [50]. They have been implicated in the response of internodes to wounding, nodule formation, and plantlet regeneration from these nodules. The rapid increase on LOX activity parallel with elevated levels of lipid peroxides in response to wounding may be involved in the synthesis of the wound-healing factor, traumatin, and jasmonates that in turn can trigger the expression of wounding/defense-related genes as previously suggested [55]. Lipoxygenase isoenzymes were shown to be de novo synthesised during induction and formation of organogenic nodules. Immunogold labelling studies showed that LOX isoenzymes were located in peroxisomes, plastids, and lipid bodies of nodular cells. In addition, two ESTs coding for LOXs were found to be differentially expressed during organogenic nodule culture [23]. Their transcriptional profile differs between them suggesting that these enzymes are located in different compartments and/or have different metabolic activities [50]. The localization of LOX preferentially in areas with high meristematic activity suggested an active role in growth and development during this morphogenic process [50]. Interestingly, also increased contents in α-linolenic acid, choline, and a short chain fatty acid were found when comparing organogenic nodules to control explants cultured in medium without growth regulators [23].
The localization of LOX in peroxisomes of nodular cells could be related to entrance of these cells in programmed cell death as it might be a source of ROS. The degradation of peroxisomal membrane and the leakage of their contents might lead to nodules separation into “daughter nodules” [50]. In fact, plant peroxisomes constitute a source of signalling molecules such as ROS [56].
The localization of LOX in lipid bodies suggests the involvement of LOX in the mobilization of storage lipids to be used as a respiratory substrate in this high energy-requiring process of nodule formation [50]. Besides the involvement of LOX, the expression of other genes and activity of enzymes involved in lipid metabolism seems to be essential for morphogenesis to occur. For instance, other ESTs coding for enzymes involved in lipid metabolism were found, namely, lipid transfer proteins and epoxide hydrolase [23]. The induction of such genes was related to the formation of a thick cutin layer of smooth texture specific to morphogenic regions of nodular structures [57]. This cutin layer may prevent water loss, thus influencing the regeneration capacity of nodular cells.
6. Growth Regulators
6.1. Allene Oxide Cyclase and Jasmonates
Allene oxide cyclase (AOC) is a key enzyme in jasmonates biosynthesis, catalysing the formation of cis-(+)-12-oxophytodienoic acid (OPDA) a stereoisomeric percursor of JA. It appears to be upregulated in diverse developmental processes as well as in response to wounding and other abiotic and biotic stresses [25, 58].
Jasmonates are oxylipins (oxygenated compounds derived from polyunsaturated fatty acids) which have attracted interest as signaling molecules activating gene expression in different plant responses, such as to wounding [25, 58]. Jasmonates include JA and its methyl ester, and octadecanoids comprising OPDA and its derivatives.
Changes of AOC expression and localization were reported during wound-induced organogenic nodule formation and plantlet regeneration [59]. Allene oxide cyclase transcripts and protein were shown to rapidly accumulate upon wounding of the explant indicating wound-induced de novo AOC synthesis. During formation of nodules, AOC was immunolocalized to the amyloplasts present in the nodules and additionally in chloroplasts of differentiated nodular cells. This localization of AOC was related to JA generation, which could be involved in conditioning storage or mobilization of sugars occurring in growth and differentiation processes. An increased level of sucrose followed by increased cell expansion seems to occur in response to JA [60].
The increase in JA and OPDA levels within the first 24 h after wound treatment of the cultured explants (internodes) was suggested to be involved in downregulation of photosynthetic genes during inhibition of chlorophyll synthesis and amyloplasts differentiation observed in the early steps after culture initiation [23, 59]. During plant regeneration from organogenic nodules, a new rise of those compounds was measured, suggesting that these morphogenic processes might be affected by JA and OPDA as reported for somatic embryos [61].
6.2. Arginine Decarboxilase and Polyamines
Arginine decarboxilase (ADC; EC 4·1.1·19), is one of the main three enzymes controlling the biosynthesis of polyamines, together with ornithine decarboxylase (ODC; EC 4·1.1·17) and S-adenosyl-L-methionine decarboxylase (SAMDC; EC 4·1.1·50). Arginine decarboxilase is involved in putrescine biosynthesis, the precursor of spermine and spermidine.
Polyamines have been implicated in a wide range of biological processes, including cell growth and division, stabilization of nucleic acids and membranes, protein synthesis and chromatin function, and biotic and abiotic stresses responses [62–64]. Polyamines are also implicated in programmed cell death [65].
During organogenic nodule formation in hop, two isoforms of ADC were detected [51]. The increase in the levels of two ADC isoenzymes with the concomitant decrease in arginine levels during prenodular and nodular formation were related to increased polyamine synthesis and their role in morphogenesis [23, 51]. In accordance, polyamines have been related to the formation and development of globular embryos and organogenesis [64, 66].
The involvement of polyamines in several biological functions may rely on the differential subcellular location of ADC, among other enzymes. In hop, ADC is located in three different compartments: nucleus, plastids, and cytosol, depending on the tissue under analysis. One isoform seems to be induced by the wounding of internodes. The high increase in total polyamines content measured four days after culture initiation was related to the onset of active cambial and cortical cells division of internodes. Moreover, polyamines might exert a protective effect on the cell membranes against ROS-induced lipid peroxidation [67, 68]. The fact that polyamines content kept increasing from start of culture up to nodule formation reaching high values at this time point infers an important role for these compounds in hop morphogenesis. For instance, polyamines have been implicated in the regulation of polar auxin transport during the induction of vascular tissue [69]. Thus, polyamines can eventually be important for the formation of vascular centers in organogenic nodules.
Since polyamines are thought to be involved in the regulation of transcription and translation, increased polyamine synthesis and metabolism may be related to selective protein synthesis occurring during organogenesis. Polyamines are also known to enhance ERK activity playing a role in signal transduction events leading to cell growth and differentiation [65].
Increased transcription of genes coding for SAM synthetase and SAMDC suggested increased S-adenosyl-L methionine synthesis [23]. In hop, a gene coding for SAMDC was upregulated during nodule formation in accordance with increased spermidine synthesis at this stage. The increase of polyamines concomitantly to the expression of SAMDC was also reported during shoot organogenesis [70]. Interestingly, gamma-aminobutyric acid, a product of polyamine oxidation, was found to accumulate throughout hop culture in particular during nodule formation [23]. This compound has been related to stress response and may be produced in certain nodular cells where oxidative stress is increased.
Besides its involvement in polyamine biosynthesis, SAM also provides methyl groups in many biological methylations, namely, in the synthesis of phenolic compounds which increase during hop morphogenesis in particular in prenodules [23].
6.3. Auxin Transport and Signalling
The plant hormone indole-3-acetic acid (IAA) is at the center of many key morphogenic events in plants, such as embryogenesis, organogenesis, root elongation, and growth responses to environmental stimuli [71]. Auxin is actively pumped from cell to cell by the action of import and export proteins. Experimental evidence suggests that it is the control of auxin transport by these transport proteins that is central to many patterning processes involving auxin [72]. Members of the PIN protein family determine in Arabidopsis the direction and rate of intercellular auxin flow through their polar plasma membrane localization [73, 74] and thus are necessary for cell polarity and patterning.
The endogenous IAA content is higher in organogenic nodules than in internodes. Moreover, the formation of nodules is impaired by inhibitors of auxin synthesis and transport [75]. In parallel, pharmacological manipulation of polar auxin transport abolishes auxin gradients and results in defective embryogenesis, shoot organogenesis, and root meristem formation [76]. Consequently, polar auxin transport seems to be a major determinant of the asymmetric auxin distribution that functions upstream of many auxin-dependent developmental processes. These results indicate that auxin synthesis and its polar transport may play a pivotal role in nodules formation and later in regeneration of plantlets. The ability of auxin to act as a morphogen is also seemingly convincing in the case of wood formation [77, 78]. In Scots pine (Pinus sylvestris L.), auxin concentration gradient coincides with the developmental gradient of secondary xylem cell specification [77]. Although auxin-responsive genes may be more sensitive to changes in auxin concentration than to the absolute concentration of auxin, this phytohormone appears to be involved in regulating the cambial cell divisions to specify secondary xylem formation [78].
Leaf protoplasts of alfalfa (Medicago sativa L.) cultured on medium with different 2,4-dichlorophenoxyacetic acid concentrations develop into either embryogenic or nonembryogenic cell types [79]. This suggests that both the concentration and the ratio of exogenous hormones are important to determine cell fate during de novo organogenesis. An important mode of auxin action is through its effects on gene expression [71]. Transcriptomic studies gave further support that changes in auxin signalling may be involved in organogenic nodule formation [23]. Two classes of auxin regulated transcripts were found: auxin-repressed protein (ARP) coding gene and Aux/IAA early auxin-response gene. The transcription of the Aux/IAA gene family presents a high heterogeneity in response to auxin depending on both tissue type as well as duration and concentration of auxin exposure [80]. During organogenic nodule formation, there is downregulation of two ARP proteins when compared to the control (noncultured internodes). Nevertheless, this repression seems to be released with the ongoing of this morphogenic process. These results suggested that ARP genes must be downregulated for auxin-mediated responses to occur. In parallel, during wood formation in hybrid aspen plants (Populus tremula × Populus tremuloides), perturbations of auxin signalling by reducing auxin responsiveness reduced the cambial cell division activity, caused spatial deregulation of cell division of the cambial initials, and led to reductions not only in radial but also in axial dimensions of fibers and vessels [78].
7. Morphogenesis-Associated Signal Transduction Pathways
The perception of hormone stimuli and/or secondary messengers like ROS and calcium may trigger various signal transduction cascades in several developmental processes. In carrot, calcium was found to be essential for morphogenesis of undifferentiated cells into somatic embryos (reviewed by [81]). An important role of calcium on organogenic nodule formation in hop has also been previously suggested [82]. In this study, X-ray spectra collected from organogenic nodules revealed higher levels of calcium on peripheral regions where regeneration was occurring. Moreover, calcium seemed to be mobilized in several directions, from inner regions of nodules towards their periphery at the onset of plantlet regeneration. During any calcium-mediated signal transduction event, an increase in the cytosolic calcium occurs and is perceived by calcium-binding proteins which undergo conformational changes and interact with a wide range of regulatory proteins. Calmodulin is an important protein involved in mediation of calcium signalling. Transcriptomic analysis of organogenic nodule formation revealed increased transcript abundance at prenodular stage of genes coding for a small G-protein ROP9, a RAC-like G-protein and calmodulin, suggesting that this proteins may participate in the determination of prenodular cells to develop into nodules [23]. ROPs are believed to influence polar growth by the ROP-dependent formation of ROS that either activates Ca2+ channels or leads to cell-wall loosening [83, 84]. Thus, the observed upregulation of this “hub” could be related to cell division and cellular rearrangement of the cytoskeleton. Indeed, active calcium/calmodulin complexes have also been detected in the meristematic regions of heart- and torpedo-stage embryos, suggesting a regulatory role of activated calmodulin in embryonal regions showing rapid cell divisions (reviewed by [81]). Rac/Rop GTPases play important roles in defense response, establishment of cell polarity, and hormone signalling [85]. Thus, hop RopGTPases may be directly or indirectly regulating diverse signaling pathways in a tissue- and temporal-specific context.
Transcriptional profiling during organogenic nodule formation also revealed upregulation of genes coding for a putative phosphatase 2A inhibitor, MAPK-like protein, zinc finger protein 4, AN1-like zinc finger, zinc finger (DHHC type) family protein, and a putative plant homeodomain finger and bromo adjacent homology domain protein [23]. Zinc finger proteins constitute an abundant family of nucleic acid binding proteins in the genomes of higher and lower eukaryotes, constituting the most abundant family of putative transcriptional regulators in Arabidopsis [86]. AN1 zinc finger proteins are involved in abiotic stress response. The overexpression of a rice A20/AN1-type zinc finger protein was reported to mediate high tolerance to cold, drought, and salt stress [87]. The upregulation of the hop ortholog during prenodule and nodule formation could help avoid stress-associated injuries and better recovery from stress.
During somatic embryo development in cotton, genes coding for zinc finger homeodomain protein, MAPK, calcium-transporting ATPase, and Argonaute were modulated [88]. A gene of the Argonaute family was proved to be essential for normal somatic embryo development in Picea glauca [89]. It is emerging the view that RNAi controlled gene expression is required for somatic embryogenesis and it may also be the case of organogenic nodule formation.
In our transcriptional profiling study, a gene coding for a putative receptor serine/threonine kinase was upregulated at prenodular stage [23]. In Arabidopsis, a SOMATIC EMBRYOGENESIS RECEPTOR KINASE1 (SERK1) plays a major role in the acquisition of embryogenic competence in culture [90]. In fact, the embryogenic competence of callus derived from seedlings over expressing AtSERK1 was three-four times higher when compared with the wild-type callus. Similarly, this gene mediates acquisition of embryogenic competence in the egg cell during zygotic embryogenesis [90]. The product of this gene is a protein that carries leucine rich repeat (LRR) domains exposed at the cell membrane that should be responsible for the interaction with other signal molecules, external signal perceptions, and signal transduction during somatic embryogenesis induction [90, 91]. This protein belongs to the receptor-like kinase-LRR (RLK) family with a serine-threonine kinase site [91]. A correlation between SERK gene expression and somatic embryogenesis has been demonstrated for many other plant species in recent years, for example, in Helianthus annuus [92], Oryza sativa [93], or Vitis vinifera [94] but also plays a role in other developmental or physiological processes, for example, adventitious shoot regeneration in Helianthus annuus [92], formation of symbiotic nodules in Medicago truncatula [95], or host defence response in Oryza sativa [96].
SERK1 was shown to interact with the KINASE-ASSOCIATED PROTEIN PHOSPHATASE that is postulated to play a role in receptor internalization [97]. This phosphatase has been reported to interact with WALL-ASSOCIATED KINASE1, among other proteins (reviewed by [98]. More recently, the MADS box transcription factor AGAMOUS-LIKE15 and an uncharacterized zinc finger protein, a member of the CONSTANS family involved in maintaining circadian oscillation, were identified as part of the SERK1 complex [99]. In hop cultures, a gene coding for a wall-associated protein kinase was upregulated in organogenic nodules when compared to control samples cultured in medium without growth regulators [23]. By interacting with cell wall pectins in the presence of calcium [100], wall-associated kinases may play a role in cell elongation and cell differentiation during morphogenesis.
It is also intriguing and deserves further studies whether the LEAFY COTYLEDON (LEC) transcription factors which are markers of embryogenesis do play a role in organogenic nodule formation, a structure that resembles an embryo without polarization since plant regeneration occurs from many regions of the nodule. LEAFY COTYLEDON transcription factors were suggested to create an environment in somatic cells that make them competent to respond to auxin and undergo somatic embryogenesis [101]. Whereas LEC transcription factors are involved in the induction of somatic embryogenesis, SERK1 increases the competence of tissues to undergo this morphogenic process (reviewed by [101]).
When organogenic nodules are compared to symbiotic nodules, differences in gene expression and metabolism should be expected since in the latter there is interaction with another organism and symbiotic nodules are involved in nitrogen fixation. Additionally, somatic embryos present polarization whereas organogenic nodules are not polarized structures. However, many features are common to all the morphogenic processes. It will be interesting to study the expression of genes such as the ones encoding POKY POLLEN TUBE and EARLY FLOWERING 3 (ELF3) during development of symbiotic nodules and somatic embryos since these genes were not reported so far for these morphogenic processes but have been found in organogenic nodule formation in hop [23].
In Arabidopsis, POK/VPS52 is associated with pollen tube growth [102], and belongs to the GARP (Golgi-associated retrograde protein)/VFT (Vps Fifty Three) complex. The poky pollen tube (pok) male gametophytic mutant exhibits very short pollen tubes. A more general role for POK in polar growth beyond the pollen tube elongation process was suggested [102]. Intense membrane trafficking is expected to occur during hop nodule organogenesis. The involvement of POK/VPS52 protein in retrograde transport towards the Golgi apparatus is suggestive of the importance of regulating some steps of vesicle trafficking during organogenesis, likely contributing with material for the cell-wall and membrane during growth and/or for the recycling of signaling components to and from the plasma membrane. This may also play an important role in establishment of the embryo axis during somatic embryogenesis.
Previously, we reported that light plays a major role in induction of organogenic formation, eventually by modulating the endogenous circadian clock which controls various cellular processes ranging from photosynthesis to stress responses [103]. In hop, a gene coding for an EARLY FLOWERING 3 (ELF3) protein was upregulated in prenodules and organogenic nodules [23]. ELF3 nuclear protein is an evening-specific repressor that represses light input to the circadian clock. Its activity is thought to be required by the core oscillator to produce circadian rhythms regulating growth responses [104]. The mutations on ELF3 Arabidopsis gene cause light-dependent circadian dysfunction, elongated hypocotyls, and early flowering. Variation on ELF3 expression might results in promotion or repression of flowering [105, 106]. Circadian rhythms are highly relevant to a wide range of biological processes and it may also be the case of development of symbiotic nodules and embryos.
8. Common Features in Diverse Morphogenic Processes
One of the fundamental questions in morphogenesis research is understanding why and how only certain cells of an organism are able to create complex forms and patterns to give rise to a new organ, given that every cell has the same genetic code. As fascinating and intriguing is the observation that separated tissues and cells can give rise to identical adult plant, but if left together, these same tissues and cells somehow “know” about each other and develop into a single organism. Rather than cell lineage, it is the positional information that determines cell fate in plants. The identification of common regulators of the different morphogenic processes might accelerate the establishment of guidelines for early diagnosis of morphogenic potential.
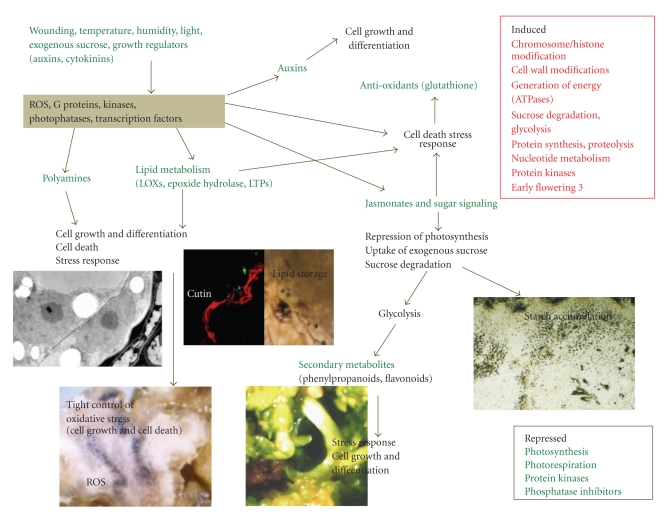
Hop organogenic nodule initiation and formation is characterized by changes in transcripts and metabolites involved in carbohydrate metabolism, hormone response, signal transduction cascades, defence, and cell division among others (Figure 3, [23]). Analysis of the datasets suggests that this process shares many similarities to the induction phase of somatic embryogenesis [49, 107] and nitrogen-fixing root nodules [108–110]. All these morphogenic processes rely on the capability to reverse their state of differentiation (first dedifferentiation and then re-differentiation). This seems to depend on the activity of genes that determine and maintain the meristematic state of cells, the level of hormones in the cells, and their sensitivity to hormones. A putative molecular and genetic regulatory network during reprogramming of somatic to embryonic cells has been proposed using Arabidopsis datasets and used to transfer information on genes between genomes based on orthologic relationships [96].
Figure 3.
Main pathways leading to formation of hop organogenic cultures. Abiotic factors such as light, wounding, sucrose and auxins induce the early expression of MAPKs. Signaling processes still not fully elucidated are responsible for inducing a stress response in which lipoxygenases, jasmonates, and polyamines as well as an increase in glutathione levels play an important role. Cell division is also induced by MAPKs and involves auxins and polyamines. Sugars and jasmonates may lead to starch accumulation and phenylpropanoid synthesis.
As with the initiation of hop organogenic nodules, the initiation of somatic embryogenesis requires a signal that induces recapitulation the embryogenic potential of the mitotically quiescent somatic cells. In many forms of somatic embryogenesis, the induction signal is exogenously applied auxin. Also de novo organogenesis leading to root nodule formation starts with the perception of an external stimulus. In this case, the signals are emitted by the symbiont rhizobial bacteria (Nod factors) upon sensing of flavonoids present in host root exudates. Nod factors are recognized by the host's root and epidermal infection stimulates cortical cells division near the infection point and neighboring the root protoxylem pole [33]. Similarly, after incision of internodes and placement in culture media, hop responds to this stimulus by intense cortical and cambial cells divisions. In both cases, the combinatorial interactions of an external cue and changes in diverse signaling pathways define cells that are dividing rapidly to establish the nodule primordium, that is, events that specify the cells that will become part of an organ.
In nitrogen-fixing root nodules, formation of nodule primordia involves dedifferentiation and reactivation of cortical root cells. Both gain- and loss-of-function mutants of Lotus japonicus have shown that cytokinin signaling through the cytokinin receptor (Lhk1) is important for reactivation of the cell cycle in cortical cells [111]. A yet unidentified ortholog of this receptor in hop may be expected to play an important role in organogenic nodule formation since benzylaminopurine addition to culture medium is determinant for morphogenesis to occur [8].
Host flavonoids have also a role in cell proliferation by inhibiting polar auxin transport [112]. This has been demonstrated by silencing of the chalcone synthase in Medicago truncatula which induced flavonoid deficiency, inhibited nodulation and disabled the inhibition of auxin transport by rhizobia [113]. The increase of dihydrophenylpropanoids and concomitant upregulation of trancripts for cinnamic acid hydroxylase and flavonol synthase [23] indicate that flavonoids may be relevant to the induction and formation of the organogenic nodules. It remains though to be examined if flavonoids regulate auxin transport and formation of auxin gradients [34] in an analogous way to nitrogen-fixing nodule.
Application of one and/or several stresses (wounding, osmotic, heavy metal ion, and dehydration stress), are known to induce or promote somatic embryogenesis [79, 114]. The cell fate switch during somatic embryogenesis, organogenic nodule formation and nitrogen-fixing nodules has been related to a tight control of defense and antioxidation mechanisms. It is well established that auxin and light can induce an oxidative burst in the target tissue by generating ROS. Similarly, oxidative burst, in form of H2O2 and oxygen singlet, has been detected during the infection by the symbionts and later in infected cells of young nodules for nodule formation [115]. Comparatively, the oxidation of nitro blue tetrazolium due to presence of ROS could be detected around wounded areas of the explant and further on at nodule separation into “daughter nodules” (Figure 3 [51]).
The accumulation of ROS/RNS in these areas might be fundamental for cellular growth. This is well illustrated by experiments that use of scavenger of ROS resulting in the impairment of hop nodule formation and indeterminate nodule formation [51, 116]. Thus, the tight control of when, where, and to what levels ROS/RNS are present might be pivotal to trigger morphogenic events. As they can provoke cellular damage, the whole antioxidant defence is essential for progression of morphogenic programmes. The hop organogenic and legumes nodules have high thiol content, namely, in the form of glutathione suggesting an active glutathione-ascorbate cycle [23, 117, 118]. Transcripts for glutathione-S-transferase genes have been shown to accumulate in somatic embryos after auxin stimulation [49] and in hop organogenic nodules up to nodular stage [23]. Glutathione-S-transferase can catalyze glutathione-dependent peroxidase reactions that scavenge toxic organic hydroperoxides and protect from oxidative damage. Thus, rise on glutathione-S-transferase transcripts or activity may accompany an increase in glutathione levels. This major antioxidant may have an important antioxidant role in morphogenesis. Finally, the production of ROS/RNS might also be important in the process of senescence and cell death occurring for instance in the separation of “daughter nodules” in hop or in the senescence of infected cells in the later stages of the nodulation process. Also, in soybean, transcriptional profiling during somatic embryogenesis suggested that that the arrangement of new cells into organized structures might depend on a genetically controlled balance between cell proliferation and cell death [49].
Because plant cells are constrained by their cell walls and cannot migrate, the tight regulation of division planes during cell proliferation and of the direction of elongation is of vital importance to place cells into the correct positions during embryogenesis and organogenesis. Rearrangements of the cytoskeleton from perinuclear radiating microtubules and actin microfilaments to cortical arrangements seem to be essential for the development of somatic embryos [119]. Similarly, in Nod factor-induced nodule cell differentiation, subtle changes in the cytoskeleton network were observed; the increase in the fine bundles of actin and decrease in the microtubuli growth rate [120]. The changes in the organization of the cytoskeleton may also involve a change in the predominant actin isoform of which the cytoskeleton is composed. Although changes in the histological and cellular organization of cytoskeleton are yet to be analyzed in hop organogenic nodules, the upregulation of a transcript for a tubulin isoform observed in hop organogenic nodule in comparison with nonmorphogenetically competent tissue [23] suggests that particular cytoskeleton changes are specific to the morphogenic events.
Hormonal systems provide coordination of processes in the organism through metabolic, morphogenetic, and genetic processes. In carrot, it was proven that single cultured cells require 2,4-dichlorophenoxyacetic acid to initiate embryo development. However, the continuous presence of this artificial auxin blocks further development and results in the accumulation of the already determined proembryogenic cell mass in the cultures [121]. The pivotal role of auxin and cytokinin in morphogenesis has been for long recognized. Notwithstanding, growth regulators such as jasmonic acid and polyamines, among others, have been related to the translation of diverse stimuli in to growth and differentiation. Pronounced changes in the levels of polyamines and their biosynthetic enzymes demonstrate the importance of these growth regulators in promotion of somatic embryogenesis [64, 66], nitrogen-fixing root nodules [122] and hop organogenic nodules [51]. Analysis of transcript accumulation for the ADC, which catalyzes the first step of polyamine biosynthesis have shown in Lotus japonicus that the maximum of expression occurs in young nodules, a developmental stage characterized by rapid nodule growth whereas the transcript of these enzymes rapidly declined during later stages of nodulation [122]. Moreover, the authors also reported a correlation between expression of polyamine biosynthetic genes and those involved in cell division and expansion. This points to a primary role for polyamines in cell expansion, since changes in the levels of various polyamines have been correlated with the developmental stage rather than the period of growth in the maturation medium.
Jasmonic acid, its immediate precursor OPDA and its derivates (methyl jasmonate), emerge as regulators of specific developmental processes and plant adaptation to environment by controlling responses to external biotic or abiotic stimuli [25, 58]. These growth regulators generally rise upon wounding stimulation of explants such as in the case of organogenic nodules of hop (Figure 3, [59]). Concomitant with the jasmonate levels, the accumulation of AOC mRNA was transiently induced after wounding. Similarly, jasmonates were shown to build up during the early steps of the symbiosis but suppressed during the nodule formation [123, 124].
Upstream in the biosynthetic pathway of JA are LOXs that catalyze the hydroxyperoxidation of fatty acids. Lipoxygenases are accumulated upon stress conditions [55]. The function of LOX in wounding seems to be related to the synthesis of a number of different groups of oxylipins with signalling activity. Hop organogenic nodules and in rhizobia-induced nodules have a characteristic early and great induction of LOX activity in the growth and development of specific cells within the nodules [23, 50, 110, 125]. The variety of different LOXs found in both organogenic processes indicate that they may function in the formation of specific tissues [110]. However, during soybean somatic embryogenesis of [49], the late upregulation of lipoxygenases indicates that they may be associated to storage rather than to signalling processes.
9. Concluding Remarks
Higher plants are rich sources of medicinal compounds and of industrial usage. However, many plants are still harvested in the wild. The increased demand, both in quantity as well as in quality, and drastic reduction in plant availability increase the pressure to produce medicinal compounds via alternative ways, especially using tissue cultures and transgenic plants. The current advancements in understanding and manipulating the molecular and cellular biology of secondary metabolism provide a basis for the commercial production of secondary products in tissue cultures and/or transgenic plants. Still, the progress in this field has been hindered by technical difficulties of in vitro propagation and regeneration of several plants. The identification of triggering factors and deciphering the genetic and biochemical networks leading to in vitro regeneration is fundamental for appointing guidelines for tissue culture and genetic engineering of plants.
In this paper, we review the studies carried out in hop in order to establish a model for organogenic nodule formation. Jasmonates, octadecanoids, polyamines, auxins, and cytokinins seem to be involved in response to stress caused by wounding and/or in formation of organogenic nodules and plantlet regeneration. Morphogenic structures accumulate large amount of starch and seem to gain energy through a heterotrophic, transport-dependent and sugar-degrading anaerobic metabolism. Oxidative stress caused by wounding and by in vitro culture conditions may induce a morphogenic response in hop internodes leading to formation of nodules and plantlets. This response is likely to involve signal components such as kinases and transcription factors that are also involved in the expression of competence in somatic embryogenesis.
Altogether, these results provide clues on how to induce competent cells and morphogenesis in plant tissues, either by manipulating the content of growth regulators, sugars, or/and the redox status of plant cells. This subject deserves further attention especially for plant species emerging as suitable for medicinal use and with economic impact worldwide such as hop.
Acknowledgments
The authors would like to thank Portuguese Foundation for Science and Technology (FCT) for providing financial support through Post-Doc fellowships to A. M. Fortes (SFRH/BPD/13850/2003 and SFRH/BPD/27122/2006). The authors are very grateful to Dr. Marta S. Silva (Dep. Chemistry and Biochemistry, Science Faculty of Lisbon), and Dr. Joana Costa (IMM, Medicine Faculty of Lisbon) for kindly providing the images on MAPK activity.
Abbreviations
- ADC:
Arginine decarboxilase
- AMPK:
AMP-activated protein kinase
- AOC:
Allene oxide cyclase
- ARP:
Auxin-repressed protein
- ELF:
Early flowering
- EST:
Expressed sequence tag
- ERK:
Extracellular signal regulated kinases
- IAA:
Indole-3-acetic acid
- JA:
Jasmonic acid
- LEC:
Leafy cotyledon
- LOX:
Lipoxygenase
- LRR:
Leucine rich repeat
- MAPK:
Mitogen activated protein kinases
- ODC:
Ornithine decarboxylase
- OPDA
12-oxophytodienoic acid
- POK:
Poky pollen tube
- RLK:
Receptor-like kinase
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- SAM:
S-adenosyl-L-methionine
- SAMDC:
S-adenosyl-L-methionine decarboxylase
- SERK:
Somatic embryogenesis receptor-like kinase
- SNF1:
Sucrose nonfermenting 1
- SnRK:
SNF1-related kinase.
References
- 1.Van Cleemput M, Cattoor K, De Bosscher K, Haegeman G, De Keukeleire D, Heyerick A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. Journal of Natural Products. 2009;72(6):1220–1230. doi: 10.1021/np800740m. [DOI] [PubMed] [Google Scholar]
- 2.Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. Journal of Ethnopharmacology. 2008;116(3):383–396. doi: 10.1016/j.jep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Magalhães PJ, Carvalho DO, Cruz JM, Guido LF, Barros AA. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Natural Product Communications. 2009;4(5):591–610. [PubMed] [Google Scholar]
- 4.Whittock SLG, Jakše J, Javornik B, et al. Use of diversity array technology (DART) for genotyping of Humulus lupulus. In: De Keukeleire D, Hummer KE, Heyerick A, editors. In: II International Humulus Symposium; 2009; Ghent, Belgium. ISHS Acta Horticulturae 848. [Google Scholar]
- 5.Batista D, Sousa MJ, Salome Pais M. Plant regeneration from stem and petiole-derived callus of Humulus lupulus L. (Hop) clone Braganca and var. Brewer’s Gold. In Vitro Cellular and Developmental Biology—Plant. 1996;32(1):37–41. [Google Scholar]
- 6.Smýkalová I, Ortová M, Lipavská H, Patzak J. Efficient in vitro micropropagation and regeneration of Humulus lupulus on low sugar, starch-Gelrite media. Biologia Plantarum. 2001;44(1):7–12. [Google Scholar]
- 7.Rakousky S, Matousek J. Direct organogenesis in hop—a prerequisite for an application of A.tumefaciens-mediated transformation. Biologia Plantarum. 1994;36(2):191–200. [Google Scholar]
- 8.Fortes AM, Pais MS. Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. American Journal of Botany. 2000;87(7):971–979. [PubMed] [Google Scholar]
- 9.Batista D, Ascensão L, Sousa MJ, Pais MS. Adventitious shoot mass production of hop (Humulus lupulus L.) var. Eroica in liquid medium from organogenic nodule cultures. Plant Science. 2000;151(1):47–57. [Google Scholar]
- 10.McCown B, Zeldin E, Pinkalla H, Dedolph R. Nodule culture: a developmental pathway with high potencial for regeneration, automated micropropagation, and plant metabolite production from woody plants. In: Hanover J, Keathley D, editors. Genetic Manipulation of Woody Plants. New York, NY, USA: Plenum; 1988. pp. 149–166. [Google Scholar]
- 11.Warrag E, Lesney MS, Rockwood DJ. Nodule culture and regeneration of Eucalyptus grandis hybrids. Plant Cell Reports. 1991;9(10):586–589. doi: 10.1007/BF00232338. [DOI] [PubMed] [Google Scholar]
- 12.Teng W-L. An alternative propagation method of Ananas through nodule culture. Plant Cell Reports. 1997;16(7):454–457. doi: 10.1007/BF01092765. [DOI] [PubMed] [Google Scholar]
- 13.Piéron S, Belaizi M, Boxus P. Nodule culture, a possible morphogenetic pathway in Cichorium intybus L. propagation. Scientia Horticulturae. 1993;53(1-2):1–11. [Google Scholar]
- 14.Batista D, Fonseca S, Serrazina S, Figueiredo A, Pais MS. Efficient and stable transformation of hop (Humulus lupulus L.) var. Eroica by particle bombardment. Plant Cell Reports. 2008;27(7):1185–1196. doi: 10.1007/s00299-008-0537-6. [DOI] [PubMed] [Google Scholar]
- 15.Ionkova I. Biotechnological approaches for the production of lignans. Pharmacognosy Reviews. 2007;1:57–68. [Google Scholar]
- 16.Pšenáková I, Gašpárková l, Faragó J. Polyphenol and flavonoid contents of hop callus and cell suspension cultures. In: Proceedings of the IHGC Scientific Commission; June 2009; Leon, Spain. [Google Scholar]
- 17.Yeoman MM, Yeoman CL. Manipulating secondary metabolism in cultured plant cells. New Phytologist. 1996;134(4):553–569. doi: 10.1111/j.1469-8137.1996.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 18.Matoušek J, Novák P, Bříza J, Patzak J, Niedermeierová H. Cloning and characterisation of chs-specific DNA and cDNA sequences from hop (Humulus lupulus L.) Plant Science. 2002;162(6):1007–1018. [Google Scholar]
- 19.Okada Y, Saeki K, Inaba A, Suda N, Kaneko T, Ito K. Construction of gene expression system in hop (Humulus lupulus) lupulin gland using valerophenone synthase promoter. Journal of Plant Physiology. 2003;160(9):1101–1108. doi: 10.1078/0176-1617-01116. [DOI] [PubMed] [Google Scholar]
- 20.Nagel J, Culley LK, Lu Y, et al. EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol. Plant Cell. 2008;20(1):186–200. doi: 10.1105/tpc.107.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matoušek J, Kocábek T, Patzak J, et al. Cloning and molecular analysis of H/bZip1 and H/bZip2 transcription factors putatively involved in the regulation of the lupulin metabolome in hop (Humulus lupulus L.) Journal of Agricultural and Food Chemistry. 2010;58(2):902–912. doi: 10.1021/jf9043106. [DOI] [PubMed] [Google Scholar]
- 22.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- 23.Fortes AM, Santos F, Choi YH, et al. Organogenic nodule development in hop (Humulus lupulus L.): transcript and metabolic responses. BMC Genomics. 2008;9, article 445 doi: 10.1186/1471-2164-9-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasternack C, Hause B. Jasmonates and octadecanoids: signals in plant stress responses and development. Progress in Nucleic Acid Research and Molecular Biology. 2002;72:165–221. doi: 10.1016/s0079-6603(02)72070-9. [DOI] [PubMed] [Google Scholar]
- 25.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100(4):681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14:S185–S205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany. 2006;57(3):449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- 28.Baier MC, Barsch A, Küster H, Hohnjec N. Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiology. 2007;145(4):1600–1618. doi: 10.1104/pp.107.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baena-González E, Sheen J. Convergent energy and stress signaling. Trends in Plant Science. 2008;13(9):474–482. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jossier M, Bouly J-P, Meimoun P, et al. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant Journal. 2009;59(2):316–328. doi: 10.1111/j.1365-313X.2009.03871.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiessen A, Prescha K, Branscheid A, et al. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant Journal. 2003;35(4):490–500. doi: 10.1046/j.1365-313x.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- 32.Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytologist. 2008;179(4):1004–1016. doi: 10.1111/j.1469-8137.2008.02511.x. [DOI] [PubMed] [Google Scholar]
- 33.Crespi M, Frugier F. De novo organ formation from differentiated cells: root nodule organogenesis. Science Signaling. 2008;1(49):p. re11. doi: 10.1126/scisignal.149re11. [DOI] [PubMed] [Google Scholar]
- 34.Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science. 2007;12(12):556–563. doi: 10.1016/j.tplants.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Dettmer J, Elo A, Helariutta Y. Hormone interactions during vascular development. Plant Molecular Biology. 2009;69(4):347–360. doi: 10.1007/s11103-008-9374-9. [DOI] [PubMed] [Google Scholar]
- 36.Zwerger K, Hirt H. Recent advances in plant MAP kinase signalling. Biological Chemistry. 2001;382(8):1123–1131. doi: 10.1515/BC.2001.142. [DOI] [PubMed] [Google Scholar]
- 37.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270(5244):1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 38.Silva M. Expression of mitogen-activated protein kinases during morphogenic processes in plants. Lisbon, Portugal: Science Faculty of Lisbon, University of Lisbon; 2003. Ph.D. thesis. [Google Scholar]
- 39.Takahashi F, Yoshida R, Ichimura K, et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19(3):805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitzschke A, Hirt H. Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiology. 2009;149(2):606–615. doi: 10.1104/pp.108.131557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19(1):63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Liu Y, Bruffett K, et al. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 2008;20(3):602–613. doi: 10.1105/tpc.108.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hord CL, Sun YJ, Pillitteri LJ, et al. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Molecular Plant. 2008;1(4):645–658. doi: 10.1093/mp/ssn029. [DOI] [PubMed] [Google Scholar]
- 44.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 45.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2001;24(4):405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- 46.Meskiene I, Hirt H. MAP kinase pathways: molecular plug-and-play chips for the cell. Plant Molecular Biology. 2000;42(6):791–806. doi: 10.1023/a:1006405929082. [DOI] [PubMed] [Google Scholar]
- 47.Sousa Silva M, Fortes AM, Testillano PS, Risueño MD, Pais MS. Differential expression and cellular localization of ERKs during organogenic nodule formation from internodes of Humulus lupulus var. Nugget. European Journal of Cell Biology. 2004;83(8):425–433. doi: 10.1078/0171-9335-00397. [DOI] [PubMed] [Google Scholar]
- 48.Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouysségur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. The EMBO Journal. 1999;18(3):664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiology. 2003;132(1):118–136. doi: 10.1104/pp.103.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fortes AM, Coronado MJ, Testillano PS, Risueño MDC, Pais MS. Expression of lipoxygenase during organogenic nodule formation from hop internodes. Journal of Histochemistry and Cytochemistry. 2004;52(2):227–241. doi: 10.1177/002215540405200211. [DOI] [PubMed] [Google Scholar]
- 51.Fortes A, Costa J, Santos F, et al. Arginine Decarboxilase expression, polyamines biosynthesis and reactive oxygen species during organogenic nodule formation in hop. doi: 10.4161/psb.6.2.14503. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 53.Sparla F, Costa A, Lo Schiavo F, Pupillo P, Trost P. Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis. Plant Physiology. 2006;141(3):840–850. doi: 10.1104/pp.106.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosahl S. Lipoxygenases in plants–their role in development and stress response. Zeitschrift für Naturforschung C. 1996;51(3-4):123–138. doi: 10.1515/znc-1996-3-401. [DOI] [PubMed] [Google Scholar]
- 55.Porta H, Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiology. 2002;130(1):15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nyathi Y, Baker A. Plant peroxisomes as a source of signalling molecules. Biochimica et Biophysica Acta. 2006;1763(12):1478–1495. doi: 10.1016/j.bbamcr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 57.Fortes AM, Testillano PS, Del Carmen Risueño M, Pais MS. Studies on callose and cutin during the expression of competence and determination for organogenic nodule formation from internodes of Humulus lupulus var. Nugget. Physiologia Plantarum. 2002;116(1):113–120. doi: 10.1034/j.1399-3054.2002.1160114.x. [DOI] [PubMed] [Google Scholar]
- 58.Balbi V, Devoto A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytologist. 2008;177(2):301–318. doi: 10.1111/j.1469-8137.2007.02292.x. [DOI] [PubMed] [Google Scholar]
- 59.Fortes AM, Miersch O, Lange PR, et al. Expression of allene oxide cyclase and accumulation of jasmonates during organogenic nodule formation from hop (Humulus lupulus var. nugget) internodes. Plant and Cell Physiology. 2005;46(10):1713–1723. doi: 10.1093/pcp/pci187. [DOI] [PubMed] [Google Scholar]
- 60.Cenzano A, Vigliocco A, Kraus T, Abdala G. Exogenously applied jasmonic acid induces changes in apical meristem morphology of potato stolons. Annals of Botany. 2003;91(7):915–919. doi: 10.1093/aob/mcg098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koda Y. Possible involvement of jasmonates in various morphogenic events. Physiologia Plantarum. 1997;100(3):639–646. [Google Scholar]
- 62.Alcázar R, Marco F, Cuevas JC, et al. Involvement of polyamines in plant response to abiotic stress. Biotechnology Letters. 2006;28(23):1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 63.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cellular and Molecular Life Sciences. 2001;58(2):244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vuosku J, Jokela A, Läärä E, et al. Consistency of polyamine profiles and expression of arginine decarboxylase in mitosis during zygotic embryogenesis of Scots pine. Plant Physiology. 2006;142(3):1027–1038. doi: 10.1104/pp.106.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachrach U, Wang Y-C, Tabib A. Polyamines: new cues in cellular signal transduction. News in Physiological Sciences. 2001;16(3):106–109. doi: 10.1152/physiologyonline.2001.16.3.106. [DOI] [PubMed] [Google Scholar]
- 66.Gemperlová L, Fischerov L, Cvikrová M, et al. Polyamine profiles and biosynthesis in somatic embryo development and comparison of germinating somatic and zygotic embryos of Norway spruce. Tree Physiology. 2009;29(10):1287–1298. doi: 10.1093/treephys/tpp063. [DOI] [PubMed] [Google Scholar]
- 67.Tadolini B, Hakim G. Interaction of polyamines with phospholipids: spermine and Ca2+ competition for phosphatidylserine containing liposomes. Advances in Experimental Medicine and Biology. 1988;250:481–490. doi: 10.1007/978-1-4684-5637-0_42. [DOI] [PubMed] [Google Scholar]
- 68.Moschou PN, Sarris PF, Skandalis N, et al. Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiology. 2009;149(4):1970–1981. doi: 10.1104/pp.108.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clay NK, Nelson T. Arabidopsis thickvein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport. Plant Physiology. 2005;138(2):767–777. doi: 10.1104/pp.104.055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu W-W, Gong H, Pua E-C. Modulation of SAMDC expression in Arabidopsis thaliana alters in vitro shoot organogenesis. Physiologia Plantarum. 2006;128(4):740–750. [Google Scholar]
- 71.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology. 2006;7(11):847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 72.Benková E, Ivanchenko MG, Friml J, Shishkova S, Dubrovsky JG. A morphogenetic trigger: is there an emerging concept in plant developmental biology? Trends in Plant Science. 2009;14(4):189–193. doi: 10.1016/j.tplants.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Wisniewska J, Xu J, Seifartová D, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312(5775):p. 883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 74.Petrášek J, Mravec J, Bouchard R, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312(5775):914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 75.Santos F. Studies of polar auxin transport in Humulus lupulus morphogenic process and isolation of proteins interacting with PIN1- a putative auxin efflux carrier of Arabidopsis thaliana. Lisbon, Portugal: Science Faculty of Lisbon, University of Lisbon; 2006. Ph.D. thesis. [Google Scholar]
- 76.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cellular and Molecular Life Sciences. 2006;63(23):2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(17):9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson J, Karlberg A, Antti H, et al. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell. 2008;20(4):843–855. doi: 10.1105/tpc.107.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasternak TP, Prinsen E, Ayaydin F, et al. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiology. 2002;129(4):1807–1819. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paponov IA, Paponov M, Teale W, et al. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Molecular Plant. 2008;1(2):321–337. doi: 10.1093/mp/ssm021. [DOI] [PubMed] [Google Scholar]
- 81.Chugh A, Khurana P. Gene expression during somatic embryogenesis—recent advances. Current Science. 2002;83(6):715–730. [Google Scholar]
- 82.Fortes AM, Pais MS. An electron probe X-ray microanalysis study during organogenesis from internode-derived nodules of Humulus lupulus var. Nugget. Plant Science. 2001;160(5):933–941. doi: 10.1016/s0168-9452(01)00334-x. [DOI] [PubMed] [Google Scholar]
- 83.Mori IC, Schroeder JI. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology. 2004;135(2):702–708. doi: 10.1104/pp.104.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liszkay A, Van Der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2H·−, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology. 2004;136(2):3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: an old new master regulator for plant signaling. Current Opinion in Plant Biology. 2004;7(5):527–536. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Englbrecht CC, Schoof H, Böhm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics. 2004;5, article 39 doi: 10.1186/1471-2164-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mukhopadhyay A, Vij S, Tyagi AK. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):6309–6314. doi: 10.1073/pnas.0401572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu X, Li F, Zhang C, Liu C, Zhang X. Differential gene expression of cotton cultivar CCRI24 during somatic embryogenesis. Journal of Plant Physiology. 2009;166(12):1275–1283. doi: 10.1016/j.jplph.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Tahir M, Law DA, Stasolla C. Molecular characterization of PgAGO, a novel conifer gene of the ARGONAUTE family expressed in apical cells and required for somatic embryo development in spruce. Tree Physiology. 2006;26(10):1257–1270. doi: 10.1093/treephys/26.10.1257. [DOI] [PubMed] [Google Scholar]
- 90.Hecht V, Vielle-Calzada J-P, Hartog MV, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiology. 2001;127(3):803–816. [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124(10):2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- 92.Thomas C, Meyer D, Himber C, Steinmetz A. Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiology and Biochemistry. 2004;42(1):35–42. doi: 10.1016/j.plaphy.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222(1):107–117. doi: 10.1007/s00425-005-1534-4. [DOI] [PubMed] [Google Scholar]
- 94.Schellenbaum P, Jacques A, Maillot P, et al. Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.) Plant Cell Reports. 2008;27(12):1799–1809. doi: 10.1007/s00299-008-0588-8. [DOI] [PubMed] [Google Scholar]
- 95.Nolan KE, Kurdyukov S, Rose RJ. Expression of the SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. Journal of Experimental Botany. 2009;60(6):1759–1771. doi: 10.1093/jxb/erp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng F, Zhang X, Cheng L, et al. A draft gene regulatory network for cellular totipotency reprogramming during plant somatic embryogenesis. Genomics. 2007;90(5):620–628. doi: 10.1016/j.ygeno.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Shah K, Russinova E, Gadella TWJ, Jr., Willemse J, de Vries SC. The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes and Development. 2002;16(13):1707–1720. doi: 10.1101/gad.220402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Becraft PW. Receptor kinase signaling in plant development. Annual Review of Cell and Developmental Biology. 2002;18:163–192. doi: 10.1146/annurev.cellbio.18.012502.083431. [DOI] [PubMed] [Google Scholar]
- 99.Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18(3):626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Decreux A, Messiaen J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant and Cell Physiology. 2005;46(2):268–278. doi: 10.1093/pcp/pci026. [DOI] [PubMed] [Google Scholar]
- 101.Braybrook SA, Harada JJ. LECs go crazy in embryo development. Trends in Plant Science. 2008;13(12):624–630. doi: 10.1016/j.tplants.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 102.Lobstein E, Guyon A, Férault M, Twell D, Pelletier G, Bonhomme S. The putative Arabidopsis homolog of yeast Vps52p is required for pollen tube elongation, localizes to golgi, and might be involved in vesicle trafficking. Plant Physiology. 2004;135(3):1480–1490. doi: 10.1104/pp.103.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dodd AN, Salathia N, Hall A, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309(5734):630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 104.Thines B, Harmon FG. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3257–3262. doi: 10.1073/pnas.0911006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA. ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell. 2001;13(6):1305–1315. doi: 10.1105/tpc.13.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu XL, Covington MF, Fankhauser C, Chory J, Wagner DR. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell. 2001;13(6):1293–1304. doi: 10.1105/tpc.13.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singla B, Tyagi AK, Khurana JP, Khurana P. Analysis of expression profile of selected genes expressed during auxin-induced somatic embryogenesis in leaf base system of wheat (Triticum aestivum) and their possible interactions. Plant Molecular Biology. 2007;65(5):677–692. doi: 10.1007/s11103-007-9234-z. [DOI] [PubMed] [Google Scholar]
- 108.Lohar DP, Sharopova N, Endre G, et al. Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiology. 2006;140(1):221–234. doi: 10.1104/pp.105.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colebatch G, Kloska S, Trevaskis B, Freund S, Altmann T, Udvardi MK. Novel aspects of symbiotic nitrogen fixation uncovered by transcript profiling with cDNA arrays. Molecular Plant-Microbe Interactions. 2002;15(5):411–420. doi: 10.1094/MPMI.2002.15.5.411. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi S, Gresshoff PM, Kinkema M. Molecular analysis of lipoxygenases associated with nodule development in soybean. Molecular Plant-Microbe Interactions. 2008;21(6):843–853. doi: 10.1094/MPMI-21-6-0843. [DOI] [PubMed] [Google Scholar]
- 111.Tirichine L, Sandal N, Madsen LH, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315(5808):104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 112.Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant Journal. 1998;14(1):23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 113.Wasson AP, Pellerone FI, Mathesius U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 2006;18(7):1617–1629. doi: 10.1105/tpc.105.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karami O, Saidi A. The molecular basis for stress-induced acquisition of somatic embryogenesis. Molecular Biology Reports. 2010;37(5):2493–2507. doi: 10.1007/s11033-009-9764-3. [DOI] [PubMed] [Google Scholar]
- 115.Pauly N, Pucciariello C, Mandon K, et al. Reactive oxygen and nitrogen species and glutathione: key players in the legume-Rhizobium symbiosis. Journal of Experimental Botany. 2006;57(8):1769–1776. doi: 10.1093/jxb/erj184. [DOI] [PubMed] [Google Scholar]
- 116.Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biology. 2007;7, article 21 doi: 10.1186/1471-2229-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harrison J, Jamet A, Muglia CI, et al. Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. Journal of Bacteriology. 2005;187(1):168–174. doi: 10.1128/JB.187.1.168-174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puppo A, Groten K, Bastian F, et al. Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytologist. 2005;165(3):683–701. doi: 10.1111/j.1469-8137.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 119.Šamaj J, Baluška F, Pretová A, Volkmann D. Auxin deprivation induces a developmental switch in maize somatic embryogenesis involving redistribution of microtubules and actin filaments from endoplasmic to cortical cytoskeletal arrays. Plant Cell Reports. 2003;21(10):940–945. doi: 10.1007/s00299-003-0611-z. [DOI] [PubMed] [Google Scholar]
- 120.Timmers ACJ. The role of the plant cytoskeleton in the interaction between legumes and rhizobia. Journal of Microscopy. 2008;231(2):247–256. doi: 10.1111/j.1365-2818.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 121.Dijkema C, de Vries S, Booij H, Schaafsma T, van Kammen A. Substrate utilization by suspension cultures and somatic embryos of Daucus carota L. measured by 13C NMR. Plant Physiology. 1988;88:1332–1337. doi: 10.1104/pp.88.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flemetakis E, Efrose RC, Desbrosses G, et al. Induction and spatial organization of polyamine biosynthesis during nodule development in Lotus japonicus. Molecular Plant-Microbe Interactions. 2004;17(12):1283–1293. doi: 10.1094/MPMI.2004.17.12.1283. [DOI] [PubMed] [Google Scholar]
- 123.Nakagawa T, Kawaguchi M. Shoot-applied MeJA suppresses root nodulation in Lotus japonicus. Plant and Cell Physiology. 2006;47(1):176–180. doi: 10.1093/pcp/pci222. [DOI] [PubMed] [Google Scholar]
- 124.Sun J, Cardoza V, Mitchell DM, Bright L, Oldroyd G, Harris JM. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant Journal. 2006;46(6):961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 125.Bueno P, Soto MJ, Rodríguez-Rosales MP, Sanjuan J, Olivares J, Donaire JP. Time-course of lipoxygenase, antioxidant enzyme activities and H2O2 accumulation during the early stages of Rhizobium-legume symbiosis. New Phytologist. 2001;152(1):91–96. doi: 10.1046/j.0028-646x.2001.00246.x. [DOI] [PubMed] [Google Scholar]