Abstract
Decorin, a small leucine-rich proteoglycan, is a natural inhibitor of transforming growth factor beta (TGFβ). Myofibroblast and haze formation in the cornea have been attributed to TGFβ hyperactivity released from corneal epithelium following injury to eye. This study tested the hypothesis that decorin gene transfer inhibits TGFβ-driven myofibroblast and haze formation in the cornea. Human corneal fibroblast (HSF) cultures generated from donor human corneas were used. Decorin cDNA was cloned into mammalian expression vector. Restriction enzyme analysis and DNA sequencing confirmed the nucleotide sequence of generated vector construct. The decorin gene cloned into mammalian expression vector was introduced into HSF with lipofectamine transfection kit. Expression of decorin in selected clones was characterized with RT-PCR, immunocytochemistry and western blotting. Phage contrast microscopy and trypan blue exclusion assay evaluated the effects of decorin gene transfer on HSF phenotype and viability, respectively. Real-time PCR, western blot and immunocytochemistry were used to analyze inhibitory effects of decorin gene transfer on TGFβ-induced myofibroblast formation by measuring differential expression of alpha smooth muscle actin (SMA), a myofibroblast marker, mRNA and protein expression. Analysis of variance (ANOVA) and the Bonferonni-Dunn adjustment for repeated measures were used for statistical analysis. Our data indicate that decorin-gene transfer into HSF do not alter cellular phenotype or viability. Decorin-overexpressing HSF clones grown in the presence of TGFβ1 under serum-free conditions showed a statistically significant 80–83% decrease in SMA expression (p value <0.01) compared to naked-vector transfected clones or un-transfected HSF controls. Decorin-transfected, naked-vector transfected and un-transfected HSF grown in the absence of TGFβ1 showed no or extremely low expression of SMA. Furthermore, decorin over-expression did not affect HSF phenotype and decreased TGFβ-induced RNA levels of profibrogenic genes such as fibronectin, collagen type I, III, and IV that play important role in stromal matrix modulation and corneal wound healing. The results of study suggest that decorin gene transfer effectively prevents TGFβ-driven transformation of keratocyte and corneal fibroblast to myofibroblasts. We postulate that decorin gene therapy can be used to treat corneal haze in vivo.
Keywords: Decorin, gene transfer, corneal scarring/haze, extracellular matrix, TGFβ
1. Introduction
Blindness due to corneal scarring or haze is third leading cause of global blindness [Congdon et al., 2004; Kempen et al., 2004]. Corneal haze is also a common complication of refractive laser surgery, photorefractive keratectomy, used widely throughout the world to correct refractive errors in humans [Fong C.S., 2007; Seiler and McDonnell, 1995]. Injury and/or infections to the eye have been shown to cause corneal haze [Seiler and McDonnell, 1995; Azar, 2006]. The molecular mechanisms for the development of corneal haze have been extensively studied using various experimental models [Jester et al., 1999; Wilson et al., 2001; Mohan et al., 2003; Netto et al., 2006; Stramer et al., 2003]. Scores of growth factors and cytokines have been found to play role in corneal wound healing, myofibroblast and haze development [Imanishi et al., 2000]. Out of many cytokines TGFβ has been found to play a central role in the development of corneal haze following injury by facilitating transdifferentiation of keratocyte to myofibroblasts [Imanishi et al 2000]. The corneal fibroblasts grown in presence of TGFβ under serum-free conditions expressed α-smooth muscle actin (α-SMA), a biochemical marker for myofibroblasts, and acquired fibroblastic phenotype but grown in the absence of TGFβ neither expressed α-SMA nor acquired fibroblastic morphology [Jester et al., 1996]. A reduction in corneal fibrosis by the topical application of TGFβ neutralizing antibodies on keratectomy wound in rabbit eyes has also been reported [Jester et al., 1997].
Small leucine rich proteoglycans such as decorin and betaglycan are known to modulate growth factor action, cell cycle machinery, tissue development and remodeling [Kalamajski and Oldberg, 2010; Schaefer and Iozzo, 2008]. Decorin is a small leucine-rich proteoglycan and has been shown to play an important role in the organization of extra cellular matrix [Reed and Iozzo, 2002; Jarvelainen et al., 2006; Ferdous et al., 2007]. It consists of a 40kD core protein and a glycosaminoglycan side chain [Iozzo1997, Reed and Iozzo, 2002]. Decorin has been reported to be involved in extracellular matrix metabolism and fibrillogenesis [Reed and Izzo, 2002; Douglas et al., 2006]. Numerous studies showed that decorin interact with matrix molecules such as collagen, fibronectin, thrombospondin, and growth factors like TGFβ [Yamaguchi, 1990; Neame et al., 2000; Ferdous et al., 2007]. The interactions of decorin with TGFβ appear to be more complex than binding to a single isolated peptide sequence. Its binding to TGFβ predominantly occurs through core protein as lack of glycosaminoglycan chain did not affect binding activity [Hildebrand et al. 1994; Cheifetz and Massague 1989]. TGFβ protein is released into the intercellular space, binds to latency-associated protein and forms small latent complex. Subsequently, this complex binds to latent TGFβ binding protein and forms a large latent complex, which is secreted to the extracellular matrix [Rifkin, 2005; Stander et al 1999]. The interactions of this large TGFβ complex to decorin or collagen-bound decorin lead to anti-fibrotic response [Stander et al 1999]. Decorin binds with all three isoforms of TGFβ(1–3) with similar efficiency [Yamaguchi et al, 1990; Hildebrand et al., 1994]. The expression of decorin in corneal stromal has been reported [Axelsson and Heinegard, 1975; Hassell et al., 1979; Brown et al., 2002]. It was also identified that the biological function of decorin in the cornea is regulated by the core protein and not by the glycosaminoglycan side chain [Rada et al., 1993].
Decorin over-expression in many non-ocular tissues has been shown to reduce TGFβ-induced fibrosis effectively in vivo in scores of experimental models [Giri et al., 1997; Shimizukawa et al., 2003; Isaka, et al., 1996; Margetts et al., 2002; Zhao et al., 1999; Kolb et al., 2001]. However, the mechanism of TGFβ inactivation is still unclear (Markmann et al., 2000). Decorin has also been shown to regulate collagen fiber formation in vivo as decorin-deficient transgenic mice showed fragile and low tensile strength in the skin (Danielson et al., 1997). Binding of decorin to fibrillar collagens and inhibition of fibrillogenesis by decorin was confirmed using in vitro model (Keene et al., 2000). Decorin and lumican have been shown to retard corneal collagen fibrillogenesis in vitro [Reed and Iozzo, 2002; Neame et al., 2000]. Based on the facts that corneal scarring occur due to TGFβ hyperactivity and decorin is a natural inhibitor of TGFβ it was hypothesized that decorin gene transfer could prevent myofibroblast and haze development in the cornea. This study tested our hypothesis using an in vitro corneal scarring model.
2. Materials and methods
2.1 Reagents
The cell culture reagents, pcDNA3.1/V5-HisA,B,C mammalian gene expression vector, transfection reagents, and secondary antibodies were purchased from Invitrogen, San Diego, CA, USA. The RNA extraction kit was purchased from Qiagen Inc., Valencia, CA and ImProm-II Reverse Transcription kit to prepare cDNA was obtained from Promega, Madison, WI. The iQ SYBR green super mix and JumpStart PCR mix were obtained from Bio-Rad Laboratories, Hercules, CA and Sigma-Aldrich, St Louis, MO, USA, respectively. TGFβ1 was purchased from PeproTech Inc, Rocky Hill, NJ, USA. The decorin and SMA primary antibodies were either purchased from Santa Cruz Boitechnology, Santa Cruz, CA or R&D System Minneapolis, MN. The decorin antibody was also procured from Dr. Larry W Fisher, National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA. The DAPI containing mounting medium was purchased Vector Laboratories, Inc., Burlingame, CA, USA.
2.2 Human Corneal Fibroblasts Culture
Primary HSF cultures were generated from donor human corneas obtained from eye banks using method described previously [Sharma et al., 2009]. Briefly, The epithelium and endothelium of the cornea were removed by gentle scraping with scalpel blade after washing the tissue with Minimum Essential Medium (MEM). The cornea was cut into small pieces, placed on culture dishes, and incubated in humidified CO2 (5%) incubator at 37°C in MEM supplemented with 10% fetal bovine serum for 3–5 weeks to obtain human corneal fibroblasts. Seventy percent confluent HSF cultures (1–3 passage) were used for experiments. Myofibroblasts were produced by culturing HSF cultures under serum free conditions in presence of TGFβ1 (1ng/ml). TGFβ1 was purchased from PeproTech Inc, Rocky Hill, NJ and cell culture reagents were purchased from Invitrogen, San Diego, CA, USA or Sigma-Aldrich, St Louis, MO, USA.
2.3 Vector generation, transfection and selection of stable clones
The pcDNA3.1/V5-HisB mammalian gene expression vector system (Invitrogen, San Diego, CA) was used. A PCR-amplified human decorin (Accession # NM_001920) cDNA (~1.1Kb) was cloned into pcDNA3.1/V5-His A vector employing standard molecular biological techniques. Restriction mapping and DNA sequencing were used to confirm the nucleotide sequence of the pcDNA3.1/V5-HisB-decorin (hereafter denoted as pcDNA3.1-decorin) vector construct. The transfection of pcDNA3.1-decorin plasmid into HSF was performed with Lipofectamine 2000 (Invitrogen) following vendor’s instructions. Briefly, for each transfection, 10µg plasmid DNA in 100µl of MEM was added to 100µl Lipofectamine™ 2000 diluted with MEM, and incubated at room temperature for 20 minutes or until solution became cloudy. The DNAL-ipofectamine solution was added drop wise to the cells containing 1.5ml medium by rocking the plate back and forth. Cultures were incubated at 37°C in a CO2 (5%) incubator for 6–8 hours, washed with medium, and incubated 48–72hrs for transient transgene expression. The stably transfected clones were identified by growing cultures in presence of MEM medium supplemented with 10% serum and geneticin (250µg/ml).
2.4. Cellular morphology and viability
The cellular morphology of clones was monitored with Leica DMIL phase-contrast microscope equipped with Leica DFC290 imaging system. Cultures were visualized at different time intervals and their phenotype was recorded using digital camera. Trypan blue assay was performed as reported earlier following manufacturer’s instructions (BRB 2010). Briefly, at selected time points, cells were trypsinised and mixed with equal amounts of 0.4% trypan blue solution (Invitrogen). Dead cells with ruptured membranes stained blue and live cells white were counted with Neubauer’s chamber. Cellular viability was calculated as a percent.
2.5 RNA Extraction, cDNA Synthesis and Quantitative Real Time PCR
Total RNA from the cells was extracted using RNeasy kit (Qiagen Inc., Valencia, CA, USA) and was reverse-transcribed to cDNA following vendor’s instructions (Promega, Madison, WI, USA). Real-time PCR was performed using iQ5 real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and hot start PCR were performed using JumpStart Taq DNA polymerase (Sigma, St Louis, MO, USA). A fifty microliters real-time PCR reaction mixture containing 2µl cDNA (250ng), 2µl forward (200nM) 2µl reverse primer (200nM) and 25µl 2X iQ SYBR green super mix (Bio-Rad Laboratories) was run at universal cycle (95°C for 3 min, 40 cycles of 95°C 30 sec followed by 60°C 60 sec) following vendor’s instructions [Sharma et al 2009]. A fifty microliters conventional hot start polymerase chain reaction containing cDNA (250ng), forward primers (36 pg/ml), reverse primers (36 pg/ml), dNTP (400 mM of each), and JumpStart Taq polymerase (4 units) in a 10 mM Trizma–HCl, pH 8·3, 50 mM KCl, 1·5 mM MgCl2, and 0·001% gelatin was run using cycle conditions (95°C for 4 min, followed by 40 cycles of 95°C for 1 min, 55°C for 30 sec, and 72°C for 1 min, with a final cycle at 72°C for 10 min) reported earlier [Mohan et al., 2003]. The forward and reverse primers used in this study are given in Table-1. Beta actin was used to test the quality of cDNA and as a house keeping gene in real-time PCR. The threshold cycle (Ct) was used to detect the increase in the signal associated with an exponential growth of PCR product during the log-linear phase. The relative expression was calculated using the following formula, 2−ΔΔCt. The ΔCt validation experiments showed similar amplification efficiency for all templates used (difference between linear slopes for all templates less than 0.1). Three independent experiments were performed and the average (± SEM) results are presented in graphic form.
Table 1.
| Gene | Forward primer sequence (5’-3’) |
Reverse primer sequence (5’-3’) |
Product size |
Accession Number |
|---|---|---|---|---|
| Decorin | ATGCAGCTAGCCTGAA AGGACTGA |
TGTTGTCCAAGTGAAG CTCCCTCA |
123bp | NM_001920 |
| pcDNA3.1- decorin |
TAATACGACTCACTAT AGGG |
CCTCGACTGTGCCTTCT A |
1.3kb | |
| Collagen type I |
TGTGGCCCAGAAGAAC TGGTACAT |
ACTGGAATCCATCGGT CATGCTCT |
89bp | NM_000088.3 |
| Collagen type III |
TATCGAACACGCAAGG CTGTGAGA |
GGCCAACGTCCACACC AAATTCTT |
94bp | NM_000090.3 |
| Collagen type IV |
AGGTGTTGACGGCTTA CCTG |
TTGAGTCCCGGTAGAC CAAC |
132bp | NM_001845 |
| Fibronectin | CGCAGCTTCGAGATCA GTGC |
TCGACGGGATCACACT TCCA |
142bp | NM_002026 |
| SMA | TGGGTGACGAAGCACA GAGC |
CTTCAGGGGCAACACG AAGC |
138bp | NM_001613 |
| Beta actin | CGGCTACAGCTTCACC ACCA |
CGGGCAGCTCGTAGCT CTTC |
143bp | x00351 |
2.6 Protein extraction and Immunoblotting
Cells were washed with ice-cold PBS, lysed with 0.6ml RIPA buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 1% NP40, 0.5% sodium deoxycholate) containing protease inhibitor cocktail (Roche Applied Sciences, Indianpolis, IN, USA) and transferred to 1·5 ml microfuge tubes. DNA was sheared by passing cell lysates through a 21-gauge needle at least ten times and debris was removed by centrifugation at 14 000g at 4°C. The protein concentration of the lysates was determined using the Bio-Rad assay [Sharma et al., 2009].
The western blotting was performed by denaturing protein samples in Laemmli’s sample buffer containing β-mercaptoethanol at 70°C for 10 min. Proteins were resolved on 4–10% SDS–PAGE, and electrophoretically transferred to a 0.45-µm pore size PVDF membrane using Xcell-II blot module (Invitrogen). The membrane was blocked with 5% nonfat dry milk in TBST for 1 hr and probed with SMA, decorin or GAPDH primary antibodies (1:100 dilution) followed by secondary anti-mouse-or goat antibodies (1:2000 dilution). The bands were visualized by NBT/BCIP.
2.7 Immunocytochemistry
Immunofluorescent staining for SMA, a myofibroblast marker responsible for corneal fibrosis, was performed using mouse monoclonal antibody for SMA. Myofibroblast formation in decorin-transfected, naked vector-transfected and un-transfected HSF was stimulated by culturing clones in the presence of TGFβ1 (1ng/ml) under serum-free conditions. At study end-point, cultures were washed twice with PBS and incubated at room temperature with a mouse monoclonal antibody for SMA (DAKO) at a 1:200 dilution in 1X PBS for 90 minutes and with secondary antibody Alexa 488 or 594 goat anti-mouse IgG (Invitrogen) at a dilution of 1:500 for 1 hour. Cells were mounted with Vectashield containing DAPI (Vector Laboratories) to allow visualization of nuclei. Irrelevant isotype-matched primary antibody, secondary antibody alone, and tissue sections from naïve eyes were used as negative controls. Culture slides were visualized under Leica fluorescent microscope (Leica, Wetzlar, Germany) and photographed with a digital camera (SpotCam RT KE, Diagnostic Instruments Inc., Sterling Heights, MI, USA) equipped to microscope. The SMA-stained cells in ten randomly selected areas were counted per 200× and/or 400× magnification field.
2.8 Statistical analysis
Statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test for cell toxicity assay. The results were expressed as mean ± standard error of the mean (SEM). The real-time PCR data was analyzed using one-way ANOVA followed by Tukey’s multiple comparison tests. A “p value” <0.05 was considered significant. The immunoblotting data was analyzed using image J 1.38 × image analysis software (NIH, USA).
3. Results
3.1 Detection of decorin Transcript in HSF clones
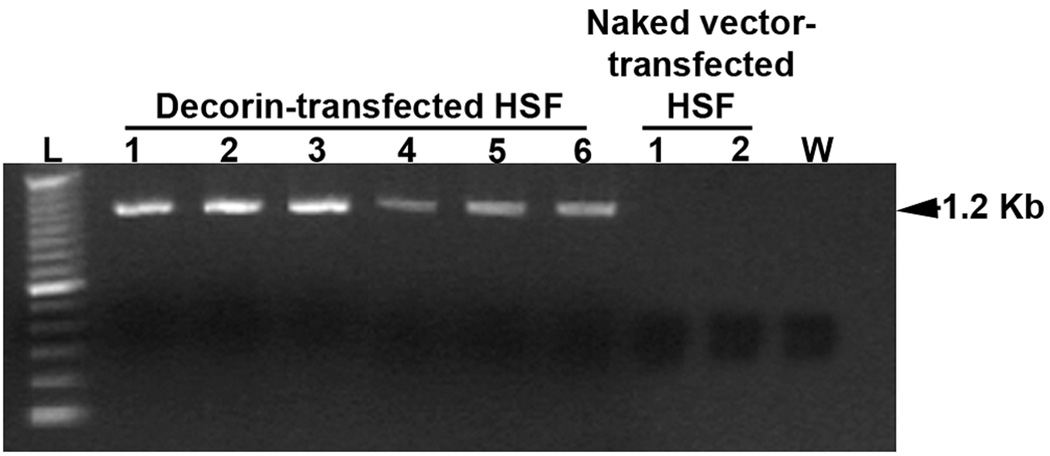
Various combinations of forward and reverse primers designed from decorin and vector DNA sequences were used in PCR to amplify decorin cDNA originating from RNA in HSF transfected with pcDNA3.1-decorin or control vector. Figure 1 shows the amplification bands detected in tested clones. Detection of DNA fragment of about 1.2 Kb corresponding to expected amplification product size confirmed new decorin mRNA transcription from the characterized decorin over-expressing clones. Control cultures showed no decorin amplification indicating that decorin expression in.
Fig. 1.
PCR analysis showing delivery of plasmid containing decorin gene into several identified HSF clones. After reverse transcribing total RNA from HSF cultures transfected with pcDNA3.1-decorin (6 clones) or naked vector (2 clones) were amplified using primers sets prepared from decorin and vector DNA sequences. An expected 1.2 Kb band for decorin was detected after 21 cycles of amplification. The “L” represents 100 bp DNA ladder and “W” is negative control reaction ran without cDNA.
3.1 Immunodetection of decorin transfected HSF clones
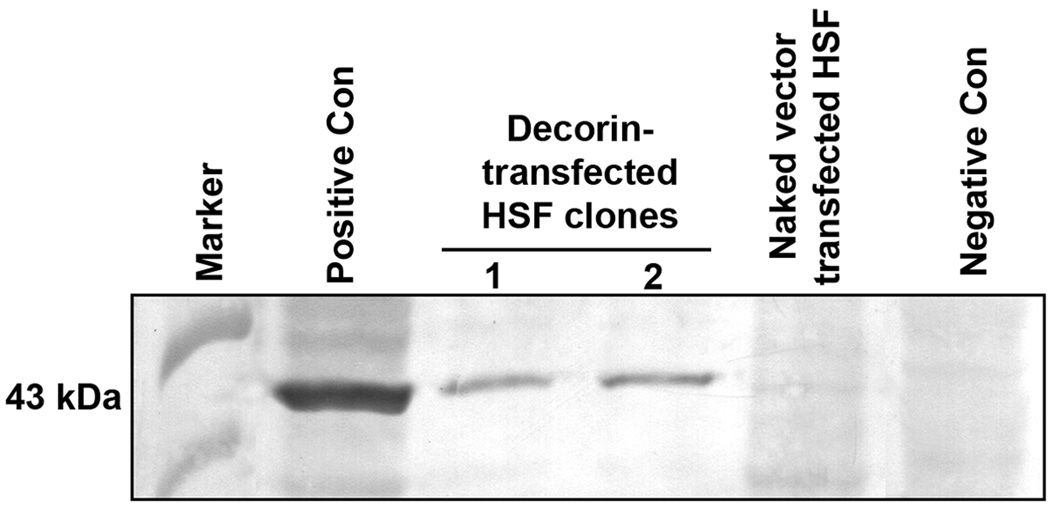
The protein cell lysates prepared from HSF clones transfected with pcDNA3.1-decorin or control vector were analyzed by western blotting using anti-decorin antibodies. Figure 2 shows representative immunoblotting data. The HSF clones trasfected with pcDNA3.1-decorin revealed a positive signal for decorin while no decorin signal was detected in clones transfected with naked vector.
Fig. 2.
Immunoblotting analysis showing expression of decorin in two decorin-transfected HSF clones. Total protein extracts prepared from HSF transfected with pcDNA3.1-decorin or naked vector were analyzed. A protein band at 43 kDa, specific for decorin, was detected in decorin-transfected HSF clones. The naked vector transfected HSF clone did not show any band. Commercially available decorin-transfected and non-transfected 293T cell lysates were used as positive and negative controls, respectively.
3.3 Cellular morphology and viability
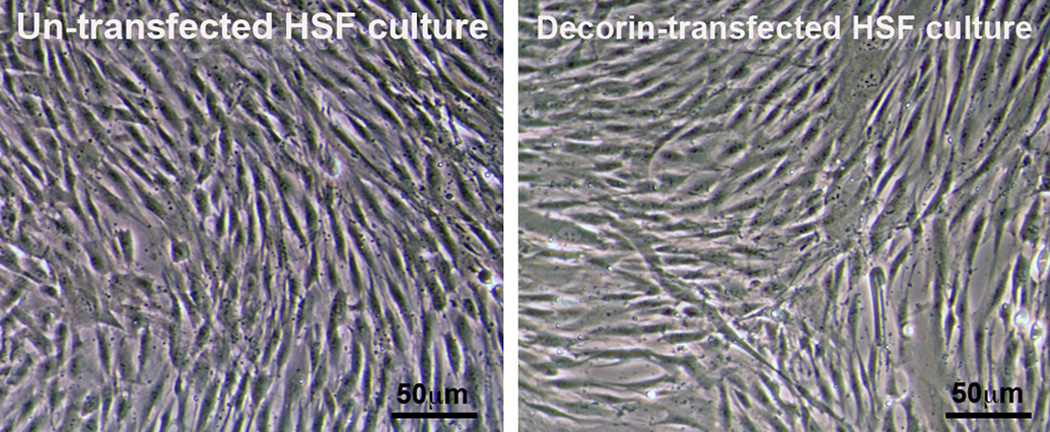
The cellular morphology of HSF clones transfected with pcDNA3.1-decorin or control vector were examined under phase-contrast microscopy. Both, decorin- and naked-vector transfected HSF, clones presented phenotype exhibited by normal human corneal fibroblasts. The characterized clones were long, spindle-shaped and possessed typical fibroblastic morphology when grown to confluence in medium containing 10% serum. Figure 3 shows cellular morphology of HSF clones transfected with pcDNA3.1-decorin or naked vector in confluent cultures. This data suggest that decorin gene transfer into HSF do not alter cellular morphology.
Fig. 3.
Representative phase-contrast light microscopy images showing un-transfected and decorin-transfected HSF clones phenotype. The 80–90% confluent decorin-transfected (left panel) HSF clone showed morphology similar to un-transfected HSF clones (right panel). This suggests that decorin gene transfer does not alter HSF phenotype. Scale bar denotes 50 µm.
The effect of decorin gene transfer on HSF viability was determined by trypan assay. Tested decorin-and naked-vector transfected clones showed varied cellular viability. Both, the decorin- and naked-vector transfected HSF clones at early passages (up to 3) showed >93% viable cells very similar to normal untransfected HSF (data not shown). A gradual decrease in cellular viability (10% or more) was observed in decorin- and naked-vector transfected tested clones in later passages. The clones were not sub-cultured after passage 6. This data suggest that decorin over-expression in HSF does not affect its viability.
3.4 Effect of decorin trasfection on fibrosis-related genes in HSF
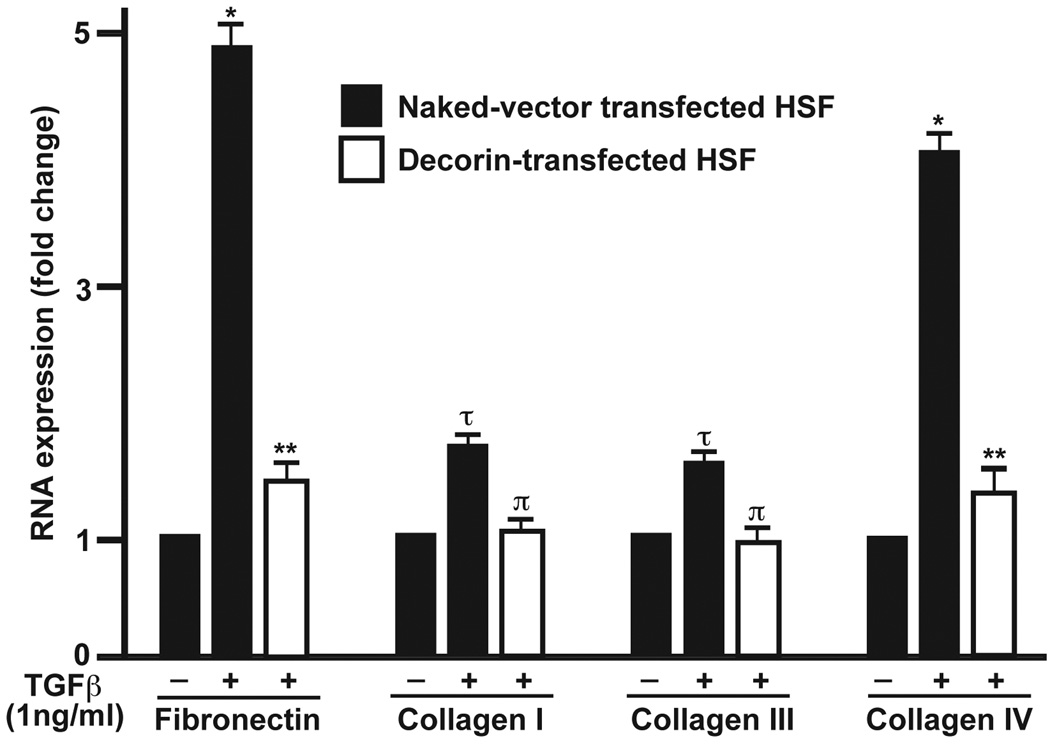
Quantitative PCR investigated mRNA expression of the extracellular matrix (ECM) components namely fibronectin, collagen type I, III and IV in decorin- or naked-vector transfected HSF clones grown in +/− of TGFβ1 (1ng/ml) under serum-free conditions or 10% serum-containing medium. Figure 4 presents quantitative mRNA measurements performed with real-time PCR. The TGFβ1 stimulation caused statistically significant increase in the expression of tested pro-fibrogeneic genes fibronectin (4.8-fold; p<0.01), collagen type I (1.6-fold; p<0.05), collagen type III (1.4-fold; p<0.05), and collagen type IV (4.1-fold; p<0.01) in naked vector-transfected HSF cultures. The decorin transfection to HSF significantly inhibited TGFβ1-driven increase of ECM as decorin-transfected HSF cultures showed significant decrease in fibronectin (3.1-fold; p<0.001), collagen type I (1-fold; p<0.01), collagen type III (0.9-fold; p<0.01), and collagen type IV (3.2-fold; p<0.001) RNA levels compared to the naked-vector transfected HSF cultured under similar conditions (Figure 4). No significant differences in the fibronectin, collagen type I, III or IV RNA levels were detected between the un-transfected normal and naked-vector transfected HSF cultures (data not shown).
Fig. 4.
Real-time quantitative PCR showing analysis of pro-fibrogenic genes, fibronectin, alpha collagen type-I, -III, and -IV expression in un-transfected, naked vector-transfected, and decorin-transfected HSF cultures grown in presence or absence of TGFβ1 (1ng/ml) under serum-free conditions. The TGFβ1 caused 1.1–4.8-fold increase in tested genes in un-transfected or naked-vector transfected HSF cultures grown in presence of TGFβ1 compared to cultures grown in absence of TGFβ1. Decorin transfection showed statistically significant inhibition of TGFβ1-induced expression of fibronectin, collagen type-I, -III and -IV. *=p<0.01 and τ=p<0.05 (−TGFβ1 naked-vector transfected HSF vs +TGFβ1 naked-vector transfected HSF), **=p<0.001 and π=p<0.01 (−TGFβ1 naked vector-transfected vs +TGFβ1 decorin-transfected HSF). No significant differences in the RNA levels of fibronectin, collagen type I, III or IV were detected between the un-transfected normal and naked-vector transfected HSF. (Data not shown)
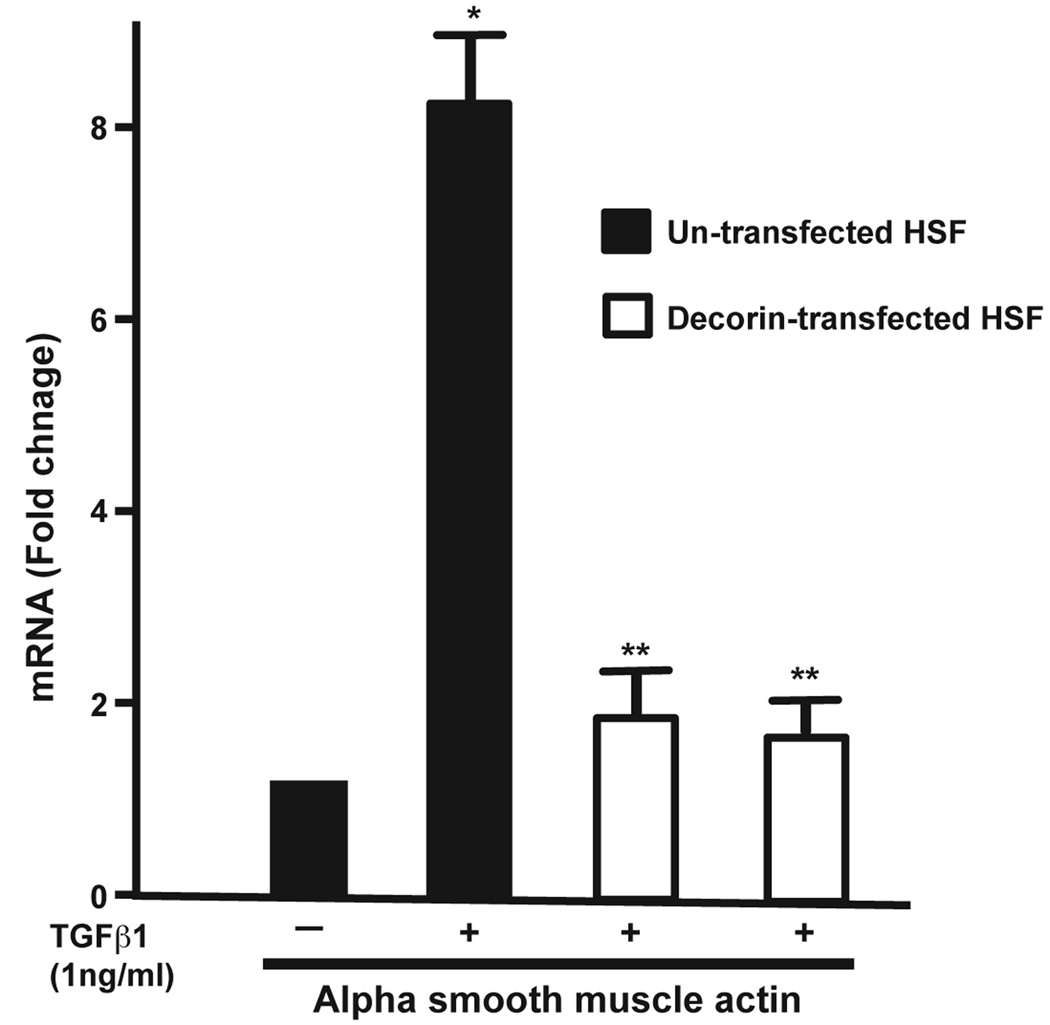
3.5 Effect of decorin transfection on TGFβ1-driven myofibroblast modulation
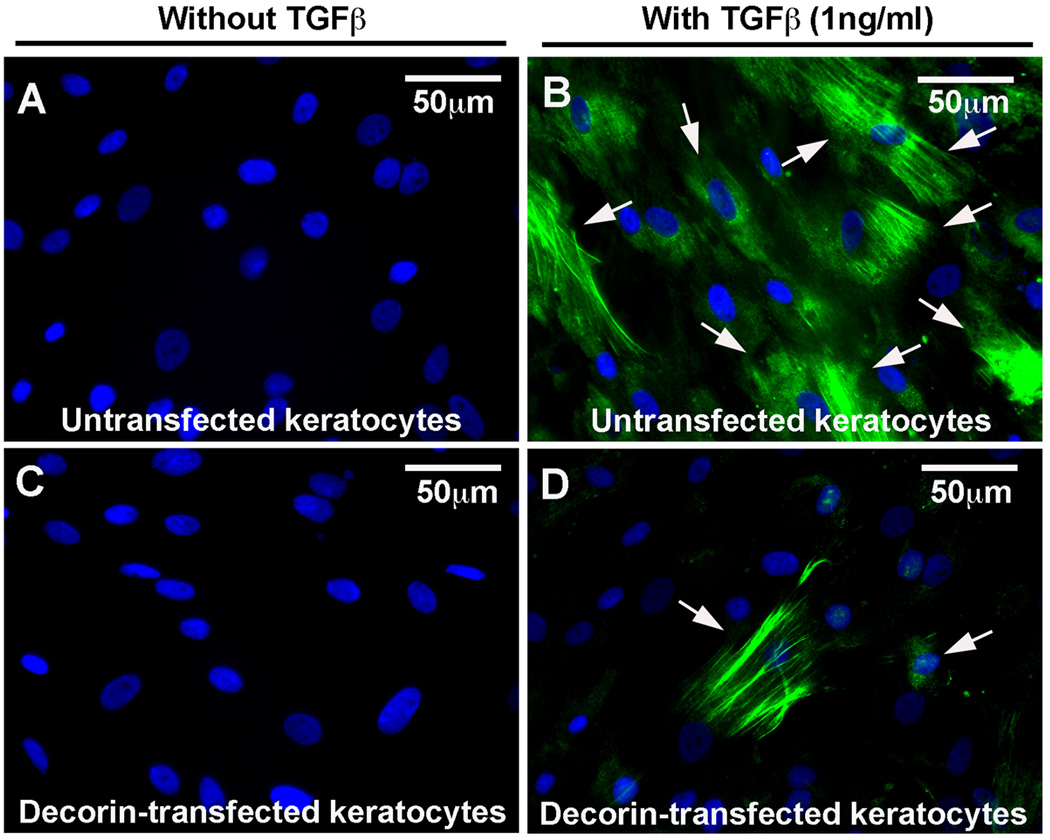
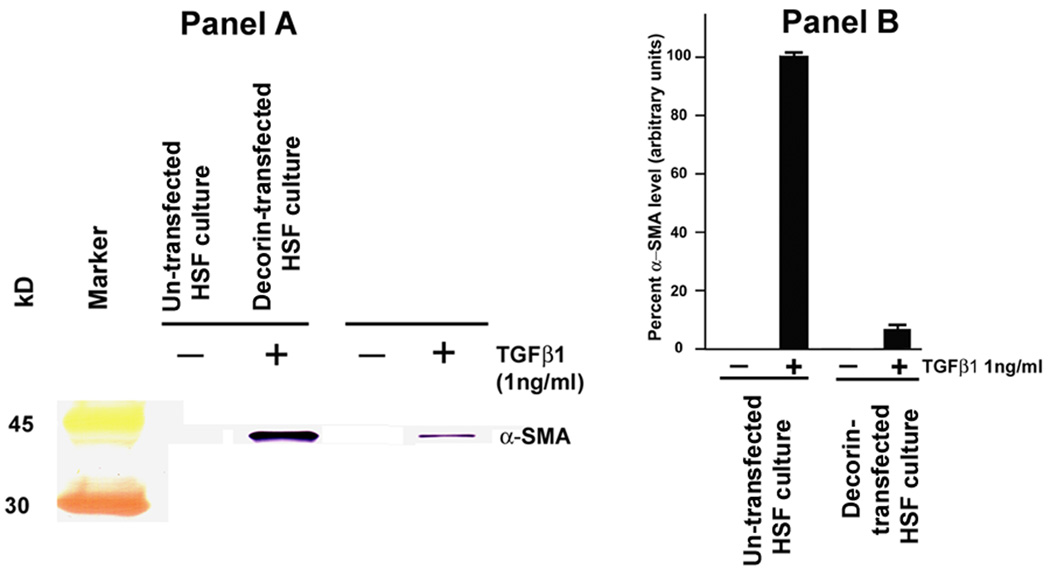
To test the hypothesis that decorin gene transfer inhibits myofibroblast formation in the cornea, the effect of decorin on TGFβ1-stimulated transformation of in corneal fibroblast to myofibroblasts was examined. The levels of α-smooth muscle actin (SMA), a myofibroblast marker, mRNA and protein in decorin-transfected and untransfected HSF clones grown in presence or absence of TGFβ1 (1ng/ml) under serum-free conditions were quantified with immunostaining and immunoblotting. Figure 5 shows quantitative measurement of SMA RNA in decorin-transfected and un-transfected HSF cultures. As evident from the data TGFβ1 stimulated un-transfected HSF culture showed 8.3 fold (p<0.01) increase in the levels of SMA gene expression compared to HSF not stimulated to TGFβ1, and decorin-transfected HSF cultures demonstrated significant decrease in SMA (more than 6-fold; p value <0.001) under similar conditions (Figure 5). Figures 6 and 7 show analysis of SMA protein performed with immunocytochemistry and western blot, respectively. As evident from the immunocytochemistry data shown in Figure 5, decorin-transfected HSF clone (panel D) showed a statistically significant lowering in SMA-stained cells (~79%, p value <0.01) compared to the untransfected normal HSF grown in the presence of TGFβ1 (panel B) under serum-free conditions. Conversely, neither untransfected normal (panel A) nor decorin-transfected (panel C) cultures grown in absence of TGFβ1 showed any SMA-stained cells (Figure 6). Similar results were observed with western blot analysis and are shown in Figure 7. The whole cell lysates of decorin-transfected HSF cultures showed a statistically significant decrease in SMA levels (~83%, p value <0.01) compared to un-transfected HSF cultures. These findings support our hypothesis that decorin gene transfer could inhibit myofibroblast and haze development in the cornea in vivo.
Fig. 5.
Real-time quantitative PCR showing analysis of SMA RNA levels in un-transfected and decorin-transfected HSF cultures grown in presence or absence of TGFβ1 (1ng/ml) under serum-free conditions. A statistically significant more than 8-fold (p<0.01) increase in the SMA gene was detected in un-transfected HSF cultures grown in the presence of TGFβ1 compared to cultures grown in the absence of TGFβ1. Decorin transfected HSF samples showed a statistically significant decrease (>6-fold; p<0.001) in SMA compared to un-transfected HSF cultures grown under similar conditions. The * indicates a p<0.01 compared to negative control (no TGFβ1) and the ** indicates a p<0.001 of un-transfected HSF versus decorin-transfected HSF grown in the presence of TGFβ1.
Fig. 6.
Representative images showing immunocytochemistry of SMA, a myofibroblast marker, done in un-transfected HSF and decorin-transfected HSF cultures grown in presence or absence of TGFβ1 (1ng/ml) under serum-free conditions. Decorin-transfected HSF (D) showed a significant decrease (79%, p<0.01) in SMA-positive cells compared to the un-transfected HSF (B) grown in the presence of TGFβ1. No SMA-positive cells were detected in un-transfected (A) or decorin-transfected cultures (C) grown in the absence of TGFβ1. Scale bar denotes 50 µm.
Fig. 7.
Western blot analysis (panel A) and digital quantification (panel B) of SMA expression detected in un-transfected HSF and decorin-transfected HSF grown in the presence or absence of TGFβ1 under serum-free conditions. Decorin-transfected HSF cultures showed a significant decrease in SMA-expression compared to un-transfected HSF grown in the presence of TGFβ1 (~83%, p<0.01). Neither un-transfected nor decorin-transfected HSF cultured in the absence of TGFβ1 expressed SMA.
4. Discussion
The results of this study show that decorin transfection inhibits TGFβ driven elevated expression of profibrogenic genes, and formation of myofibroblasts in human corneal fibroblasts. TGFβ regulates a diverse set of essential cellular processes. The pathogenic role for TGFβ in many corneal diseases and dystrophies including corneal scarring has been demonstrated in many experimental models [Jester et al. 1997]. It is also implicated in the development of haze in the cornea following refractive laser eye surgery. Formation of corneal haze involves keratocyte apoptosis, proliferation and transformation of corneal keratocytes to fibroblasts and myofibroblasts as well as increase in the expression of smooth muscle actin and deposition of disorganized extra-cellular matrix proteins [Jester et al., 1999; Wilson et al., 2001; Mohan et al., 2003; Netto et al., 2006; Stramer et al., 2003]. Numerous studies showed up-regulation of TGFβ RNA and its receptors are after surface laser ablation in laser eye surgery photorefractive keratectomy [Imanishi, 2000]. We observed a time-dependent increase in the expression of TGFβ1 and TGFβ2 in the corneal stroma of rabbit eye subjected to −9 diopter photorefractive keratectomy with excimer laser [Mohan et al Unpublished data]. The blocking of TGFβ activity with antibodies, or antisense RNA specific to TGFβ1 has been shown to reduce synthesis of ECM proteins and corneal scarring in animal models [Jester et al., 1997; Nakamura, 2004]. It was postulated that administration of anti-TGFβ genes such as proteoglycan decorin in the cornea repressing TGFβ activity is an attractive approach to control corneal scarring. Decorin gene transfer has been shown to inhibit fibrosis in many non-ocular tissues [Border et al.1992; Giri et al. 1997; Isaka et al. 1996; Kolb et al 2001].
Proteoglycans have ability to modulate activities growth factors. The lumican, keratocan, and decorin proteoglycans are important components of extracellular matrix of the cornea [Tanihara et al., 2002; Chakravarti, 2002; Kao and Liu, 2002; Funderburgh,1989; Funderburgh, 2001]. In corneal stroma, proteoglycans regulate collagen fibril organization, thus playing a critical role in corneal transparency [Funderburgh, 2001]. The structural abnormalities in the corneas of proteoglycan null mice have been demonstrated [Chakravarti, 2002; Kao and Liu, 2002]. Funderburg et al showed a reduction in keratin sulfate and an increase in dermatan sulfate gylcosaminoglycans in rabbit cornea. It is also evident from numerous studies that proteoglycans can act as signal molecule for kinases and growth factor receptors that influence cellular functions such as cell migration, proliferation and apoptosis [Iozzo, 1997; Schaefer and Iozzo, 2008]. These cellular functions are essential components of corneal healing and emphasize the role of proteoglycans in corneal wound healing. Indeed, mice lacking proteoglycans such as lumican or keratocan have shown delayed corneal healing [Tanihara et al., 2002; Chakravarti, 2002]. Decorin, a small leucine rich proteoglycans, has been shown to modulate the fibrillar organization of collagen and regulate collagen fibrillogenesis [Reed and Iozzo, 2002]. Impaired angiogenesis in injured cornea of the decorin-deficient mice suggests its role in corneal angiogenesis [Schonherr et al., 2004]. Increased production of decorin in human mesangial cells has been shown to downregulate expression of the growth factors like TGFβ, PGDFβ, extracellular matrix components such as fibronectin, tenascin, collagens are type I, and collagen type IV suggesting that decorin plays important role in regulating wound healing and fibrosis. Collagen type I and III are commonly affected during fibrosis, however, modulation of collagen type IV in the laser ablated cornea following photorefractive keratectomy and laser in situ keratomileusis surgeries has also been reported [Mohrenfels et al. 2002a; Mohrenfels et al. 2002b; Kaji et al. 2001]. A decrease of collagen I, III and IV RNA expression detected in decorin over-expressing clones suggest that decorin plays important role corneal matrix organization and TGFβ-induced wound healing. Furthermore, suppression of glomerular matrix production and accumulation in the injured glomeruli of rat by decorin injections provide strong support to our hypothesis that decorin gene transfer in the cornea may prove to be clinically useful in treating corneal diseases associated with hyperactivity of TGFβ.
Successful restoration of vision in human patients with gene therapy affirms its promise to cure ocular diseases and prevent blindness [Bainbridge et al., 2008; Maguire et al., 2008]. The feasibility of gene therapy to treat corneal scarring has already been demonstrated in experimental animals by delivering herpes simplex virus thymidine kinase or cyclin G1 gene into rabbit keratocytes with retrovirus vector [Seitz, 1998; Behrens et al., 2002]. The potential of decorin gene therapy to inhibit TGFβ-driven fibrosis has been demonstrated in many non-ocular tissues employing various in vivo and in vitro experimental models [Giri et al., 1997; Shimizukawa et al., 2003; Isaka et al., 1996; Margetts et al 2002; Zhao et al 1999; Kolb et al., 2001]. Nevertheless, no studies have been performed to examine the potential of decorin gene therapy in modulating wound healing and curing fibrosis in the cornea. This study is the first to investigate the effect of decorin gene transfer in human corneal fibroblast cultures. The results of our study demonstrate that increased production of decorin in HSF, obtained by the decorin transfection, down-regulates collagen type IV and fibronectin gene expression indicating modulation of matrix accumulation and retardation of fibrosis. Additionally studies with HSF exhibiting decorin production detected a significant reduction in TGFβ-stimulated transformation of HSF to myofibroblasts suggesting that decorin is capable of treating fibrosis in the cornea. Thus, we postulate that decorin gene therapy can cure corneal fibrosis in vivo. This is a promising approach that deserves further investigation.
Acknowledgements
The work was supported by the RO1EY17294 (RRM) grant from the National Eye Institute, National Institutes of Health, Bethesda, MD and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Authors thank Heartland Eye Bank, St. Louis, Missouri for donor human corneas. Thanks are due to Jason M. Newman medical student (M-3) for his help in cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial Relationship: R. R. Mohan, None; R. Gupta, None; M. K. Mehan, None; J. W. Cowden, None; S. Sinha, None.
References
- Axelsson I, Heinegard D. Fractionation of proteoglycans from bovine corneal stroma. Biochem J. 1975;145:491–500. doi: 10.1042/bj1450491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar DT. Corneal angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing. Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Behrens A, Gordon EM, Li L, Liu PX, Chen Z, Peng H, La Bree L, Anderson WF, Hall FL, McDonnell PJ. Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze. Invest Ophthalmol Vis Sci. 2002;43:968–977. [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Brown CT, Lin P, Walsh MT, Gantz D, Nugent MA, Trinkaus-Randall V. Extraction and purification of decorin from corneal stroma retain structure and biological activity. Protein Expr Purif. 2002;25:389–399. doi: 10.1016/s1046-5928(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- Cheifetz S, Massagué J. Transforming growth factor-beta (TGF-beta) receptor proteoglycan. Cell surface expression and ligand binding in the absence of glycosaminoglycan chains. J Biol Chem. 1989;264:12025–12028. [PubMed] [Google Scholar]
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Taylor HR, Mitchell P. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7:2388–2393. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor-interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- Fong CS. Refractive surgery: the future of perfect vision? Singapore Med J. 2007;48:709–718. [PubMed] [Google Scholar]
- Funderburgh JL, Chandler JW. Proteoglycans of rabbit corneas with nonperforating wounds. Invest Ophthalmol Vis Sci. 1989;30:435–442. [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54:1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Lozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Newsome DA, Hascall VC. Characterization and biosynthesis of proteoglycans of corneal stroma from rhesus monkey. J Biol Chem. 1979;254:12346–12354. [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- Jarvelainen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV, Sage EH, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997;16:177–187. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog. Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010 Jan 18; doi: 10.1016/j.matbio.2010.01.001. [PMID: 20080181] [DOI] [PubMed] [Google Scholar]
- Kaji Y, Soya K, Amano S, Oshika T, Yamashita H. Relation between corneal haze and transforming growth factor-beta1 after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1840–1846. doi: 10.1016/s0886-3350(01)01141-5. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. Decorin binds near the C terminus of type I collagen. 2000;29:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Congdon NG, O'Colmain BJ. Eye Diseases Prevalence Research Group. Prevalence of refractive errors among adults in USA, Western Europe & Australia. Arch Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, Gauldie J. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, V.olpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;22:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetts PJ, Gyorffy S, Kolb M, Yu L, Hoff CM, Holmes CJ, Gauldie J. Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol. 2002;13:721–728. doi: 10.1681/ASN.V133721. [DOI] [PubMed] [Google Scholar]
- Markmann A, Hausser H, Schönherr E, Kresse H. Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction. Matrix Biol. 2000;7:631–636. doi: 10.1016/s0945-053x(00)00097-4. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Possin DE, Mohan RR, Sinha S, Wilson SE. Development of genetically engineered tet HPV16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Exp Eye Res. 2003;77:395–407. doi: 10.1016/s0014-4835(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BY. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004;10:703–711. [PubMed] [Google Scholar]
- Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E, Sunderkötter C, Schaefer L, Thanos S, Grässel S, Oldberg A, Iozzo RV, Young MF, Kresse H. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res. 2004;6:499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function IUBMB Life. 2008;60:729–733. doi: 10.1002/iub.115. [DOI] [PubMed] [Google Scholar]
- Seiler T, McDonnell PJ. Excimer laser PRK. Surv. Ophthalmol. 1995;40:89–118. doi: 10.1016/s0039-6257(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Seitz B, Moreira L, Baktanian E, Sanchez D, Gray B, Gordon EM, Anderson WF, McDonnell PJ. Retroviral vector-mediated gene transfer into keratocytes in vitro and in vivo. Am J Ophthalmol. 1998;126:630–639. doi: 10.1016/s0002-9394(98)00205-0. [DOI] [PubMed] [Google Scholar]
- Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR. Trichostatin-A inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci. 2009;50:2695–2701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizukawa M, Ebina M, Narumi K, Kikuchi T, Munakata H, Nukiwa T. Intratracheal gene transfer of decorin reduces subpleural fibroproliferation induced by bleomycin. Am J Physiol Lung Cell Mol Physiol. 2003;284:526–532. doi: 10.1152/ajplung.00131.2002. [DOI] [PubMed] [Google Scholar]
- Ständer M, Naumann U, Wick W, Weller M. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221–227. doi: 10.1007/s004410051283. 1999. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Koga T, Yano T, Kimura A. Proteoglycans in the eye. Cornea. 2002;21:62–69. doi: 10.1097/01.ico.0000263121.45898.d2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hong JW, Lee JS, Choi R, Mohan RR. The wound healing response after laser in situ keratomileusis and photorefractive keratectomy: elusive control of biological variability and effect on custom laser vision correction. Arch Ophthalmol. 2001;119:889–896. doi: 10.1001/archopht.119.6.889. [DOI] [PubMed] [Google Scholar]
- Winkler von Mohrenfels C, Reischl U, Lohmann CP. Corneal haze after photorefractive keratectomy for myopia: role of collagen IV mRNA typing as a predictor of haze. J Cataract Refract Surg. 2002a;28:1446–1451. doi: 10.1016/s0886-3350(02)01273-7. [DOI] [PubMed] [Google Scholar]
- Winkler von Mohrenfels CW, Reischl U, Gabler B, Lohmann CP. Corneal haze after photorefractive keratectomy. Role of individual collagen type IV synthesis on postoperative corneal opacity. Ophthalmologe. 2002b;99:532–537. doi: 10.1007/s00347-001-0559-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sime PJ, Bringas P, Jr, Gauldie J, Warburton D. Adenovirus-mediated decorin gene transfer prevents TGF-beta-induced inhibition of lung morphogenesis. Am J Physiol. 1999;277:412–422. doi: 10.1152/ajplung.1999.277.2.L412. [DOI] [PubMed] [Google Scholar]