Abstract
Malalignment is known to impact the medial-to-lateral load distribution in the tibiofemoral joint. In this longitudinal study, we test the hypothesis that subchondral bone surface areas functionally adapt to the load distribution in malaligned knees.
Alignment (hip-knee-ankle angle) was measured from full limb films in 174 participants with knee osteoarthritis. Coronal MR images were acquired at baseline and 26.6±5.4 months later. The subchondral bone surface area of the weight-bearing tibiofemoral cartilages was segmented, with readers blinded to the order of acquisition. The size of the subchondral bone surface areas was computed after triangulation using proprietary software.
The hip-knee-ankle angle showed a significant correlation with the tibial (r2=0.25, p<0.0001) and femoral (r2=0.07, p<0.001) ratio of medial-to-lateral subchondral bone surface area. In the tibia, the ratio was significantly different between varus (1.28:1), neutral (1.18:1) and valgus (1.13:1) knees (ANOVA; p<0.00001). Similar observations were made in the weight-bearing femur (0.94:1 in neutral, 0.97.1 in varus, 0.91:1 in valgus knees; ANOVA p=0.018). The annualized longitudinal increase in subchondral bone surface area was significant (p<0.05) in the medial tibia (+0.13%), medial femur (+0.26%) and lateral tibia (+0.19%). In the medial femur, the change between baseline and follow-up was significantly different (ANOVA; p=0.020) between neutral, varus and valgus knees, the increase in surface area being significantly greater (p=0.019) in varus than in neutral knees.
Tibiofemoral subchondral bone surface areas are shown to be functionally adapted to the medial-to-lateral load distribution. The longitudinal findings indicate that this adaptational process may continue to take place at advanced age.
Keywords: Functional adaptation, Subchondral Bone, Knee, Alignment, Magnetic Resonance Imaging
Introduction
The ability of tissues to emerge and maintain their structure in accordance with specific environmental requirements has been termed “functional adaptation” [7;9;28;39;46], and the adaptation of musculoskeletal tissues to mechanical loading has been a key topic in mechanobiology research: Physical exercise has been shown to increase bone and muscle mass (i.e. body building), whereas states of inactivity or microgravity have been associated with tissue atrophy [2;27]. In bone, functional adaptation of tissue to mechanics has been termed Wolff’s law [46] and has been characterized mathematically [7], more recently as a cell-mediated process in which osteocytes act as mechanical sensors and orchestrate the function of other (bone forming and bone resorbing) cells through biochemical signalling [20].
Malalignment is known to alter the load distribution between the medial and lateral tibiofemoral (FT) compartment, with greater loads being transferred through the medial FT compartment in neutral and varus knees, and with relatively greater loads through the lateral compartment in valgus knees [1;8;25;31;34;41]. Hurwitz et al. [24] were the first to report a significant relationship of the “dynamic” medio-lateral load distribution in the tibiofemoral joint, as predicted from gait measurement, with bone mineral content measured in the proximal tibia using dual X-ray absorptiometry (DXA). Müller-Gerbl [35] used computed tomography (CT) to study subchondral density in maligned patients and described characteristic density patterns for varus knees, which became more similar to those of normal knees one year after correction osteotomy. Recently, Thorp et al. [42] confirmed that both static markers (knee alignment angle) and dynamic markers of knee loads (knee adduction angular momentum for the entire stance phase) each explained 18% of the variability of proximal tibial bone mineral density (BMD measured with DXA) in subjects with mild and moderate knee OA.
Bone density, however, is only one aspect of adaptation (remodeling), whereas bone is also able to change it’s geometry/form (modeling) [7]. Whether, however, the size of the subchondral bone surface area is phenotypically plastic and able to functionally adapt to joint loading has not been clearly established: Lieberman et al. [30] reported that diaphyseal cross sectional geometry significantly differed between sedentary and exercised sheep of various ages, but found no significant differences in subchondral bone surface areas. Plochocki et al. [37], in contrast, showed the size of the femoral head surface to be significantly larger in growing mice with voluntary access to physical activity than in controls without such access. Investigating directional asymmetry in human skeletons, Plochocki [36] found the subchondral bone surface areas of the right upper limb to be significantly larger than those of the left upper limb. In the human second metacarpal, Lazenby et al. [29] recently also observed the medio-lateral (but not the dorso-palmar) dimensions of the subchondral bone surfaces to be significantly larger on the right hand side, but pointed out that the side-differences in subarticular bone microarchitecture and diaphyseal bone geometry were quantitatively more important.
In a previous study [15], we have measured joint surface areas based on MR images and found triathletes to exhibit larger knee joint surfaces than sedentary subjects. It was concluded that adaptation of subchondral bone surface area may keep stresses in synovial joints within acceptable limits of cartilage tissue tolerance, as higher load are distributed over a wider area. Here we test the hypothesis that the ratio of medial-to-lateral subchondal bone area in the tibia and femur is adapted to alignment and shows significant differences between neutral, varus and valgus knees. Secondly, we test the hypothesis that subchondral bone surface areas increase with aging, but that the increase is significantly impacted also by knee alignment.
Materials and Methods
The participants were from a community-recruited cohort of a natural history study of knee osteoarthritis (MAK −2= Mechanical Factors in Arthritis of the Knee, second cycle). The participants had definite tibiofemoral osteophytes in one or both knees (Kellgren/Lawrence [K/L] grade ≥2, as determined from anterior-posterior weight-bearing radiographs acquired in a semi-flexed position with fluoroscopic control [4]), and a Likert category of at least “a little difficulty” for two or more items in the WOMAC physical function scale. Approval was obtained from the Office for the Protection of Research Subjects-Institutional Review Boards of Northwestern University and Evanston Northwestern Healthcare. Written consent was obtained from all participants.
To assess the hip-knee-ankle angle, a single anterior-posterior radiograph of both legs was obtained using a 51×14 inch graduated grid cassette, to include the full limb of the participants [33]. All radiographs were obtained in the same unit by two trained technicians. The tibial tubercle was used as positioning landmark, since it is adjacent to the knee but not distorted by OA. Participants stood without footwear, with tibial tubercles facing forward. The x-ray beam was centered at the knee at a distance of 2.4 m. Alignment was analyzed as a continuous variable at baseline, by measuring the angle formed by the intersection of the line connecting the centers of the femoral head and intercondylar notch with the line connecting the centers of the surface of the ankle talus and tips of the tibial spines (hip-knee-ankle angle). Knees with a hip-knee-ankle angle of −2° to +2° were classified as neutral, those with >2° as varus, and those with <−2° as valgus.
The participants had MRI of both knees using a commercial knee coil and one of two whole-body scanners, either a 1.5 Tesla (T) Symphony at Northwestern University (Siemens, Erlangen, Germany) or a 3T Genesis Signa Scanner at Evanston Health Care (GE Healthcare Technologies, Waukesha, WI). Baseline and follow-up images for each individual patient were always acquired with the same piece of equipment. The mean [±standard deviation] observation interval was 26.6 [±5.4] months, with a range of 14 to 50 months. For quantitative measurement of the tibiofemoral subchondral bone surface areas, a previously validated [12–14;19] coronal spoiled gradient echo (SPGR) sequences with water excitation (we) were acquired with a slice thickness of 1.5 mm and an in-plane resolution of 0.31 mm (field of view 16cm, 512 × 512 matrix, NEX =1). The repetition time (TR), echo time (TE) and flip angle were 18.6 ms/9.3ms/15° on the 1.5T scanner, and 12.2 ms/5.8 ms/9° on the 3T scanner, respectively. The MR image data were sent to the image analysis center on CD ROM, quality controlled, and converted to a proprietary format (Chondrometrics GmbH, Ainring, Germany).
One knee per participant was studied: When baseline and follow-up data sets of good quality and without artifacts of both knees were available, the dominant knee was included, the dominant leg being determined by asking with which leg the participant would kick a ball. In total, 174 knees were selected (157 dominant, 17 non-dominant knees, 145 right knees, 29 left knees). The 174 participants (76% women, 24% men) were aged 66±11.1 years (mean± standard deviation) and had a BMI of 30.1±5.9 kg/m2. 74 knees showed neutral alignment (range −2° to +2°; mean±SD = 0.2±1.3°), 57 varus malalignment (+6.5±4.1°, maximum=19°), and 43 valgus malalignment (−5.3±2.5°, minimum=−13°).
Segmentation of the subchondral bone surface area was performed manually by twelve readers with formal training in knee cartilage segmentation, using proprietary software (Chondrometrics GmbH, Ainring, Germany). The total subchondral bone surface area (tAB) of the medial tibia (MT), the lateral tibia (LT), the central (weight bearing) medial femoral condyle (cMF) and the central lateral femoral condyle (cLF) were traced (Figures 1,2), including denuded areas, but excluding osteophytes [10]. The weight-bearing region of the femoral condyles was analyzed between the intercondylar notch and 60% of the distance to the posterior end of the femoral condyles [16;17]: because posteriorly the femoral condyles curve upwards, partial volume effect become to high here for the subchondral bone surface interface to be clearly delineated in coronal images [16]. Quality control of all segmentations was performed by one reader (F.E.), reviewing all segmented slices of all data set [13;16]. The segmentations were then used to compute the total area of subchondral bone surface by triangulation (tAB).
FIG 1.
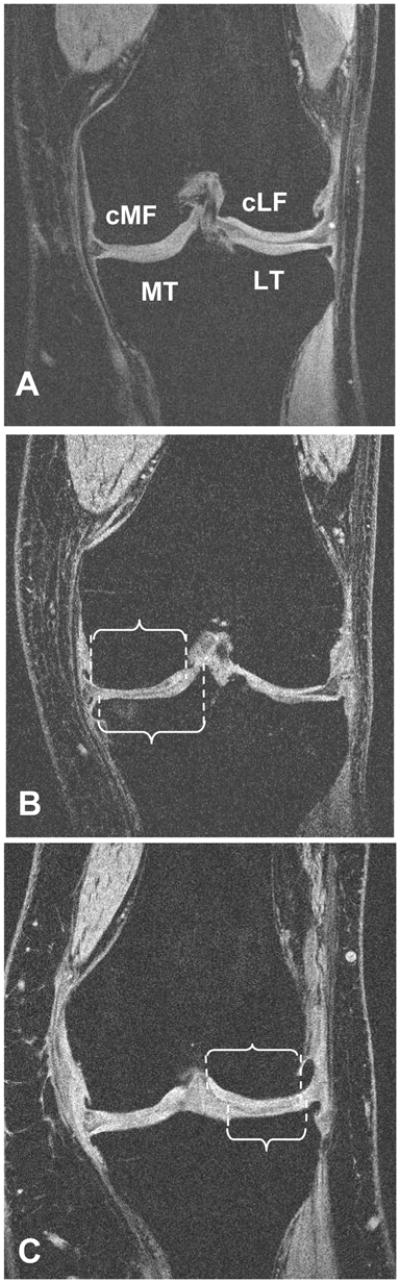
Coronal MR image, acquired with an SPGR sequence with water excitation. The image shows the tibiofemoral cartilage plates: MT= medial tibia, LT= lateral tibia, cMF= medial weight-bearing femur; cLT= lateral weight-bearing femur, and the subchondral bone area that was segmented in these areas
a) Neutrally aligned knee: MT = medial tibia, cMF = central (weight-bearing) medial femur, LT = lateral tibia, cLF = central (weight-bearing) lateral femur
b) Knee with varus malignment: lines and brackets indicate the subchondral bone contour over which the subchondral bone area is measured in the medial femorotibial compartment
c) Knee with valgus malignment: lines and brackets indicate the subchondral bone contour over which the subchondral bone area is measured in the lateral femorotibial compartment
FIG 2.
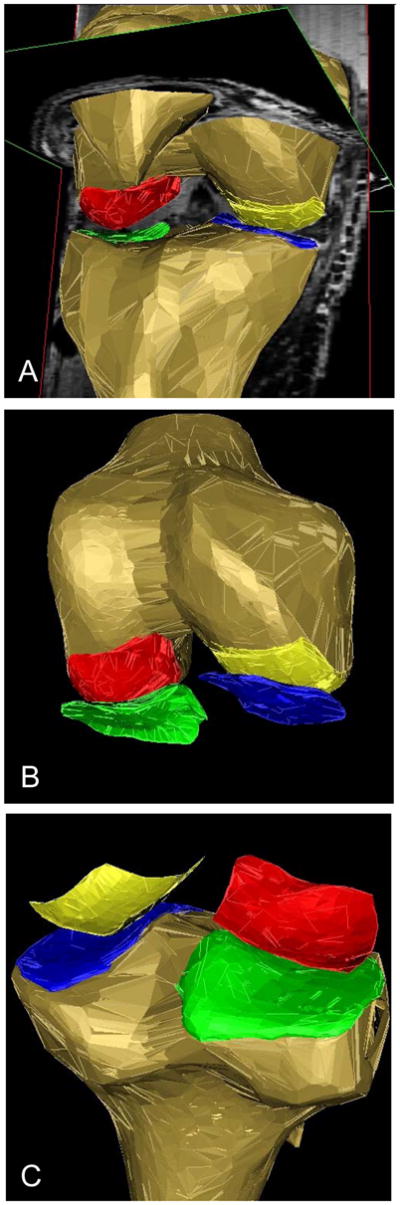
3D reconstruction of the knee with subchondral bone areas (tAB) being shown in color
a) Graph showing a reconstruction of the tibial, patellar and femoral bones (brown color), a coronal and a (secondary) axial MR slice: medial tibia (MT) = blue; central (weight-bearing) medial femur (cMF) = yellow, lateral tibia (LT) = green, central (weight-bearing) lateral femur (cLF) = red
b) Reconstruction of the femoral bone only and of the tAB of the above cartilages
c) Reconstruction of the tibial bone only and of the tAB of the above cartilages
The ratio of medial-to-lateral tibial (MT/LT) and femoral (cMF/cLF) subchondral bone surface area at baseline was computed for each participants, correlated with the hip-knee-ankle angle (as a continuous variable) by determining the coefficient of determination (r2), and then compared between neutral, varus and valgus knees using a one-way ANOVA with knee joint alignment as the “between subject” variable. When the ANOVA indicated a significant effect of alignment, unpaired two-sided t-tests were used to identify between which of the groups significant differences were present, with the number of (parallel) tests being restricted to varus vs. neutral and valgus vs. neutral, respectively. The longitudinal change in subchondral bone surface area from baseline to follow-up was determined in each knee after adjusting individual changes to a 12 month observation period and a paired t-test was used to identify whether the changes between baseline and follow-up were statistically significant. A one-way ANOVA with knee joint alignment as the between-subject independent variable was used to compare changes in MT, LT, cMF and cLF between varus, valgus and neutral knees. Again, when ANOVA indicated a significant effect of alignment, unpaired two-sided t-tests were used to identify between which of the groups (varus vs. neutral, valgus vs. neutral) significant differences were present.
Results
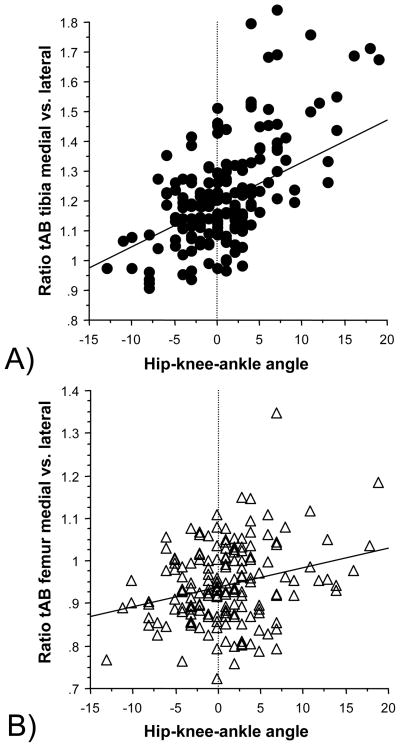
The hip-knee-ankle angle showed a significant correlation with the baseline values of the tibial medial-to-lateral ratio of subchondral bone surface area (r2=0.25, p<0.0001, 95% confidence interval= 0.14 to 0.36). The correlation with the weight-bearing part of the femoral medial-to-lateral bone area was also significant, but somewhat weaker (r2=0.07, p<0.001, 95% confidence interval= 0.01 to 0.16) (Fig. 3).
FIG 3.
Scatter plots showing the correlation between the hip-knee-ankle angle (alignment) and the ratio of the subchondral bone surface area (tAB) between the medial and lateral part of the tibiofemoral joint:
A) Tibia
B) Femur
Negative values for the hip-knee-ankle angle show valgus malalignment, and positive values varus malalignment
The size of the subchondral bone surface areas in the medial and lateral tibia and in the medial and lateral weight-bearing femur for valgus, neutral and varus knees at baseline are shown in Table 1. The ratio between the medial-to-lateral tibial subchondral bone surface area at baseline (1.18±0.12 in neutral 1.28±0.17 in varus and 1.13±0.13 in valgus knees) differed significantly (p<0.00001) between alignment groups (ANOVA). Paired t-tests revealed that the medial-to-lateral tibial ratio was significantly (p=0.00017) higher in varus than in neutral knees, and significantly (p=0.014) lower in valgus than in neutral knees. Similar observations were made in the weight-bearing femur (0.94±0.08 in neutral, 0.97±0.11 in varus, and 0.91±0.07 in valgus knees), with ANOVA revealing significant difference between alignment groups (p=0.018). However, t-tests failed not reveal significant differences in femoral subchondral bone surface ratio between varus and neutral, and valgus and neutral knees, respectively.
Table 1.
Size of the tibiofemoral subchondral bone areas in neutral, varus and valgus knees
| Neutral | Varus | Valgus | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| MT (cm2) | 11.51 | 1.65 | 12.65 | 2.29 | 10.96 | 1.28 |
| LT (cm2) | 9.78 | 1.55 | 10.04 | 2.20 | 9.84 | 1.44 |
| Ratio MT/LT | 1.18 | 0.12 | 1.28 | 0.17 | 1.13 | 0.13 |
| cMF (cm2) | 5.81 | 0.85 | 6.17 | 1.03 | 5.63 | 0.75 |
| cLF (cm2) | 6.21 | 0.92 | 6.46 | 1.24 | 6.17 | 0.76 |
| Ratio cMF/cLF | 0.94 | 0.08 | 0.97 | 0.11 | 0.91 | 0.07 |
SD = standard deviation, MT = Medial tibia, LT = lateral tibia, cMF = weight bearing medial femur, cLF weight bearing lateral femur. The ratio MT/LT and cMF/cLF differed significantly between neutral, varus and valgus knees (ANOVA), MT, LT, cMF and cLF were not tested directly for significant differences between neutral varus and valgus knees, because these are more strongly affected by inter-subject differences in bone size than the MT/LT and cMF/cLF ratios.
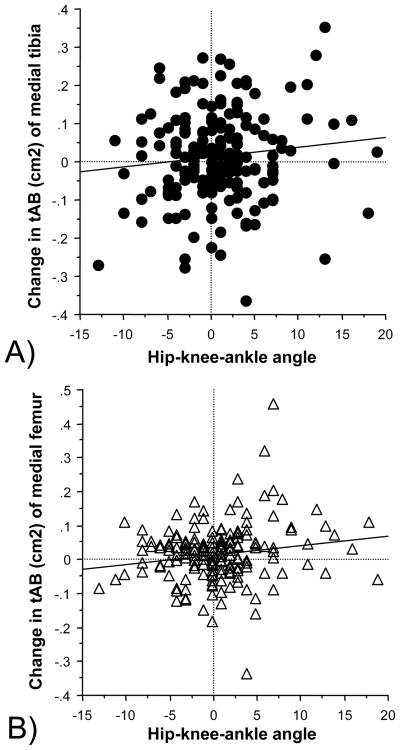
Longitudinally between baseline and follow up, the subchondral bone surface area increased significantly (p<0.05, paired t-test) across all knees in the medial tibia (+0.13±1.03% per annum), medial femur (+0.26±1.47 %) and lateral tibia (+0.19±1.07 %), but only a small (non-significant) increase was observed in the lateral femur (+0.06±1.19%). The annual percent increases in valgus, neutral and varus knees are shown in Table 2. The increase in subchondral bone surface area was significantly correlated with the hip-knee-ankle angle in the medial weight-bearing femur (r2=0.03, p<0.05, 95% confidence interval= 0.0004 to 0.10), but not in the lateral femur ot tibial surfaces (Fig. 4). The one-way ANOVA revealed that alignment (neutral, varus, valgus) significantly affected the change in the medial femoral bone surface areas between baseline and follow up (p=0.020), but not in the lateral femur (p=0.12) or in the tibial bone surfaces. The longitudinal increase in cMF was significantly (p=0.019) greater in varus than in neutral knees (t-test)..
Table 2.
Increase of the size of the tibiofemoral subchondral bone areas (% per annum) in neutral, varus and valgus knees over the observation period
| Neutral | Varus | Valgus | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Change MT | 0.17 | 0.93 | 0.18 | 1.05 | 0.00 | 1.18 |
| Change LT | 0.14 | 1.15 | 0.29 | 0.98 | 0.14 | 1.09 |
| Change Ratio MT/LT | 0.01 | 1.27 | −0.08 | 1.24 | −0.15 | 1.18 |
| Change cMF | 0.06 | 1.13 | 0.67 | 1.87 | 0.03 | 1.12 |
| Change cLF | −0.14 | 0.97 | 0.12 | 1.42 | 0.32 | 1.15 |
| Change Ratio cMF/cLF | 0.23 | 1.37 | 0.49 | 1.93 | −0.27 | 1.67 |
SD = standard deviation, MT = Medial tibia, LT = lateral tibia, cMF = weight bearing medial femur, cLF weight bearing lateral femur. The longitudinal changes in cMF and cLF differed significantly between neutral, varus and valgus knees (ANOVA), but not those in MT and LT.
FIG 4.
Scatter plots showing the correlation between the hip-knee-ankle angle (alignment) and the increase in the subchondral bone surface area (tAB) between baseline and follow up (in cm2 normalized to one year):
A) Medial tibia (MT)
B) Medial weight-bearing femur (cMF)
Negative values for the hip-knee-ankle angle show valgus malalignment and positive values varus malalignment
Discussion
While some previous research suggests that subchondral bone surfaces adapt their size in response to mechanical loading, other studies have suggested that joint morphology is highly canalized at the species level (articular contraint model – [29;30]). We here used a malalignment model to address this question, as it has been established that the ratio of medial-to-lateral load magnitudes varies substantially between neutral, varus and valgus knees and the impact on medial-to-lateral bone surface areas can be investigated in the same knee. The results of our study suggest that subchondral bone size/geometry functionally adapts to the individual load pattern of malaligned knees and thus provides an indication that articular morphology may be subject of functional adaptation and phenotypic plasticity in bones of matured humans.
Strengths of the study include a) that malalignment was determined at baseline from full limb X-rays to determine the accurate mechanical axis (hip-knee-ankle angle) rather than from knee radiographs only, b) that subchondral bone surface area was reconstructed and quantified in 3D, using an MR sequence and image analysis process that has been thoroughly validated in the context of cartilage volume and thickness measurements and measurements of subchondral bone surface areas [6;19]; c) that test-retest reproducibility errors of these measurements have previously been shown to be low [5;18], and d) that adaptational processes of the bone were not only evaluated cross-sectionally but also longitudinally over an average two year observation period.
The study also has several limitations: a) Despite the relatively large number of participants, the sample size was relatively modest in view of the relatively small effect (increase in subchondral bone surface area with time) that was investigated; b) static knee alignment was measured, but not dynamic loading [32]. It is known that static measurements predict about 50% of the variability in knee adduction moments [23], and Thorp et al. [42] found that static and dynamic measurements each explained the same proportion (18%) of the variability of proximal tibial bone mineral density in subjects with knee osteoarthritis; c) the technique used to ascertain femoral bone area based on coronal MR images may not fully capture the expansion occurring in AP direction; d) the study was limited to participants with established symptomatic and radiographic osteoarthritis, and therefore conclusions cannot be readily generalized to healthy joints. During the segmentation process, osteophytes were not included, in order to avoid the subchondral bone surface areas to be affected by osteophyte growth. A previous cross-sectional [26] and a longitudinal study [44] have described an increase in tibial bone area in osteoarthritic patients, the latter reporting relatively large increases (2.2±6.9% and 1.5±4.3% per annum in MT and LT, respectively). A later study by the same group [43] reported an increase of tibial bone area also in healthy women (1.2% per annum in the medial and 0.8% in the lateral tibia), and, in a multi-centre study, we recently found a statistically significant increase of 0.3% of the tibial and femoral subchondral bone surface area over a period of 3 months [11]. This increase did not differ significantly between healthy knees (n=97) and those with symptomatic knee osteoarthritis (n=61). An age-related expansion of bone cross sectional areas in the order of 0.18 to 0.60% per year has also been observed at other skeletal locations, such as the vertebrae, the femoral neck and the femoral shaft in a cohort of 1715 individual aged 67 to 93 years [40]. Similar increases were seen at the distal radius and distal tibia in a cohort of 696 subjects aged 20 to 97 years [38]. In view of these findings, we believe that the increase of subchondral bone surface area over time is not specific to knees with osteoarthritis [44;45] and that osteophytes did not affect the measurement. However, we cannot exclude that uni-compartimental osteoarthritis had an effect on the medial-to-lateral ratio of subchondral bone surface area at baseline and on the ratio of the increase with time, through other mechanisms. To this end, a study in non-arthritic cohort would be necessary.
One prior study has looked at the relationship of alignment, cartilage volume, tibial (but not femoral) bone area, and chondral defects in a cohort of subjects consisting of 256 non-osteoarthritic and 56 arthritic knees [47]. Alignment was determined from knee radiographs (not full limb films) and the tibial bone area from a single axially reconstructed slice of sagittal MRI scans. It is, however, somewhat unclear how the curved medial and lateral subchondral bone surface area can be accurately assessed from a single plane measurement, without including the intercondylar area. The authors reported the baseline lateral tibial bone area to be significantly higher in valgus (12.7cm2) than in normal knees (12.0cm2), but no significant differences were observed between varus and normal knees, or in the medial tibia. The change in medial tibial bone area was significantly lower (−0.2% per annum) in valgus knees than in normal (+0.6%) knees, but the authors concluded that overall there was no statistically significant association between varus/valgus alignment and change in tibial bone area (or cartilage volume loss/or progression of chondral defects). This negative finding persisted after stratifying for osteoarthritic and non-osteoarthritis subjects.
In contrast, we found a highly significant correlation of the medial-to-lateral ratio of the tibial subchondral bone surface area with hip-knee-angle angle and highly significant differences between varus, valgus and neutral knees. In the femur, the correlation was somewhat less strong, but still significant. The cartilage plates generally displayed an increase of the subchondral bone surface area over time, and in the femur the increase was significantly different between varus and valgus knees. These results are exciting, as they suggest that the size of the subchondral bone surface area adapts to mechanical loading, and that this adaptation may persist up to advanced age. Whereas from the perspective of bone fracture, adaptation by increased density provides increased structural strength of bones, adaptation of the subchondral bone surface area may be a more efficient mechanism of protecting the diarthrodial joint from undue stress. The increase in bone area may distribute the load over a wider area and thus reduces mechanical stress acting on the cartilage surface, whereas an increase in subchondral bone density (and stiffness) has no cartilage-protective effect. We have previously reported that the subchondral bone surface area is significantly higher in triathletes compared with physically inactive controls [15] and is more highly correlated to body weight across between a wide range of species than cartilage thickness (Grams, Dissertation, LMU München, Germany). Taken together, these results suggest that knee joint morphology is not constained but phenotypically plastic, and that adaptation of subchondral bone surface area to mechanical loading may be a principle of functional adaptation of synovial joints. There are, obviously, limits to the effectiveness of this process: The size of the medial tibia in varus was 10% higher than in neutral knees, whereas the increase in load is likely much higher than that [1;8;25;31;34;41]. Nevertheless, as malalignment may start with small offsets from the normal hip-knee-ankle angle and may become stronger once the cartilage and meniscus degenerate and the joint space narrows [22], adaptation of subchondral bone surface area may provide initial protection and explain the relatively high number of varus and valgus knees that do not develop osteoarthritis [3;21]. Once osteoarthritis develops, however, one has to take into account that while the subchondral area may still increase, this does not capture the curvature/congruity of the joint surfaces, and with focal remodelling and attrition the resulting contact area and mechanical stress may be negatively affected to still lead to a vicious circle of joint degeneration.
In conclusion, our findings suggest that tibiofemoral subchondral bone surface area functionally adapts to the medial-to-lateral load distribution in the knee. The longitudinal findings indicate that the ability of bone to modify its shape depending on mechanical loading may continue to take place at advanced age. This may be a general principle by which joints keep mechanical stress within the limits of cartilage tolerance.
Acknowledgments
Funding Source: NIH/NIAMS RO1 AR48216, RO1 AR48748, P60 AR48098
We would like to thank Pottumarthi Prasad for his help with the image acquisition and Wolfgang Hitzl, Research Office of Paracelsus Medical University, for his help with the statistical analysis. We would also like to thank the following readers for their dedicated help with subchondral bone surface segmentation: Julia Bula-Sternberg, Gudrun Goldmann, Linda Jakobi, Verena Lengfelder, Ilona Lipp, Manuela Kunz, Dr. Susanne Maschek, Sabine Mühlsimer, Franz Romeder, Verena Stein, Annette Thebis, and Dr. Barbara Wehr. We would like to thank NIH/NIAMS for funding this study through the following grants: RO1 AR48216, RO1 AR48748, P60 AR48098
Reference List
- 1.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25:395–403. [PubMed] [Google Scholar]
- 2.Booth FW. Terrestrial applications of bone and muscle research in microgravity. Adv Space Res. 1994;14:373–376. doi: 10.1016/0273-1177(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, Pols HA, Bierma-Zeinstra SM. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 4.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3(Suppl A):71–80. [PubMed] [Google Scholar]
- 5.Burgkart R, Glaser C, Hinterwimmer S, Hudelmaier M, Englmeier KH, Reiser M, Eckstein F. Feasibility of T and Z scores from magnetic resonance imaging data for quantification of cartilage loss in osteoarthritis. Arthritis Rheum. 2003;48:2829–2835. doi: 10.1002/art.11259. [DOI] [PubMed] [Google Scholar]
- 6.Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072–2077. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Carter DR, Wong M, Orr TE. Musculoskeletal ontogeny, phylogeny, and functional adaptation. J Biomech. 1991;24(Suppl 1):3–16. doi: 10.1016/0021-9290(91)90373-u. [DOI] [PubMed] [Google Scholar]
- 8.Cooke TD, Sled EA, Scudamore RA. Frontal plane knee alignment: a call for standardized measurement. J Rheumatol. 2007;34:1796–1801. [PubMed] [Google Scholar]
- 9.Darwin C. The origin of species. New American Library; New York: 1872. [Google Scholar]
- 10.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, Gray M, Link TM, Majumdar S, Mosher T, Peterfy C, Totterman S, Waterton J, Winalski CS, Felson D. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14:974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, Hunter D, Hutchins G, Jackson C, Byers KV, Lane NE, Link TM, Majumdar S, Mazzuca S, Prasad PV, Schnitzer TJ, Taljanovic MS, Vaz A, Wyman B, Hellio Le Graverand MP. Precision of 3.0 Tesla Quantitative Magnetic Resonance Imaging of cartilage morphology in a multi center clinical trial. Ann Rheum Dis 2008. 2008 doi: 10.1136/ard.2007.076919. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, Wirth W, Evelhoch JL. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(Suppl 1):46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Eckstein F, Faber S, Muhlbauer R, Hohe J, Englmeier KH, Reiser M, Putz R. Functional adaptation of human joints to mechanical stimuli. Osteoarthritis Cartilage. 2002;10:44–50. doi: 10.1053/joca.2001.0480. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, Eaton CB, Schneider E. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, Kunz M, Hudelmaier M, Jackson R, Yu J, Eaton CB, Schneider E. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 Tesla: a pilot study for the osteoarthritis initiative. Magn Reson Med. 2007;57:448–454. doi: 10.1002/mrm.21146. [DOI] [PubMed] [Google Scholar]
- 18.Glaser C, Burgkart R, Kutschera A, Englmeier KH, Reiser M, Eckstein F. Femoro-tibial cartilage metrics from coronal MR image data: Technique, test-retest reproducibility, and findings in osteoarthritis. Magn Reson Med. 2003;50:1229–1236. doi: 10.1002/mrm.10648. [DOI] [PubMed] [Google Scholar]
- 19.Graichen H, Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50:811–816. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 20.Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, Aliabadi P, Sack B, Zhang Y. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56:1212–1218. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, LaValley M, Guermazi A, Gale D, Felson DT. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol. 2005;32:2192–2199. [PubMed] [Google Scholar]
- 23.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31:423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 25.Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J Bone Joint Surg Br. 1980;62:346–349. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 26.Jones G, Ding C, Scott F, Glisson M, Cicuttini F. Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthritis Cartilage. 2004;12:169–174. doi: 10.1016/j.joca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Keller TS, Strauss AM, Szpalski M. Prevention of bone loss and muscle atrophy during manned space flight. Microgravity Q. 1992;2:89–102. [PubMed] [Google Scholar]
- 28.Lamarck JB. Philosophie zoologique. Baillere; Paris: 1809. [Google Scholar]
- 29.Lazenby RA, Cooper DM, Angus S, Hallgrimsson B. Articular constraint, handedness, and directional asymmetry in the human second metacarpal. J Hum Evol. 2008;54:875–885. doi: 10.1016/j.jhevol.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DE, Devlin MJ, Pearson OM. Articular area responses to mechanical loading: effects of exercise, age, and skeletal location. Am J Phys Anthropol. 2001;116:266–277. doi: 10.1002/ajpa.1123. [DOI] [PubMed] [Google Scholar]
- 31.Maquet P. Mechanics and osteoarthritis of the patellofemoral joint. Clin Orthop Relat Res. 1979:70–73. [PubMed] [Google Scholar]
- 32.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreland JR, Bassett LW, Hanker GJ. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69:745–749. [PubMed] [Google Scholar]
- 34.Morrison JB. The mechanics of the knee joint in relation to normal walking. J Biomech. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Gerbl M. The subchondral bone plate. Adv Anat Embryol Cell Biol. 1998;141:III–XI. 1–134, III–134. doi: 10.1007/978-3-642-72019-2. [DOI] [PubMed] [Google Scholar]
- 36.Plochocki JH. Bilateral variation in limb articular surface dimensions. Am J Hum Biol. 2004;16:328–333. doi: 10.1002/ajhb.20023. [DOI] [PubMed] [Google Scholar]
- 37.Plochocki JH, Riscigno CJ, Garcia M. Functional adaptation of the femoral head to voluntary exercise. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:776–781. doi: 10.1002/ar.a.20345. [DOI] [PubMed] [Google Scholar]
- 38.Riggs BL, Melton IL, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 39.Roux W. Der Kampf der Teile im Organismus. Engelmann; Leipzig: 1881. [Google Scholar]
- 40.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39:644–651. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–377. [PubMed] [Google Scholar]
- 42.Thorp LE, Wimmer MA, Block JA, Moisio KC, Shott S, Goker B, Sumner DR. Bone mineral density in the proximal tibia varies as a function of static alignment and knee adduction angular momentum in individuals with medial knee osteoarthritis. Bone. 2006;39:1116–1122. doi: 10.1016/j.bone.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Ding C, Wluka AE, Davis S, Ebeling PR, Jones G, Cicuttini FM. Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology (Oxford) 2006;45:79–84. doi: 10.1093/rheumatology/kei108. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Wluka AE, Cicuttini FM. The determinants of change in tibial plateau bone area in osteoarthritic knees: a cohort study. Arthritis Res Ther. 2005;7:R687–R693. doi: 10.1186/ar1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wluka AE, Wang Y, Davis SR, Cicuttini FM. Tibial plateau size is related to grade of joint space narrowing and osteophytes in healthy women and in women with osteoarthritis. Ann Rheum Dis. 2005;64:1033–1037. doi: 10.1136/ard.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolff J. Das Gesetz der Transformation der Knochen. Hirschwald; Berlin: 1892. [Google Scholar]
- 47.Zhai G, Ding C, Cicuttini F, Jones G. A longitudinal study of the association between knee alignment and change in cartilage volume and chondral defects in a largely non-osteoarthritic population. J Rheumatol. 2007;34:181–186. [PubMed] [Google Scholar]