Abstract
Background
Intact articular cartilage tissue is used clinically in the form of osteochondral allografts and experimentally as explants in modeling the physiologic behavior of chondrocytes in their native extracellular matrix. Long-term maintenance of allograft tissue is challenging.
Hypothesis
By carefully modulating the preservation environment, it may be possible to preserve osteochondral allograft tissue over the long term while maintaining its original mechanical and biochemical properties.
Study Design
Controlled laboratory study.
Methods
In this study, juvenile bovine, mature bovine, and canine cartilage explants were cultured in chemically defined media with or without supplementation of dexamethasone for up to 4 weeks.
Results
The mechanical properties and biochemical content of juvenile bovine explants cultured in the presence of dexamethasone were significantly enhanced after 2 weeks in culture and remained stable with sustained cell viability thereafter. In contrast, the mechanical properties and biochemical content of juvenile bovine explants cultured in the absence of the dexamethasone significantly decreased after 2 weeks of culture. The mechanical and biochemical content of mature bovine and canine explants were not significantly affected by the presence of dexamethasone and maintained initial (day 0) mechanical and biochemical properties throughout the entire culture period with or without supplementation of dexamethasone.
Conclusion
These results suggest that juvenile and mature cartilage explants respond differently to dexamethasone. The functional properties of juvenile cartilage explants can be maintained in vitro through the addition of dexamethasone to culture media. Functional properties of mature cartilage can be preserved for at least 4 weeks in culture regardless of the presence of dexamethasone.
Clinical Relevance
Biochemical and biomechanical properties of osteochondral allograft tissue may be enhanced by the addition of dexamethasone to culture media. These findings may translate to longer shelf life of preserved osteochondral allograft transplantation tissue and increased clinical availability of grafts.
Keywords: cartilage explants, osteochondral grafts, dexamethasone, tissue culture
Transplantation of fresh osteochondral allograft tissue about the knee has resulted in clinical success rates of over 75% for the treatment of femoral condyle lesions of various causes.11 Current practices to ensure safety for use of allografts require bacterial and viral testing, typically resulting in a minimum waiting period of 7 days after procurement before clinical implantation. However, concerns about chondrocyte viability and associated graft performance with prolonged storage time generally restrict clinical use to within 28 days after harvest. Therefore, the current optimal clinical window for osteochondral allografting, 7 to 28 days after harvest, severely limits the number of grafts that can be used and the number of patients who can benefit from this technique. An increase in shelf life will have a very significant effect on the treatment of cartilage lesions by expanding the availability of osteochondral allografts. In addition, a number of avenues of orthopaedic research depend on in vitro experiments on cartilage tissue that can last for weeks; maintenance of the physiologic functions of cartilage explants in the long term is vital to the validity of these experiments. Longer-term preservation of articular cartilage tissue will aid in study of chondrocytes in their native extracellular environment.2,37
Although Brighton et al,7 as well as others,17,38 showed promising findings using tissue culture techniques for cartilage maintenance, cold storage (~4°C) is the current standard for osteochondral graft preservation by tissue banks that provide allografts for clinical use. In the current study, we revisit the potential of in vitro culturing techniques for maintaining cartilage explants long term. Our previous work demonstrated that a chemically defined serum-free medium (also referred to as chondrogenic medium) adapted from a well-established formulation known to foster chondrogenesis was exceptionally effective for functional tissue engineering of cartilage and long-term maintenance of cartilage explants.5,14,28,33 It is interesting to note that this serum-free medium formulation resulted in improvements in the mechanical and biochemical aspects of the cultured cartilage explants without added growth factors (such as transforming growth factor-β 1 or insulin-like growth factor-1 typically applied). However, the formulation of the chondrogenic medium includes dexamethasone. Dexamethasone is a synthetic adrenal corticosteroid with a potent anti-inflammatory property and is used in treatment of a wide variety of inflammatory conditions such as rheumatoid arthritis.16 It has also been shown that dexamethasone has a protective effect against catabolic factor–mediated degradation of cartilage.23,35 On the other hand, dexamethasone has also been shown to induce the expression of anabolic growth and differentiation factors, such as bone morphogenetic proteins.31,36 For these reasons, we speculate that dexamethasone plays a critical role in the maintenance of the mechanical properties of the explants in serum-free medium. The objective of this study was to further investigate the effects of dexamethasone in maintaining the native properties of juvenile bovine (study 1), mature bovine (study 2), and mature canine (study 3) cartilage explants in long-term culture, with the goal of determining an optimal protocol for cartilage tissue preservation for clinical application to osteochondral allografting.
MATERIALS AND METHODS
Sample Preparation and Culturing
Study 1 (Juvenile Bovine)
Full-thickness juvenile bovine cartilage plugs (4-mm diameter) were harvested from the femoral condyles of 2- to 6-month-old calves. Explants were then cultured in the chemically defined serum-free medium (Dulbecco’s modified Eagle’s medium, 1% ITS [insulin, transferring, and selenium] + premix, 50 μg/mL L-proline, 0.9 mM sodium pyruvate) with or without supplementation of dexamethasone (0.1 μM).13 The media were also supplemented with ascorbate 2-phosphate (50 μg/mL). The serum-free medium is also referred to as chondrogenic medium (CM) for its original use in inducing chondrogenesis of stem cells. The experimental groups were as follows (n = 12 for each group at each time point): (1) explants cultured in CM with continuous supplementation of dexamethasone (Dex group), (2) explants cultured in CM with continuous dexamethasone removed at 14 days (2 weeks), and (3) explants cultured in CM without continuous dexamethasone (No Dex).
Studies 2 and 3 (Mature Bovine and Mature Canine)
In study 2, full-thickness osteochondral plugs (3-mm diameter) were harvested from mature (2- to 4-year-old) bovine femoral condyles and cleaned of bone marrow with a high-velocity water pick. In study 3, full-thickness osteochondral plugs (3-mm diameter) were harvested from mature (2- to 4-year-old) canine femoral condyles and cleaned of bone marrow. In study 2, 3 groups were established (n = 12 for each group at each time point): (1) explants cultured with continuous supplementation of 0.1 μM dexamethasone (Dex), (2) explants cultured with dexamethasone removed after 2 weeks of culture (2 weeks), and (3) explants cultured without dexamethasone (No Dex). In study 3, only Dex and No Dex groups were used (n = 5 or 6 for each group at each time point). All studies were incubated in CM at 37°C (5% CO2) for 28 days. Media were changed 3 times a week.
Mechanical Testing
The average mechanical properties of explant disks were evaluated on days 0, 14, and 28 of culture, using a custom tabletop testing device.32 Briefly, the diameter and thickness of the explant disks were measured using a digital caliper. A tare load of 5 g was then applied to the explant disks to achieve complete surface contact between the loading platen and the explant disks. Subsequently, the explant disks were subjected to unconfined compression at 10% strain during a stress relaxation test lasting for 2000 seconds. The equilibrium Young’s modulus (EY) was then determined using the equilibrium load at the end of the stress relaxation. The stress relaxation was followed by tests for dynamic modulus at 0.1, 0.5, and 1 Hz and 1% strain.
Biochemical Analysis
One half of each explant disk was weighed wet, lyophilized, reweighed dry, and digested in 0.5 mg/mL Proteinase-K (Fisher Scientific, Pittsburgh, Pennsylvania) in 50 mM Tris-buffered saline containing 1 mM ethylenediaminetetraacetic acid, 1 mM iodoacetamide, and 10 mg/mL pepstatin A, at 56°C for 16 hours. The PicoGreen assay (Invitrogen, Molecular Probes, Eugene, Oregon) was used to quantify the DNA content of the explant disks with Lambda phage DNA (0–1 mg/mL) as a standard.34 The glycosaminoglycan (GAG) content was measured using dimethylmethylene blue (Sigma Chemicals, St. Louis, Missouri) dye-binding assay with shark chondroitin sulfate (0–50 mg/mL) as a standard.18 The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio.21 The collagen and GAG contents were normalized to the disk wet weight and DNA content. Cell viability was assessed using the LIVE/DEAD Assay Kit (Molecular Probes), where live cells are stained green with calcein acetoxymethyl ester (calcein-AM) and dead cells stained red with ethidium homodimer.
Statistical Analysis
Statistica software (StatSoft, Tulsa, Oklahoma) was used to perform statistical analyses using 2-way analysis of variance and the Tukey honestly significant differences post hoc test with culture duration and culture media type as independent variables.
RESULTS
Biomechanical Testing
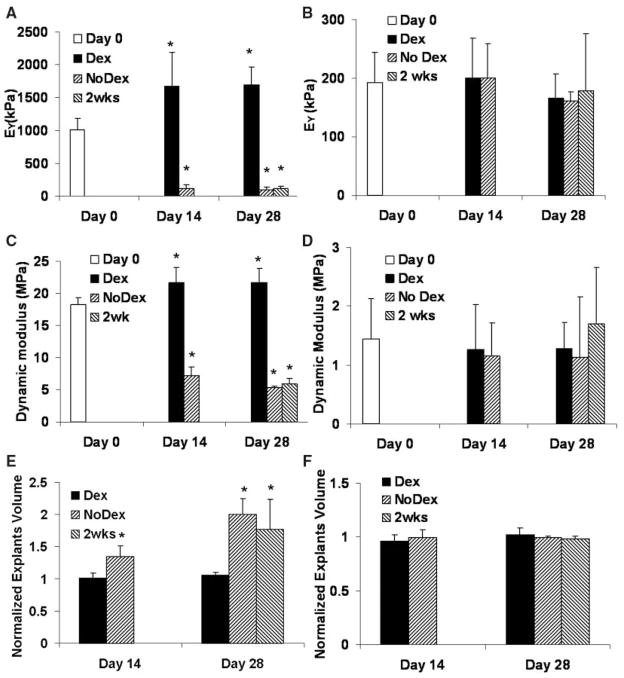
In study 1, after 28 days, the equilibrium modulus (EY) of juvenile bovine explants grown with continuous supplementation of dexamethasone (Dex group) increased by approximately 70% (reaching a value of 1700 ± 520 kPa) from the day 0 value, while that of the explants grown without dexamethasone (No Dex group) dropped to 10% of the day 0 level (decreased to 117 ± 60 kPa) (Figure 1A). Removal of dexamethasone after 14 days resulted in significant reduction in the EY of the juvenile bovine explants (Figure 1A). A similar trend was observed in the dynamic modulus of the juvenile bovine explants as in the equilibrium modulus (Figure 1C). Absence of dexamethasone resulted in a significant decrease in the dynamic modulus of the juvenile explants.
Figure 1.
Equilibrium modulus of the explants from study 1 (A, immature bovine) and study 2 (B, mature bovine); dynamic modulus of the explants from study 1 (C, immature bovine) and study 2 (D, mature bovine) obtained at a testing frequency of 0.5 Hz; and explant volume (normalized to day 0) of study 1 (E, immature bovine) and study 2 (F, mature bovine). *P <.05 versus day 0.
In study 2, the effects of dexamethasone on both EY and dynamic modulus of mature bovine cartilage were not statistically significant and all experimental groups stayed at day 0 levels (Figure 1B and 1D).
Volume Testing
In study 1, the volume of the juvenile bovine explants cultured without dexamethasone increased significantly by 35% ± 16% (No Dex) at day 14 (Figure 1E). At day 28, volume increased 99% ± 25% (No Dex) and 76% ± 47% (2 weeks) compared with initial (day 0) volume. In contrast, the volume of juvenile bovine explants remained close to the initial level in the presence of dexamethasone (Figure 1E).
In studies 2 and 3, there was no significant change in tissue volume within each group over different time points or among different groups at the same time points (Figure 1F, data not shown for study 3).
Biochemical Testing
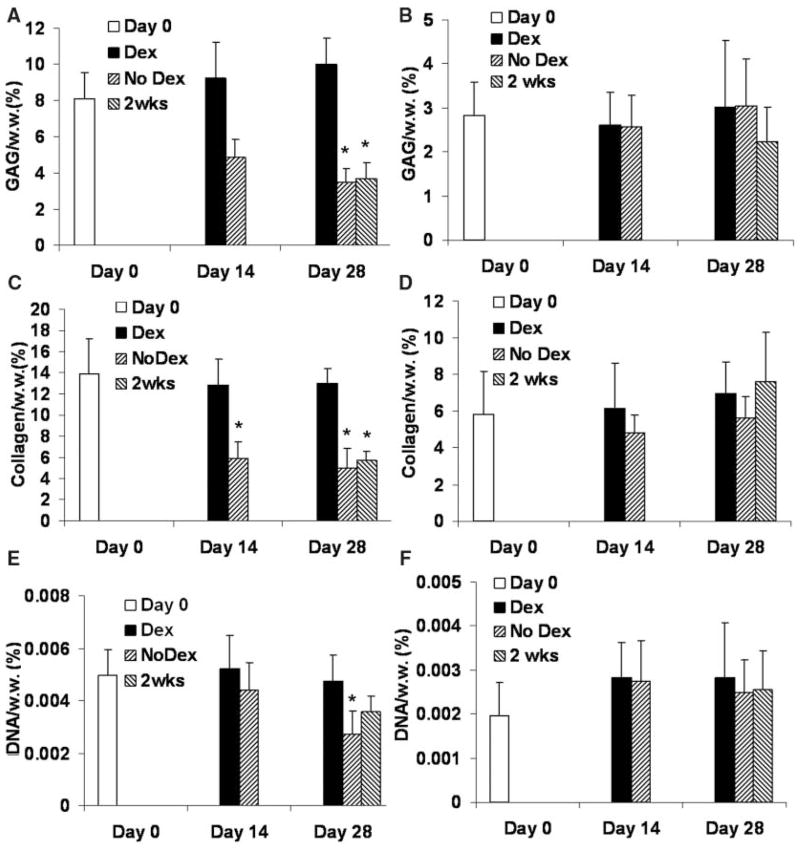
In study 1, the GAG content of the juvenile bovine cartilage grown with continuous supplementation of dexamethasone (Dex group) increased slightly from the initial level, reaching a value of 9.4% ± 1.4% (normalized by wet weight) on day 28, whereas those of the other 2 groups (No Dex, 2 weeks) decreased significantly to 3.5% ± 0.7% and 3.7% ± 0.8%, respectively (Figure 2A). The collagen content of the Dex group was maintained at the day 0 level (13.8% ± 3.4% by wet weight). In contrast, the collagen content of the No Dex group became significantly lower than the day 0 level starting from day 14 and dropped to the value of 5.0% ± 1.8% (by wet weight) (Figure 2C). Removing dexamethasone from medium after day 14 (2-weeks group) resulted in a significant decrease in collagen content (Figure 2C). The DNA content (by wet weight) of the Dex group remained at its initial level, whereas the DNA content of the No Dex group decreased over time and became significantly lower than the day 0 level (Figure 2E). There was no significant change in DNA content normalized by the dry weight of the tissue in all groups (data not shown).
Figure 2.
Glycosaminoglycan (GAG) content (normalized to the wet weight) of the explants in study 1 (A, immature bovine) and study 2 (B, mature bovine); collagen content (normalized to the wet weight) of the explants in study 1 (C, immature bovine) and study 2 (D, mature bovine); and DNA content (normalized to the wet weight) of the explants in study 1 (E, immature bovine) and study 2 (F, mature bovine). *P <.05 versus day 0.
In study 2, the effects of dexamethasone on the GAG and collagen content of the mature bovine cartilage were not significant and all experimental groups stayed at the day 0 levels (Figure 2B and 2D). There was no significant difference in the DNA content of the mature bovine explants within the same group over different time points or among different groups at the same time points (Figure 2F).
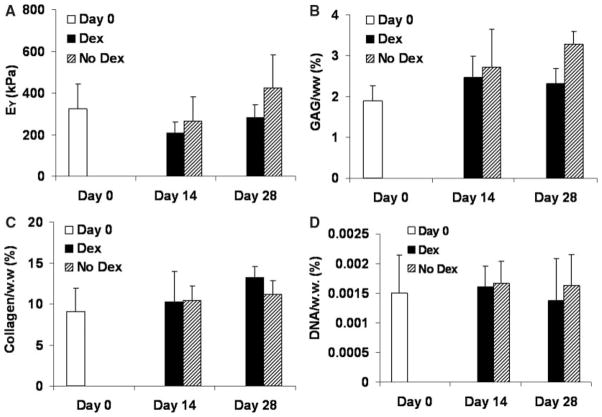
In study 3, the effects of dexamethasone on EY, GAG, collagen, and DNA content of the mature canine cartilage explants were not significant and all experimental groups stayed at the day 0 levels (Figure 3). Based on viability staining, there was no significant cell death at the end of the culture (day 28) in all studies (studies 1–3) in the absence of dexamethasone (Figure 4).
Figure 3.
Equilibrium modulus (A), glycosaminoglycan (GAG) content (B), collagen content (C), and DNA content (D) of mature canine explants from study 3 (normalized to the wet weight).
Figure 4.
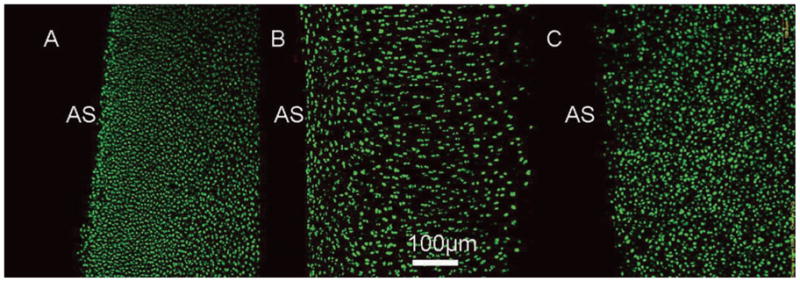
Viability staining of the cartilage explants from the No Dex groups of study 1 (A, immature bovine), study 2 (B, mature bovine), and study 3 (C, mature canine) on day 28. Green, live cells; red, dead cells; AS, articular surface.
DISCUSSION
Our results showed that the mechanical and biochemical properties of the cartilage explants of different ages and species can be successfully maintained at ‘‘fresh’’ levels in a chemically defined serum-free medium for at least 4 weeks under free-swelling conditions. This was achieved without application of dynamic mechanical loading or medium perfusion, both of which have been shown to enhance matrix synthesis and maintain the functional properties of cartilage.25,37,38 As such, our preservation technique is readily amenable for use in large-scale tissue banking. Bovine tissue was studied because it is well established and extensively used in cartilage research models, for which tissues of various ages are readily available. Canine tissue was also examined as the dog has been used widely for models of osteoarthritis and as a preclinical model of cartilage repair including treatment by osteochondral allografting.1,19 Dexamethasone proved to be necessary in maintaining the mechanical stiffness and collagen content of the juvenile bovine cartilage explants during long-term culture, whereas mature bovine and canine explants maintained their initial properties independently of use of the steroid.
Excessive cartilage swelling is closely correlated with the onset of osteoarthritis, contributing to the breakdown of the cartilage matrix.4,12,15 In study 1, the dexamethasone-free group (No Dex group) exhibited lower GAG and collagen content, which we speculate led to increased permeability and reduced tensile stiffness of the explants. Higher permeability and lower tensile stiffness can both contribute to the reduced dynamic modulus in the No Dex group (Figure 1C). Furthermore, in study 1, accompanying the matrix loss and decreasing mechanical stiffness, was a significant swelling (2-fold increase from initial volume) of juvenile bovine cartilage explants cultured without supplementation of dexamethasone. This swelling resulted in an effective dilution of biochemical constituents and reduced cell density as shown by the DNA content in the tissue. This most likely contributed to the observed drop in mechanical properties. The swelling may have also increased the permeability of the cartilage tissue, facilitating the loss of matrix components to the medium. In contrast, the volume of juvenile bovine explants cultured with supplementation of dexamethasone remained relatively constant with culture time, further demonstrating the beneficial effects of dexamethasone for preservation of juvenile cartilage in vitro.
For this study, cartilage explants included the superficial, middle, and deep zones. It is well established that the cells in different zones have different characteristics3,20,40 and can respond differently to the same external stimuli.22,27 A recent study showed that chondrocytes in one zone can modulate the behavior of chondrocytes in a different zone.24 However, a separate study (unpublished) in our laboratory using only the middle-zone portion of the juvenile bovine cartilage showed similar trends to those seen in study 1. The mechanical properties and biochemical contents (GAG and collagen) of middle-zone cartilage (juvenile bovine) decreased significantly in the absence of dexamethasone. This indicated that the effect of dexamethasone on juvenile bovine cartilage is independent of interactions among different zonal populations of chondrocytes. This finding has important implications for application of this work to osteochondral allografts with intact full-thickness articular cartilage components.
The significant effect of dexamethasone on juvenile bovine cartilage was not observed in mature bovine and mature canine cartilage explants. Mechanical stiffness and matrix molecule contents stayed at initial levels (day 0) regardless of dexamethasone supplementation. This different response to dexamethasone by cartilage of different ages may be explained by the changes in cartilage matrix structure and chondrocyte function as cartilage ages. Articular cartilage undergoes significant structural and compositional changes with age as evidenced by decreasing size of proteoglycan aggregates,8,9 increasing surface fibrillation,26 and increasing collagen cross-linking.10,39 Articular cartilage cell function also changes with age. Studies have shown that the mitotic and synthetic activities of human articular cartilage chondrocytes decline with age,6,29 and an age-related decline in response to anabolic factors like insulin-like growth factor I occurs.30 These findings indicate that immature cartilage, which is more responsive to endogenous anabolic or catabolic factors, may incur larger structural changes than mature cartilage in the absence or presence of the metabolic factors in the media. Further studies are needed to elucidate the exact molecular mechanism underlying this different response to dexamethasone by cartilage of different ages, but this information can be immediately applied to development of optimal protocols for tissue banking of articular tissues from organ donors of different ages.
In this study, we examined the effect of dexamethasone on cartilage of different ages. This was motivated by the fact that even though most of the cartilage allografts used currently are derived from adult donors, allogeneic human juvenile cartilage allograft cubes and chondrocytes are also currently being evaluated in strategies for treating cartilage defects (DeNovo NT and DeNovo ET, Zimmer Inc, and ISTO Technologies Inc). We believe our findings in this study are clinically important and can potentially contribute to better maintained cartilage allograft tissues, leading to improved clinical outcome.
CONCLUSION
This study showed that the mechanical and biochemical properties of cartilage explants of different ages and species can be successfully maintained at initial levels for at least 4 weeks in tissue culture. Dexamethasone proved to be necessary in maintaining the mechanical stiffness and the content of major matrix components of the juvenile bovine cartilage explants during long-term culture, whereas mature bovine and canine explants maintained their initial properties independently of the presence of steroid. Findings from this study will aid the optimization of culture protocols for long-term maintenance of cartilage explant culture for basic science studies and model systems for pharmacologic testing, as well as for cartilage allograft storage, to potentially allow for longer storage times and greater availability of osteochondral allografts. Clinical outcomes of osteochondral allograft transplantation may be improved by increasing the availability of these grafts.
Acknowledgments
This work was supported by National Institutes of Health grants AR46568 (C.T.H.) and AR53530 (J.L.C.), and a Mus-culoskeletal Transplant Foundation grant CU07-194 (C.T.H.). The experimental assistance from Duo Xu and David Y. Williams is also acknowledged.
Footnotes
No potential conflict of interest declared.
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56(1):188–198. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 3.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes: I. morphology and cartilage matrix production. Connect Tissue Res. 1988;18(3):205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- 4.Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000;43(10):2202–2210. doi: 10.1002/1529-0131(200010)43:10<2202::AID-ANR7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Bian L, Lima EG, Angione SL, et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Bio-mech. 2008;41(6):1153–1159. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: implications for aggregate stability. Biochem J. 1999;337(Pt 1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Brighton CT, Shadle CA, Jimenez SA, Irwin JT, Lane JM, Lipton M. Articular cartilage preservation and storage: I. application of tissue culture techniques to the storage of viable articular cartilage. Arthritis Rheum. 1979;22(10):1093–1101. doi: 10.1002/art.1780221008. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Rosenberg L. Structural changes during development in bovine fetal epiphyseal cartilage. Coll Relat Res. 1983;3(6):489–504. doi: 10.1016/s0174-173x(83)80028-4. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Rosenberg LC. Electron microscopic studies of cartilage proteoglycans: direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J Biol Chem. 1982;257(16):9830–9839. [PubMed] [Google Scholar]
- 10.Buckwalter JA, Woo SL, Goldberg VM, et al. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993;75(10):1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191–195. [PubMed] [Google Scholar]
- 12.Bush PG, Hall AC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage. 2003;11(4):242–251. doi: 10.1016/s1063-4584(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 13.Byers BA, Mauck RL, Chiang I, Tuan RS. Temporal exposure of TGF-β3 under serum-free conditions enhances biomechanical and biochemical maturation of tissue-engineered cartilage. Trans Orthop Res Soc. 2006;31:43. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo E, Palacios I, Delgado E, et al. High-resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage. 2001;9(5):463–472. doi: 10.1053/joca.2001.0413. [DOI] [PubMed] [Google Scholar]
- 16.Cuzzocrea S, Mazzon E, Paola RD, et al. Effects of combination M40403 and dexamethasone therapy on joint disease in a rat model of collagen-induced arthritis. Arthritis Rheum. 2005;52(6):1929–1940. doi: 10.1002/art.21044. [DOI] [PubMed] [Google Scholar]
- 17.Dumont J, Ionescu M, Reiner A, et al. Mature full-thickness articular cartilage explants attached to bone are physiologically stable over long-term culture in serum-free media. Connect Tissue Res. 1999;40(4):259–272. doi: 10.3109/03008209909000704. [DOI] [PubMed] [Google Scholar]
- 18.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 19.Glenn RE, Jr, McCarty EC, Potter HG, Juliao SF, Gordon JD, Spindler KP. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34(7):1084–1093. doi: 10.1177/0363546505284846. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka C, Cheng C, Alexandre D, Bhargava M, Torzilli PA. Maturational differences in superficial and deep zone articular chondrocytes. Cell Tissue Res. 2006;323(1):127–135. doi: 10.1007/s00441-005-0050-y. [DOI] [PubMed] [Google Scholar]
- 21.Hollander AP, Heathfield TF, Webber C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93(4):1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang NS, Varghese S, Lee HJ, et al. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett. 2007;581(22):4172–4178. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafari HS, Saez-Llorens X, Paris M, et al. Dexamethasone attenuation of cytokine-mediated articular cartilage degradation in experimental lapine Haemophilus arthritis. J Infect Dis. 1993;168(5):1186–1193. doi: 10.1093/infdis/168.5.1186. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, Leong NL, Mung JC, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage. 2008;16(1):70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311(1):1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 26.Koepp H, Eger W, Muehleman C, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci. 1999;4(6):407–412. doi: 10.1007/s007760050123. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Yao S, Alini M, Grad S. Different response of articular chondrocyte subpopulations to surface motion. Osteoarthritis Cartilage. 2007;15(9):1034–1041. doi: 10.1016/j.joca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Lima EG, Bian L, Ng KW, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56(4):B172–B179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15(4):491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 31.Martinovic S, Borovecki F, Miljavac V, Kisic V, Maticic D, Francetic I, Vukicevic S. Requirement of a bone morphogenetic protein for the maintenance and stimulation of osteoblast differentiation. Arch Histol Cytol. 2006;69(1):23–36. doi: 10.1679/aohc.69.23. [DOI] [PubMed] [Google Scholar]
- 32.Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 33.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10(7):580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 35.Morin I, Li WQ, Su S, Ahmad M, Zafarullah M. Induction of stromely-sin gene expression by tumor necrosis factor alpha is inhibited by dexamethasone, salicylate, and N-acetylcysteine in synovial fibro-blasts. J Pharmacol Exp Ther. 1999;289(3):1634–1640. [PubMed] [Google Scholar]
- 36.Oreffo RO, Kusec V, Romberg S, Triffitt JT. Human bone marrow osteoprogenitors express estrogen receptor-alpha and bone morphogenetic proteins 2 and 4 mRNA during osteoblastic differentiation. J Cell Biochem. 1999;75(3):382–392. doi: 10.1002/(sici)1097-4644(19991201)75:3<382::aid-jcb4>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 38.Strehl R, Tallheden T, Sjogren-Jansson E, Minuth WW, Lindahl A. Long-term maintenance of human articular cartilage in culture for biomaterial testing. Biomaterials. 2005;26(22):4540–4549. doi: 10.1016/j.biomaterials.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Verzijl N, DeGroot J, Oldehinkel E, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 40.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996;14(3):424–432. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]