Synopsis
The Slc30a8 gene encodes the islet-specific zinc transporter ZnT-8, which provides zinc for insulin-hexamer formation. Polymorphic variants in amino acid 325 of human ZnT-8 are associated with altered susceptibility to type 2 diabetes and ZnT-8 autoantibody epitope specificity changes in type 1 diabetes. To assess the physiological importance of ZnT-8, mice carrying a Slc30a8 exon 3 deletion were analyzed histologically and phenotyped for energy metabolism and pancreatic hormone secretion. No gross anatomical or behavioral changes or differences in body weight were observed between wild type and ZnT-8 −/− mice and ZnT-8 −/− mouse islets were indistinguishable from wild type in terms of their numbers, size and cellular composition. However, total zinc content was markedly reduced in ZnT-8 −/− mouse islets, as evaluated both by Timm’s histochemical staining of pancreatic sections and direct measurements in isolated islets. Blood glucose levels were unchanged in 16 week old, 6 hr fasted animals of either gender, however, plasma insulin concentrations were reduced in both female (~31%) and male (~47%) ZnT-8 −/− mice. Intraperitoneal glucose tolerance tests demonstrated no impairment in glucose clearance in male ZnT-8 −/− mice but glucose-stimulated insulin secretion from isolated islets was reduced ~33% relative to wild type littermates. In summary, Slc30a8 gene deletion is accompanied by a modest impairment in insulin secretion without major alterations in glucose metabolism.
Keywords: Autoantigen, Glucose, Zinc Transporter, Insulin, Islet, Mouse
Introduction
Zinc homeostasis is maintained by two types of proteins, namely metallothioneins and zinc transporters [1], the former being involved in the intracellular trafficking and storage of zinc. There are two classes of zinc transporters, the SCL39 (Zip) family that controls the cellular uptake of zinc and the SLC30 (ZnT) family that controls zinc efflux into the extracellular matrix and intracellular vesicles [1]. In mammalian cells the ZnT family is comprised of ten members that share a similar six transmembrane domain structure with a histidine-rich loop located between helices IV and V, with the exception of ZnT-6 which contains a serine rich loop and ZnT-10 which contains a basic amino-acid rich loop [1].
Chimienti et al. [2] had suggested that expression of the Slc30a8 gene, which encodes the zinc transporter ZnT-8, is restricted to pancreatic islets, specifically insulin secreting beta cells, but recent data suggest that it is also expressed in glucagon secreting alpha cells [3]. Recent genome wide association (GWA) studies have shown that sequence variations in SLC30A8 are linked to an increased susceptibility to the development of type 2 diabetes [4–7]. ZnT-8 [8] has also recently been identified as an autoantigen in type 1 diabetes in humans. Interestingly, the same polymorphic variant in amino acid 325 of human ZnT-8 that is associated with altered susceptibility to type 2 diabetes also affects ZnT-8 autoantibody epitope specificity in type 1 diabetes [9]. Within beta cells ZnT-8 localizes to insulin secretory granules [10] and because insulin is stored as a hexamer bound to two zinc ions, it has been proposed that ZnT-8 is important for providing zinc to allow for the proper maturation, storage and secretion of insulin [11]. In this study we examine the effect of a global Slc30a8 null mutation in vivo, a mouse model that is directly relevant to type 2 diabetes susceptibility in humans. The results are consistent with a role for ZnT-8 in islet function though surprisingly not in whole body glucose metabolism.
Materials and Methods
For details on the generation of the Slc30a8 targeting vector, generation and PCR genotyping of Slc30a8 knockout mice and the analysis of islet number, size and cellular composition in Slc30a8 knockout mice please see Supplemental Data.
Animal care
The animal housing and surgical facilities used for the mice in these studies meet the American Association for the Accreditation of Laboratory Animal Care standards. All animal protocols were approved by the Vanderbilt University Medical Center Animal Care and Use Committee. Mice were maintained on standard rodent chow (LabDiet 5001; 23% protein and 4.5% fat; PMI Nutrition International) with food and water provided ad libitum.
Breeding strategy for Slc30a8 knockout mice
F1 chimeric (129SvEvBrd x C57BL/6J) mice were interbred to generate F2 wild type, heterozygous and homozygous knockout mice. The F2 heterozygous mice were then bred with F1 hybrid (C57BL/6J X 129SvEvBrd) mice. Two male and two female heterozygous mice from this breeding, along with their offspring, were then interbred to generate a mouse colony used in the phenotypic characterization of the effect of the Slc30a8 null mutation.
Immunohistochemical staining
Pancreas tissue was fixed for 1 hr in 4 % paraformaldehyde in PBS and embedded for paraffin sectioning (8 μm). Primary antisera to insulin (Guinea pig 1:100, Dako, Denmark), glucagon (mouse 1:100, Sigma, St Louis, MO), somatostatin (rat 1:100 Abcam, Cambridge, MA) were combined with a rabbit polyclonal antibody raised to a 102 amino acid COOH terminal human ZnT-8 peptide (amino acids 268-369) (1:500) and detected with species specific secondary antibodies conjugated to Cy2, Cy3, Cy5 and AMCA (Jackson Laboratories).
Timm’s staining analyses in Slc30a8 knockout mice
The determination of zinc content in wild type and Slc30a8 knockout mouse pancreas was based on further modification of the revised Timm’s protocol described by Danscher et al. [12]. Briefly, pancreatic tissue sections (8 μm) (frozen and paraffin) fixed in 4% paraformaldehyde were placed on glass slides and immersed in 0.1% sodium sulfide in 0.15 M Sorenson Buffer (pH 7.4) for 1 hr in glass jars inside a chemical fume hood. The slides were briefly rinsed in PBS and immersed in autometallography (AMG) developer (pH 3.8) (60 ml gum arabic, 10 ml sodium citrate (25.5 g citric acid monohydrate + 23.5 g sodium citrate dihydrate in 100 mls de-ionized water), 15 ml reducing agent (0.056% hydroquinone in de-ionised water at 40°C) and 15 ml of solution containing silver ions (0.008% silver lactate in de-ionized water at 40°C) added just before use. All glassware used for AMG development was rinsed in Farmer’s solution (9 parts Sodium thiosulphate 10%, 1 part Potassium Ferricyanide 10%) and warm water. AMG development was carried out at room temperature, in the dark and with gentle shaking. The reaction was stopped after 45 min with 5% sodium thiosulfate solution for 10 min. The slides were rinsed in warm water several times, counterstained with hematoxylin and eosin and permanently mounted.
Measurement of islet zinc content
Freshly isolated islets from wild type and Slc30a8 knockout mice were washed in Ca2+ free Hanks Balanced Salt Solution and frozen down at −80°C in 20 islet aliquots. Islet pellets were lysed by re-suspension in 1 ml lysis buffer (1% TritonX-100 in 10 mM Tris HCl, pH 7.4). Zn2+ concentration in the lysate was measured using the Zn2+ sensitive fluorescent dye FluoZin-3 (Invitrogen). In the presence of 1.181 μM FluoZin-3 the fluorescent signal at the emission peak (516nm) was measured in the total sample lysate using a fluorometer (PTI Instruments). The fluorescent signal was compared with a standard curve generated from serial dilutions of ZnSO4 in lysis buffer to obtain the lysate Zn2+ concentration and thus Zn2+ content per islet. As a normalization factor, the protein content per islet was measured in the total sample lysate using the BCA protein assay (Pierce). To minimize contaminating Zn2+, all solutions were made in double distilled H2O (18.2MΩ), avoiding the use of any glassware. Blank samples were also prepared during the islet isolation to quantify any additional Zn2+ contamination.
Phenotypic analysis of Slc30a8 −/− mice
Animals were fasted for 5 hours, weighed and then one hour later anesthetized using isoflurane before collection of blood samples (~200 μl) from the retro-orbital venous plexus. Whole blood glucose concentrations were determined using an Accu-Check Advantage monitor (Roche). EDTA (5 μl; 0.5 mol/l) was then added before centrifugation to isolate plasma. Trasylol (aprotinin; 5 μl; Bayer Health Care) was added to the plasma to prevent proteolysis of glucagon. Cholesterol was assayed using the Cholesterol Reagent Kit (Raichem) whereas triacylglycerol and glycerol were assayed using the Serum Triglyceride Determination Kit (Sigma). Insulin and glucagon levels were quantitated using radioimmunoassays by the Vanderbilt Diabetes Center Hormone Assay Core.
Intraperitoneal glucose tolerance tests on overnight fasted conscious mice were performed as previously described [13].
Islet isolation and glucose-stimulated insulin secretion (GSIS) assays
Islets were isolated from ~5 month old male mice as described [14]. After isolation, islets were rinsed in three 12 mL changes of RPMI-1640 media containing 10% FBS, 100 IU/ml penicillin, 100 _g/ml streptomycin, and 11 mM glucose and then cultured in 10 cm non-treated plates overnight at 37°C. The next day islets were transferred into medium with 5 mM glucose and allowed to equilibrate for one hour at 37°C. Following the equilibration period, 50 islet equivalents (IEQs) were incubated in 5 mL of RPMI-1640 media with 5 or 11 mM glucose for 30 min at 37°C. At the end of the static incubations islets were collected into 1.5-mL tubes, washed three times with 1 mL of 1X PBS and insulin was extracted in 0.2 mL of acid alcohol for 48 h at 4°C. The media from the static incubations were harvested into 15 mL conical tubes and centrifuged at 2000 rpm. Islet insulin extracts and static incubation media were stored at −80°C until assayed for insulin by radioimmunoassay.
Statistical analyses
Data were analyzed using a Student’s t-test: two sample assuming equal variance. The level of significance was as indicated (two-sided test).
Results
Biochemical characterization of ZnT-8 −/− mice
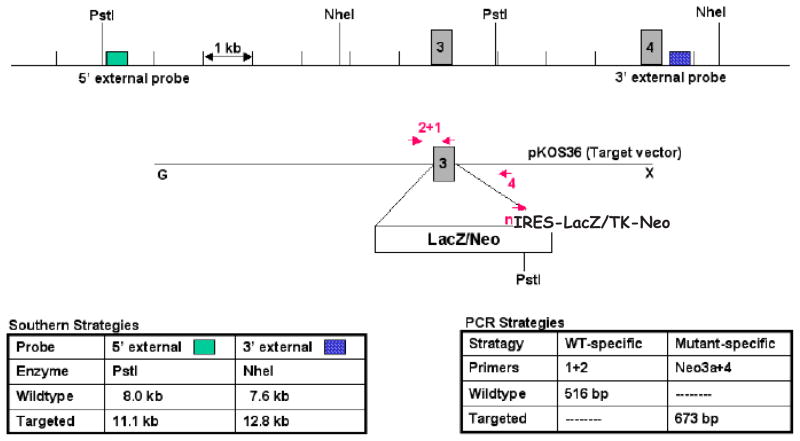
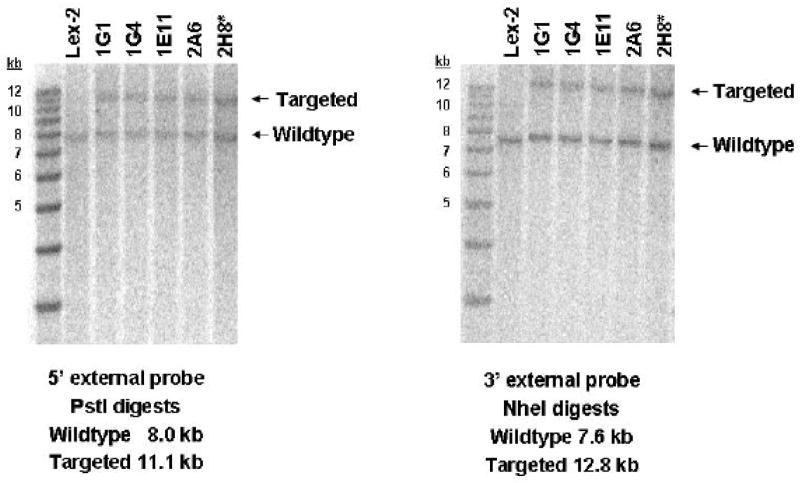
The human and mouse ZnT-8 genes contain 8 exons (Ref. [2] and data not shown). A modified mouse Slc30a8 allele, in which 135/147 bp of exon 3 and the first 10 bp of intron 3 were replaced by a LacZ/Neo cassette, was generated by homologous recombination in 129/SvEvBrd (Lex-1) ES cells (Fig. 1A). Deletion of exon 3 disrupts two putative ZnT-8 transmembrane domains [2]. Correct gene targeting was confirmed by Southern blot (Fig. 1B) and PCR (data not shown) analysis prior to injection of ES cells into C57BL/6 (albino) blastocysts and subsequent generation of ZnT-8 −/+ mice on a mixed 129/SvEvBrd X C57BL/6 background.
Figure 1. Generation and biochemical characterization of ZnT-8 −/− mice.
Panel A: Strategy used to generate ZnT-8 −/− mice by homologous recombination in ES cells. A schematic representation of the wild type murine Slc30a8 locus and the targeting construct are shown. Exon 3 was replaced with a cassette containing an IRES, the LacZ gene and a TK-neomycin selectable marker. Correctly targeted clones were identified by Southern blotting using the indicated probes and confirmed by PCR using the indicated primers. The primers represented sequences in exon 3 (primer 1), intron 2 (primer 2), intron 3 (primer 4) and the Neo gene (Neo3a primer).
Panel B: Southern blot analysis of the Slc30a8 locus using genomic DNA extracted from the indicated targeted ES cell lines, or wild type ES cell genomic DNA, designated Lex-2, as a control, using 5′ and 3′ diagnostic probes (Panel A). The sizes of the wild type locus, targeted allele and DNA markers are indicated. Clone 2H8 was used to achieve germline transmission.
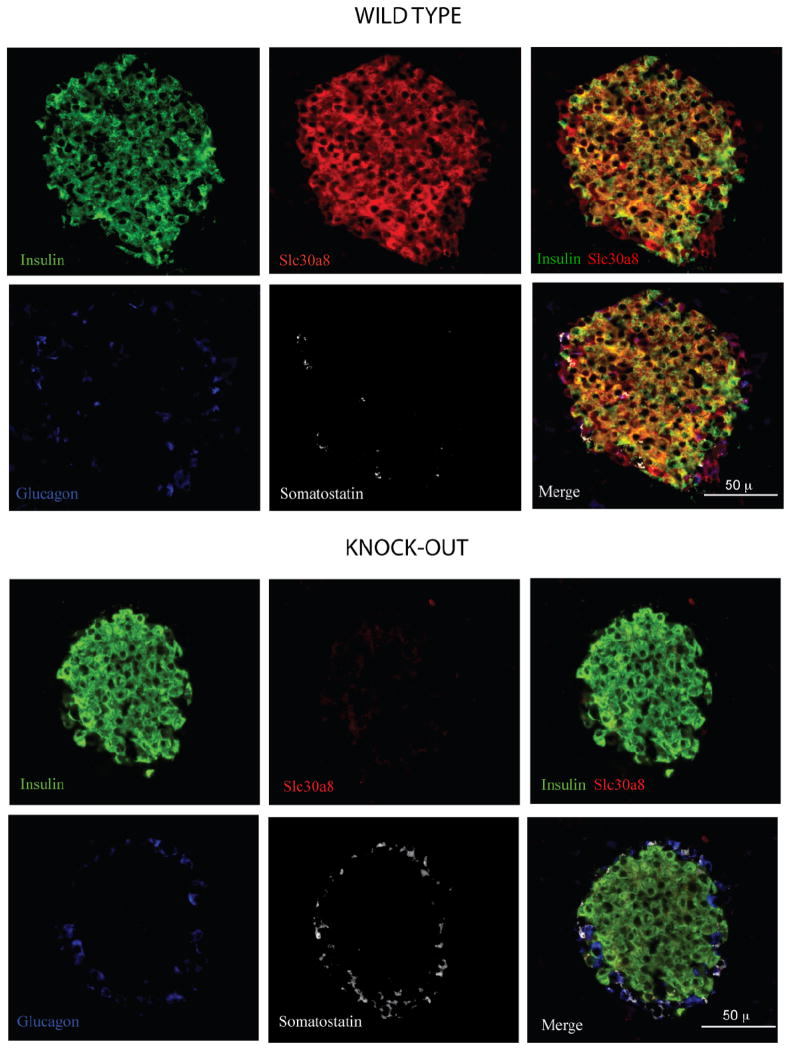
Panel C: Immunohistochemical staining of wild type and ZnT-8 −/− mouse pancreas with antisera raised to insulin, glucagon, somatostatin and ZnT-8 was performed as described in Research Design and Methods. Representative pictures (200 × magnifications) are shown. WT, wild type; KO, knockout.
To confirm that the targeting strategy had abolished ZnT-8 expression immunohistochemical staining was performed on pancreas sections prepared from a ZnT-8 −/− mouse and a wild type littermate. Figure 1C shows that ZnT-8 was detected in both alpha and beta cells in wild type but not ZnT-8 knockout mouse islets.
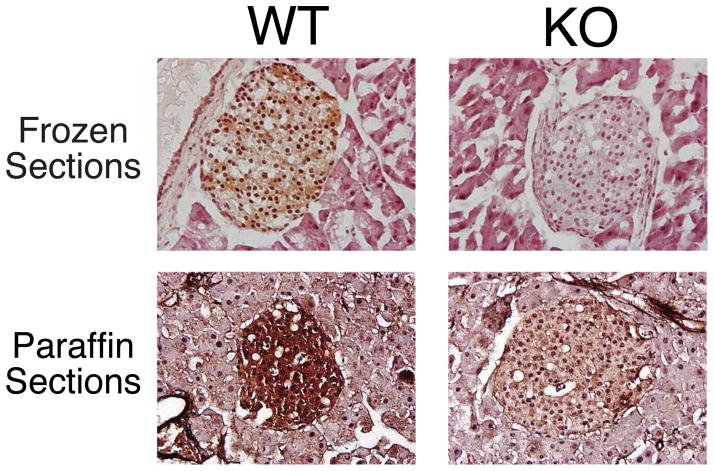
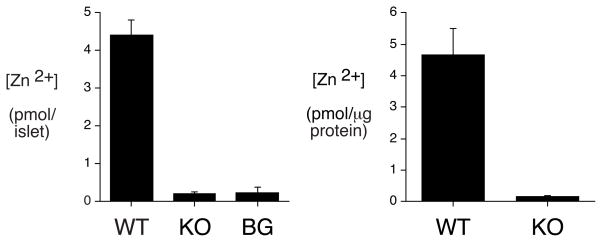
The size and number of islets in ZnT-8 −/− animals were indistinguishable from wild type littermates as were the relative numbers of and alpha and beta cells (Supplemental Fig. 1). Histological analysis of zinc content on frozen pancreatic sections using a modified Timm’s staining procedure that involves silver enhancement of metal sulphide precipitation showed that, in wild type mouse pancreas, islets contained abundant zinc relative to the exocrine tissue (Fig. 2A). This contrasted with ZnT-8 −/− mouse pancreas in which no difference was observed in Timm’s staining between islets and exocrine tissue, though gross islet morphology was preserved (Fig. 2A). These results are consistent with analyses of zinc content in isolated islets using an assay that detects free or loosely bound zinc (Fig. 2B). Figure 2B shows that zinc content was markedly reduced in islets isolated Slc30a8 knockout mice relative to those isolated from wild type mice. The concentration of zinc detected in wild type islets was similar to that previously reported in the islet-derived INS1 cell line [10].
Figure 2. Analysis of islet zinc content in ZnT-8 −/− mice.
Panel A: A modified Timm’s staining protocol was used to assess zinc content in both frozen and paraffin pancreas sections prepared from male wild type (WT) and ZnT-8 knockout (KO) mice as described in Research Design and Methods. Representative pictures (100 × magnifications) are shown.
Panel B: Zinc content in isolated islets was determined as described in Material and Methods. Results represent the mean ± S.E.M. (n= 12). *, p < 0.05 versus WT. BG, background.
Phenotypic characterization of ZnT-8 −/− mice
Genotype analysis of 383 three week old pups generated by cross breeding heterozygous ZnT-8 −/+ mice demonstrated that 83 mice were ZnT-8 +/+, 203 were ZnT-8 −/+, and 97 were ZnT-8 −/−, a distribution close to the expected pattern for Mendelian inheritance. The ratio of male to female mice was 206: 177. Cross breeding experiments revealed that both male and female homozygous ZnT-8 −/− mice are fertile.
The activity and behavior of ZnT-8 −/− mice were indistinguishable from their wild type and heterozygous littermates at all ages, from birth up to one year in age. No gross anatomical changes were observed either externally or to major internal organs and no differences were seen in the weights or lengths of ZnT-8 −/− and wild type mice (Table 1).
Table 1. Phenotypic characterization of ZnT-8 knockout mice.
At 16 weeks of age mice were fasted for 5 hours and then weighed. One hour later mice were anesthetized, their length was measured and blood isolated. Blood and plasma cholesterol, triacylglycerol, glycerol, insulin and glucagon levels were determined as described in Research Design and Methods. Results represent mean data ± S.E.M. obtained from the indicated number of animals in parentheses.
| Gender & Genotype | Weight (g) | Length (mm) | Glucose (mg/dl) | Cholesterol (mg/dl) | Triglyceride (mg/dl) | Glycerol (mg/dl) | Insulin (ng/ml) | Glucagon (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| Female WT | 23.8 ± 0.4 (29) | 98.8 ± 0.5 (28) | 110.1 ± 3.9 (29) | 75.1 ± 4.0 (26) | 49.2 ± 2.5 (27) | 2.5 ± 0.1 (27) | 0.45 ± 0.05 (19) | 70.5 ± 5.6 (23) |
| Female −/+ | 23.3 ± 0.3 (67) | 98.7 ± 0.3 (65) | 110.0 ± 1.8 (66) | 87.8 ± 2.4 (64) * | 49.5 ± 1.5 (62) | 2.6 ± 0.1 (63) | 0.34 ± 0.03 (38) | 71.8 ± 3.4 (56) |
| Female K/O | 23.5 ± 0.4 (32) | 98.8 ± 0.4 (31) | 116.9 ± 3.7 (32) | 82.5 ± 2.8 (30) | 46.1 ± 1.7 (30) | 2.6 ± 0.1 (30) | 0.31 ± 0.05 (20) * | 74.2 ± 6.3 (26) |
| Male WT | 32.4 ± 0.5 (35) | 105.5 ± 0.4 (34) | 135.7 ± 3.9 (35) | 100.7 ± 4.4 (34) | 68.4 ± 2.7 (32) | 2.5 ± 0.1 (33) | 1.50 ± 0.23 (12) | 63.2 ± 5.6 (29) |
| Male −/+ | 32.4 ± 0.4 (63) | 104.7 ± 0.3 (60) | 135.7 ± 3.0 (63) | 103.6 ± 3.2 (58) | 73.2 ± 2.0 (58) | 2.7 ± 0.1 (59) | 1.07 ± 0.22 (15) | 66.4 ± 3.5 (54) * |
| Male K/O | 31.6 ± 0.7 (34) | 105.3 ± 0.6 (32) | 137.2 ± 3.4 (34) | 111.7 ± 3.6 (33) | 70.6 ± 2.9 (32) | 2.5 ± 0.1 (31) | 0.79 ± 0.11 (16) * | 52.0 ± 4.7 (26) |
1. Insulin: *M WT vs. M KO, p<0.01; *F WT v KO, p=0.05
2. Glucagon: *M Het vs. M KO p<0.05
3. Cholesterol: *F WT vs. F Het, p<0.01
Table 1 summarizes changes in metabolic parameters in these animals assayed at 16 weeks of age following a 6 hour fast. No marked changes in plasma cholesterol, triglyceride or glycerol were observed in either male or female ZnT-8 −/− mice relative to wild type animals (Table 1). Blood glucose and glucagon concentrations were also unchanged in both male and female ZnT-8 −/− mice relative to wild type animals, however, a statistically significant difference in plasma insulin concentrations was observed (Table 1). This result suggests that while the absence of ZnT-8 might affect islet function, it has a limited effect on whole body glucose metabolism. In addition, since a statistically significant difference in plasma insulin concentrations was not observed between male or female ZnT-8 +/− mice relative to wild type animals, this suggests that loss of a single Slc30a8 allele is insufficient to affect islet function (Table 1). ZnT-8 −/− mice showed gender-related variation in the majority of these metabolic parameters that were in the same direction and of similar magnitude to the gender-related differences in wild type mice. Thus, in males versus females, insulin, triacylglycerols, cholesterol and glucose were all higher whereas glucagon was lower (Table 1).
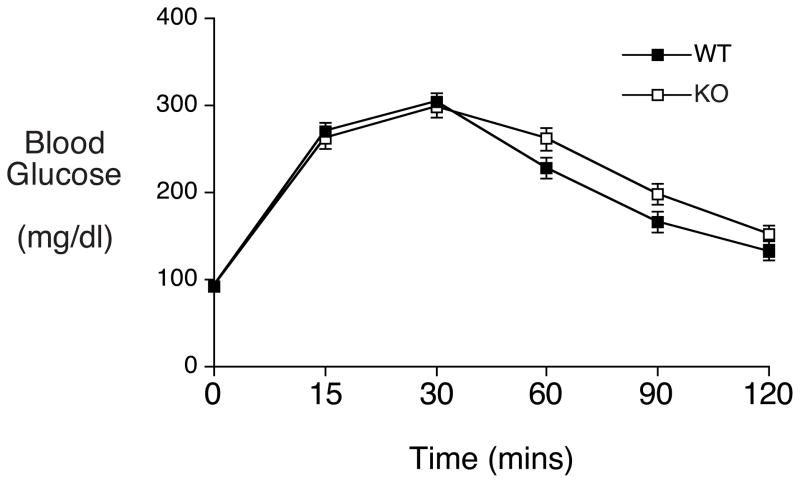
Since some metabolic disturbances only become readily apparent under stimulatory rather than basal conditions intraperitoneal glucose tolerance tests were used to provide a measure of dynamic islet function in vivo. Following glucose injection (2 g/kg) blood glucose was assessed over a 120 minute period (Fig. 3). The data show no impairment in glucose clearance between wild type and ZnT-8 −/− mice (Fig. 3). This result again suggests that while the absence of ZnT-8 might affect islet function it has a limited impact on whole body glucose metabolism, at least under the conditions examined.
Figure 3. Analysis of glucose tolerance in ZnT-8 −/− mice in vivo.
Intraperitoneal glucose tolerance tests were performed on overnight fasted conscious wild type (closed symbols) and ZnT-8 −/− (open symbols) male mice as described in Materials and Methods. Results show the mean glucose concentrations ± S.E.M. in wild type (n= 30; mean age 20 weeks) and ZnT-8 −/− (n=36; mean age 20 weeks) animals.
Insulin secretion from ZnT-8 −/− mouse islets
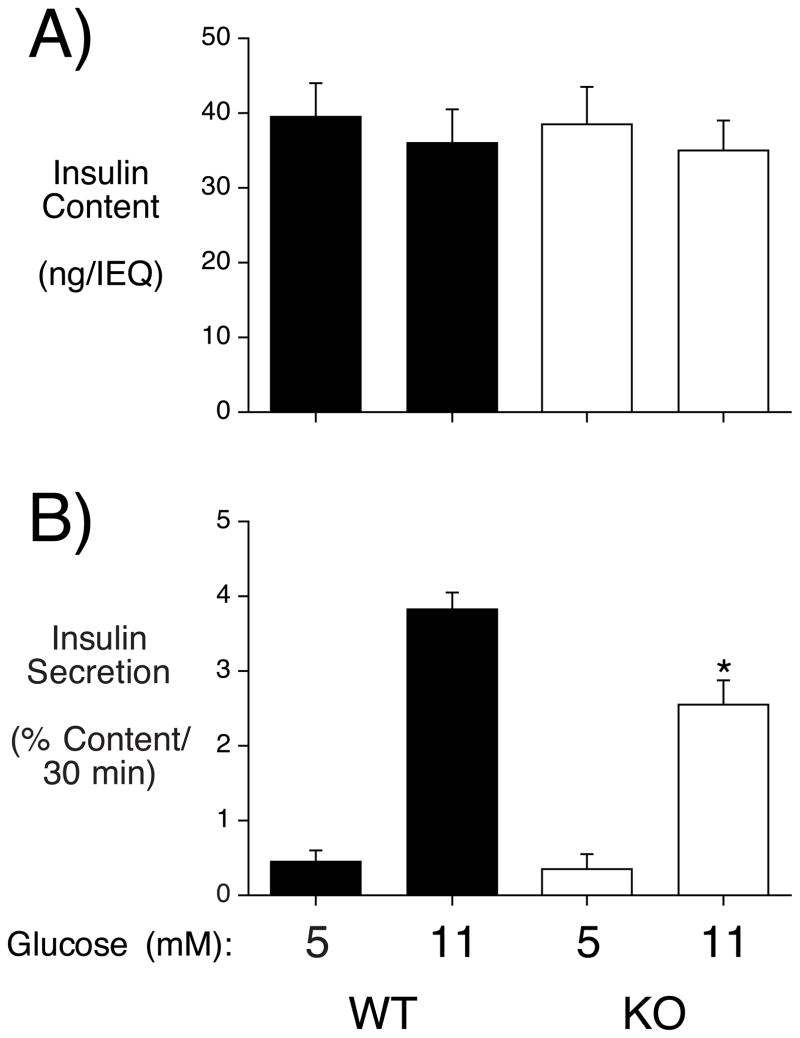
To directly assess the impact of ZnT-8 on islet function, GSIS was compared in islets isolated from male wild type and ZnT-8 −/− mice in static incubations. Figure 4A shows that insulin content did not differ between wild type and ZnT-8 −/− mouse islets whereas Figure 4B shows that GSIS from ZnT-8 −/− mouse islets was reduced ~33% relative to that from wild type mouse islets.
Figure 4. Analysis of insulin content and GSIS in isolated ZnT-8 −/− mouse islets.
Islets were isolated from wild type (WT) and ZnT-8 knockout (KO) mice and then insulin content (Panel A) and GSIS (Panel B) were assayed as described in Materials and Methods. Results show the mean data ± S.E.M. from 3-4 islet preparations. *, p < 0.05 versus WT 11 mM glucose.
Discussion
In this study we examine the effect of a global Slc30a8 null mutation in vivo, a mouse model that is directly relevant to type 2 diabetes susceptibility in humans. The study addresses the hypothesis, based on GWA data, that changes in the activity or stability of the ZnT-8 protein may result in islet dysfunction, which contributes to the pathogenesis of type 2 diabetes. The data indicate that deletion of the Slc30a8 gene results in a mild metabolic phenotype on a mixed 129SvEvBrd X C57BL/6 background. Plasma insulin is reduced in both male and female ZnT-8 −/− mice following a 6 hr fast (Table 1). Consistent with this observation, GSIS from isolated islets is impaired (Fig. 4) and islet zinc content is markedly reduced (Fig. 2), though islet size, number and cellular composition are unaffected (Supplemental Fig. 1). These observations in ZnT-8 −/− mice are consistent with the demonstration that overexpression of ZnT-8 in INS-1 cells has the opposite effects, stimulating zinc accumulation and GSIS [10]. Although the loss of ZnT-8 function only has a mild effect it may be enough to account for the small contribution of Slc30a8 mutations to type 2 diabetes, as reflected in the odds ratio of 1.12 [4–7]. Future studies will be designed to examine whether the absence of ZnT-8 affects glucose metabolism under conditions more favorable for the development of glucose intolerance, such as following high fat feeding or in older animals.
While the results indicate that ZnT-8 is important for normal islet function surprisingly whole body glucose metabolism appears unaltered based on an assessment of fasting glucose levels (Table 1) and glucose tolerance tests (Fig. 3). These observations suggest that ZnT-8 is not necessary for glucose homeostasis, at least under the conditions examined. There appear to be two possible explanations for the decrease in plasma insulin without a concomitant increase in blood glucose (Table 1). There is a statistically significant decrease in plasma glucagon (~20%) between male ZnT-8 +/− and ZnT-8 −/− mice, though not between ZnT-8 +/+ and ZnT-8 −/− mice (Table 1). If the latter simply reflects a lack of power to detect a small change in plasma glucagon, the former would imply that glucagon secretion is also impaired in male ZnT-8 −/− mice. In this event an offsetting decrease in both insulin and glucagon secretion could result in normal blood glucose. Indeed, the insulin:glucagon ratios in individual animals were not statistically different between male wild type and ZnT-8 −/− mice (data not shown). Future experiments studying glucagon secretion from isolated islets will address this possibility. In contrast, in female mice there are no statistically significant differences in glucagon levels between groups (Table 1). This suggests that there may be differences in insulin sensitivity between female wild type and ZnT-8 −/− mice. This would be consistent with the results of post-hoc QUICKI calculations [15] suggesting a statistically significant difference in insulin sensitivity between female wild type and ZnT-8 −/− mice (data not shown). Such a difference could have arisen as an adaptive change during development to compensate for low plasma insulin. Alternatively, a difference in insulin sensitivity could arise if ZnT-8 were expressed in others tissues, specifically ones which directly or indirectly modulate insulin-dependent glucose disposal. Interestingly, Murgia et al. [16] have recently demonstrated that ZnT-8 is expressed at low levels in tissues other than islets. Future experiments will directly compare insulin sensitivity in female wild type and ZnT-8 −/− mice using hyperinsulinemic clamps. Finally, zinc is required not only for stabilizing the insulin crystal within the insulin storage granule but may also be essential for the conversion of proinsulin to insulin [17]. Increased circulating proinsulin is a feature of early type 2 diabetes and impaired glucose tolerance [18, 19] and in a group genetically at risk of developing type 2 diabetes, the SLC30A8 allele was associated with reduced proinsulin to insulin conversion, though not insulin secretion [20]. Since proinsulin has only ~3% of the potency of insulin [21] if proinsulin secretion was markedly increased in the female ZnT-8 knockout mice this could also explain the reduced plasma insulin levels associated with unchanged blood glucose levels. Unfortunately, commercially available assays for murine proinsulin are unavailable at the current time.
Chimenti et al. [2] had previously reported that ZnT-8 is only expressed in pancreatic islet beta cells but using antiserum raised against a 102 amino acid COOH terminal human ZnT-8 peptide (amino acids 268-369) we found that ZnT-8 is also clearly expressed in alpha cells (Fig. 1C). This observation is consistent with the results of Gyulkhandanyan et al. [3] who examined ZnT-8 expression in dispersed islet cells. The specificity of our antiserum was confirmed by the absence of staining in sections prepared from ZnT-8 −/− mouse pancreas (Fig. 1C). The hypothesis that glucagon secretion may be impaired in male ZnT-8 −/− mice would therefore consistent with the expression of ZnT-8 in alpha cells (Fig. 1C). However, the possibility also exists that glucagon secretion from alpha cells has been indirectly affected by the absence of ZnT-8 in beta cells. Indeed, though the mechanism(s) involved are disputed, zinc release from beta cells inhibits glucagon secretion from alpha cells [3, 22, 23].
The SNP that linked the human SLC30A8 gene to increased type 2 diabetes susceptibility [4–7] is located in the C terminus of ZnT-8 and represents a non-synonymous polymorphism that changes the sequence of amino acid 325 [2]. In theory this ZnT-8 variant could represent either a gain or loss of function but because overexpression of ZnT-8 enhances GSIS [10] and because deletion of the Slc30a8 gene in mice impairs GSIS (Fig. 4) we would predict that this human sequence variant impairs ZnT-8 function. Future experiments will address this hypothesis.
Supplementary Material
Acknowledgments
We thank K. Platt and K. Rufus for assistance with this project. We also thank W. Snead, G. Poffenberger and B. Trivedi for performing insulin and glucagon assays and M. Brissova and A. Golovin for performing islet isolations and GSIS analyses. We would also like to thank C. J. Easley for helpful discussions regarding Zn2+ measurements. Research in the laboratory of R.O’B. was supported by NIH grant DK076027. Research in the laboratory of J. C. H. was supported by the Juvenile Diabetes Association Autoimmunity Prevention Center grant, NIH grant DK076027 and the Barbara Davis Center Diabetes and Endocrinology Research Center (P30 DK57516). Research in the laboratory of O. M. was supported by NIH grant DK064877. Research in the laboratory of D. W. P. was supported by NIH grants DK53434 and GM72048 and the Department of Defence Medical Free-Electron Laser Program. The Vanderbilt Hormone Assay & Analytical Services Core and the Vanderbilt Islet Procurement and Analysis Core are both supported by NIH grant P60 DK20593, to the Vanderbilt Diabetes Research Training Center (VDRTC) and by NIH grant DK59637 to the Vanderbilt Mouse Metabolic Phenotyping Center.
Abbreviations
- GWA
genome wide association
- SNP
single nucleotide polymorphism
- GSIS
glucose-stimulated insulin secretion
- X-gal
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
- AMG
autometallography
- IEQ
islet equivalents
- IRES
internal ribosome entry site
- TK
thymidine kinase
- Neo
neomycin
Footnotes
The authors have no financial interests that would result in a conflict of interest with respect to this work.
References
- 1.Chimienti F, Aouffen M, Favier A, Seve M. Zinc homeostasis-regulating proteins: new drug targets for triggering cell fate. Curr Drug Targets. 2003;4:323–338. doi: 10.2174/1389450033491082. [DOI] [PubMed] [Google Scholar]
- 2.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 3.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283:10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 4.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 6.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, Burton PR, Clayton DG, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Davison D, Easton D, Evans D, Leung HT, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskvina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop DT, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Mathew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop GM, Connell J, Dominiczak A, Braga Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hider SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Ferreira T, Pereira-Gale J, Hallgrimsdottir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Compston A, Ouwehand NJ, Samani MR, Isaacs JD, Morgan AW, Wilson GD, Ardern-Jones A, Berg J, Brady A, Bradshaw N, Brewer C, Brice G, Bullman B, Campbell J, Castle B, Cetnarsryj R, Chapman C, Chu C, Coates N, Cole T, Davidson R, Donaldson A, Dorkins H, Douglas F, Eccles D, Eeles R, Elmslie F, Evans DG, Goff S, Goodman S, Goudie D, Gray J, Greenhalgh L, Gregory H, Hodgson SV, Homfray T, Houlston RS, Izatt L, Jackson L, Jeffers L, Johnson-Roffey V, Kavalier F, Kirk C, Lalloo F, Langman C, Locke I, Longmuir M, Mackay J, Magee A, Mansour S, Miedzybrodzka Z, Miller J, Morrison P, Murday V, Paterson J, Pichert G, Porteous M, Rahman N, Rogers M, Rowe S, Shanley S, Saggar A, Scott G, Side L, Snadden L, Steel M, Thomas M, Thomas S, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 8.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 11.Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 12.Danscher G, Stoltenberg M, Bruhn M, Sondergaard C, Jensen D. Immersion autometallography: histochemical in situ capturing of zinc ions in catalytic zinc-sulfur nanocrystals. J Histochem Cytochem. 2004;52:1619–1625. doi: 10.1369/jhc.4A6371.2004. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20:1853–1866. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
- 14.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 15.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 16.Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis. 2008 doi: 10.1016/j.numecd.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 18.Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D., Jr Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30:698–702. doi: 10.1007/BF00296991. [DOI] [PubMed] [Google Scholar]
- 19.Wareham NJ, Byrne CD, Williams R, Day NE, Hales CN. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care. 1999;22:262–270. doi: 10.2337/diacare.22.2.262. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff K, Machicao F, Haupt A, Schafer SA, Tschritter O, Staiger H, Stefan N, Haring HU, Fritsche A. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 21.Ciaraldi TP, Brady D. Comparisons of insulin and biosynthetic human proinsulin actions in cultured hepatocytes. Kinetics and biologic potencies. Horm Metab Res. 1990;22:283–288. doi: 10.1055/s-2007-1004903. [DOI] [PubMed] [Google Scholar]
- 22.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;56:1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.