Abstract
Allergic asthma is caused by inhaled allergens and it is characterized by airway eosinophilia as well as mucus hypersecretion which can lead to airflow obstruction. Despite the association of increased IL-6 levels with human atopic asthma, the contribution of IL-6 to the development of allergic airway inflammation triggered by inhaled allergens remains unclear. In this study, we examined the role of IL-6 in a mouse model of allergic airway inflammation induced by direct airway exposure to extracts of Aspergillus fumigatus, a common allergen in humans. We show here that inhaled A. fumigatus extracts rapidly triggers the production of IL-6 in the airways. IL-6 appears to be dispensable for the recruitment of eosinophils to the lung during the development of allergic airway inflammation. However, IL-6 is essential for mucus hypersecretion by airway epithelial cells triggered in response to inhaled A. fumigatus antigens. Impaired mucus production caused by IL-6 deficiency correlates with a severe reduction in the levels of IL-13, a major inducer of mucin glycoproteins. Thus, IL-6 is a key regulator of specific hallmark features of allergic airway inflammation, and it could be a potential target for pulmonary diseases that are associated with goblet cell metaplasia and mucus hypersecretion.
Keywords: Lung, Allergy, Fungal, Rodent, Cytokines
INTRODUCTION
IL-6 is a multifunctional cytokine that is produced by a variety of hematopoietic and non-hematopoietic cell types in response to diverse stimuli (1). Although IL-6 was originally considered as a surrogate marker of inflammation similar to IL-1β and TNF-α, recent studies have shown that IL-6 influences the effector functions of various CD4+ T cell subsets. IL-6 inhibits Th1 differentiation through the upregulation of suppressor of cytokine signaling 1 (SOCS1) (2), and promotes Th2 differentiation by the induction of NFAT (3) and c-Maf (4) during early CD4+ T cell activation. In addition, IL-6 induces the differentiation of Th17 effector cells in the presence of TGFβ (5-7). In contrast, it inhibits regulatory T cell (Treg) development most likely by suppressing Foxp3 expression (6, 8, 9). Its role in promoting Th2 and Th17 differentiation along with inhibiting Treg activity, suggests that IL-6 might play a role in the onset and/or progression of diseases that are associated with these types of immune responses.
Allergic asthma is a chronic inflammatory disease of the airway that occurs in response to inhaled allergens such as ragweed pollen, cat dander, house dust mites, and fungi. The development of a CD4+ Th2 immune response and its associated cytokines (e.g. IL-4, IL-5, and IL-13) are known to play an important role in the pathogenesis of allergic asthma. IL-4 promotes Ig isotype switching in B cells to produce primarily IgE and IgG1 (10). IL-5 promotes eosinophil survival, differentiation and migration (11) while IL-13 induces mucus metaplasia and airway hyperresponsiveness (12-14). Despite the role of Th17 cells in inflammation (15), the function of this subset and its signature cytokines IL-17A and IL-17F in allergic airway inflammation is less clear. IL-17R deficient mice exhibit a decrease in eosinophil recruitment, Th2 cytokine production, and IgE levels during induction of allergic airway inflammation, suggesting that IL-17 is required during the initiation of allergic asthma (16, 17). However, exogenous administration of IL-17 during allergen challenge decreases local Th2 cytokine production and eosinophilia through repressing eotaxin production by lung epithelial cells (16).
Despite the role of IL-6 in Th2 and Th17 differentiation, its relative contribution to allergic airway inflammation remains unclear. Correlation studies in patients with asthma have shown increased IL-6 levels in bronchoalveolar lavage fluid (BALF) (18) and serum (19), as well as increased IL-6 production from cultured lung epithelial cells (20), suggesting a role for IL-6 in asthma pathogenesis. However, studies in mice have yielded conflictive results. Studies examining acute or chronic allergic airway inflammation induced by intraperitoneal (i.p.) ovalbumin (OVA) immunization with alum followed by challenge with aerosolized OVA in IL-6 knockout mice show increased airway eosinophilia and Th2 cytokine production, suggesting that IL-6 may negatively regulate allergic airway inflammation (21, 22). In contrast, local disruption of IL-6 signaling in the lung by intranasal administration of an IL-6Rα blocking antibody prior to OVA challenge decreases OVA-induced Th2 inflammation in vivo, suggesting that IL-6 positively regulates the Th2 immune response (23). Overall, whether IL-6 promotes or inhibits Th2 inflammation or even acts in a dual fashion in allergen-induced airway inflammation remains unclear.
In this study, we examined the role of IL-6 in allergic airway inflammation induced by repeated direct exposure of the lungs to extracts of Aspergillus fumigatus (A.f), a common spore-forming fungi and known allergen in humans (24), without previous i.p. immunization. We show that IL-6 is essential specifically for the development of mucus metaplasia in the airways in response to inhaled allergen, but it is dispensable for the recruitment of eosinophils to the lung. Thus, the elevation of IL-6 found in some respiratory diseases (e.g. COPD and allergic asthma) could contribute to the pathogenesis of the disease by promoting mucus hypersecretion.
RESULTS
IL-6 is rapidly produced in the lung in response to A. fumigatus exposure
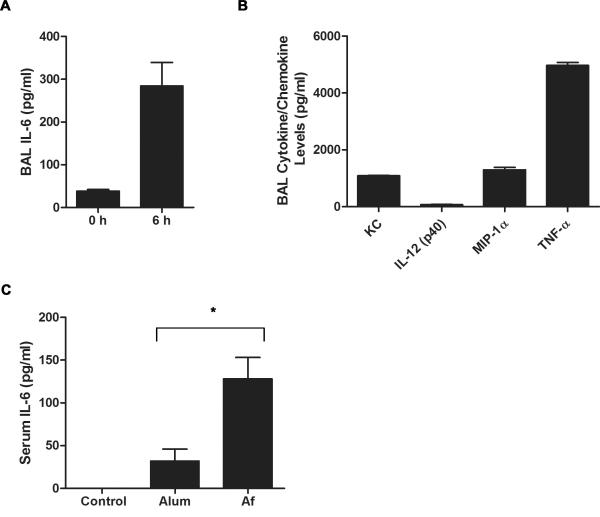
Allergen exposure in human subjects leads to increased IL-6 levels in BALF and sputum (18, 25). Several studies have shown a role of IL-6 in the differentiation of CD4+ T cells into specific effector cell subsets. Thus, IL-6 produced in the lung upon allergen exposure could modulate the type of CD4+ T cell response that develops against inhaled antigens. Fungi (e.g. A. fumigatus and A. alternata) are a commonly encountered environmental allergens and act as an asthma trigger (26). We have previously shown that direct lung exposure to A. fumigatus (A.f.) extracts by oropharyngeal (o.p.) administration in the absence of adjuvant is sufficient to induce allergic airway inflammation characterized by eosinophilia, mucus hypersecretion and Th2 cytokine production (27). In order to determine whether A.f. exposure induces IL-6 production in the lung, IL-6 levels were examined in BALF from unexposed mice and mice treated o.p. with A.f. extracts. As soon as 6 h post exposure, high levels of IL-6 were already present in BALF, suggesting that this cytokine could affect CD4+ T cell differentiation (Fig. 1A). We also examined the presence of other cytokines known to affect the differentiation of CD4+ T cells into specific effector Th cells. Although TNF-α and specific chemokines (e.g. KC and MIP-1α) were also induced (Fig. 1B), none of the established polarizing cytokines such as IL-4 (Th2 polarizing), IL-12 or IFNγ (Th1 polarizing) were detected in the BALF of A.f.-exposed mice (data not shown). Thus, direct A.f. exposure to the lung selectively triggered the production of IL-6 among the polarizing cytokines.
Figure 1.
A.fumigatus induces rapid IL-6 production in the airway and peripheral blood. (A) Wild type mice (n=4) were o.p. administered A.f. extracts. IL-6 levels in the BALF were assessed at 0 and 6 h post exposure by ELISA. The results indicate the mean ± SEM (p<0.05). (B) Wild type mice were o.p. administered A.f. extracts and 6 h later cytokine/chemokine levels in BALF were determined by Bio-Plex. (C) Wild type mice were i.p. administered OVA with alum (n=5) or A.f. (n=4) and 6 h later serum IL-6 levels were measured by ELISA (*, p<0.05). Data are representative of two or three independent experiments.
Although IL-6 is considered as an inflammatory cytokine that is induced early during the immune response by a number of adjuvants (28, 29), recent studies have shown that alum, a commonly used adjuvant, activates the inflammasome pathway and induces IL-1β and IL-18, but has minimal effect on IL-6 (30, 31). To further show the specific effect of A.f. on IL-6 production during the initiation of the immune response, mice were i.p. immunized with OVA together with alum or A.f. extracts and IL-6 levels in serum were determined. In agreement with previous studies on alum, the level of IL-6 in the serum of mice administered the adjuvant was minimal at 6 h post injection (Fig. 1C) and undetectable after 12 h (data not shown). In contrast, i.p. immunization with A.f. extracts induced significant IL-6 production (Fig. 1C). Thus, components within natural allergens favor IL-6 production.
Airway eosinophilia does not require IL-6
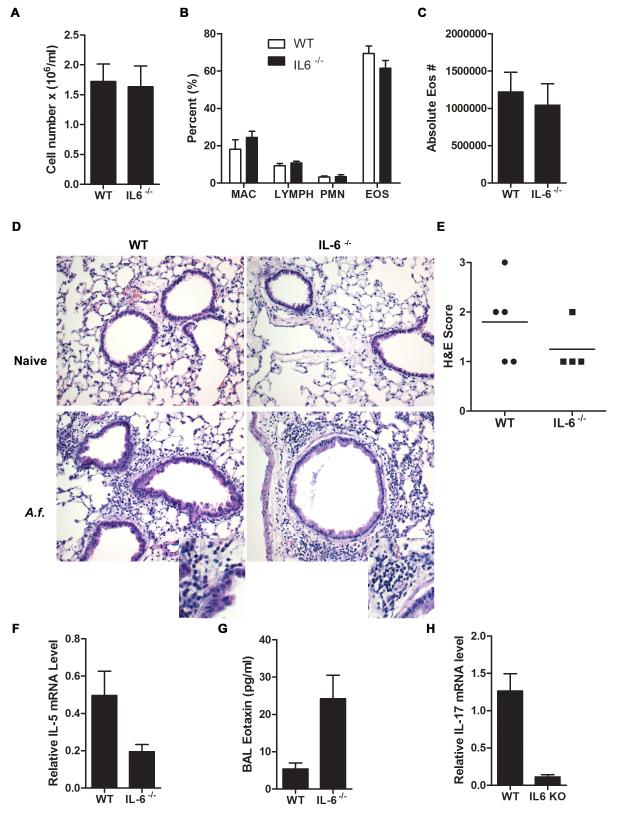
The above studies indicate that direct airway exposure to allergens such as A.f., triggered rapid production of IL-6 in the mouse, as previously described in human subjects. Considering the known role of IL-6 in Th1, Th2, and Th17 differentiation, we investigated the contribution of this cytokine to the different pathological features of allergic airway inflammation induced by inhalation of A.f. extracts. We used IL-6 deficient (IL-6−/−) mice that were previously generated by deletion of exons I, II, and III of the IL-6 gene (32). Wild type and IL-6−/− mice were administered A.f. extracts o.p. on day 0, 7, and 14. Allergic airway inflammation was examined 48 h after the last dose. The total cell count in the BALF was similar in A.f.-exposed wild type and IL-6−/− mice (Fig. 2A). Cell differential analysis in BALF (Fig. 2B) and total eosinophil count (Fig. 2C) showed that the accumulation of these cells were also comparable in wild type and IL-6−/− mice. In addition, H&E staining analysis showed similar peribronchial and perivascular eosinophilic inflammation (Fig. 2D and 2E). Thus, IL-6 deficiency does not interfere with the recruitment of eosinophils to the lung.
Figure 2.
IL-6 does not contribute to eosinophilic infiltration in the lung. (A-C) Wild type and IL-6−/− mice (n=4) were administered A.f. extracts o.p. on day 0, 7, and 14. BALF was collected 48 h after last A.f. dose. (A) Total cell count in BALF was determined (p=0.89). (B) Relative percentage of macrophages (MAC), lymphocytes (LYMPH), neutrophils (PMN), and eosinophils (EOS) in BALF (p>0.05). (C) Absolute number of eosinophils in BALF (p=0.89). (D) H&E staining of paraffin embedded lungs sections from unexposed (naïve) and A.f.-exposed wild type and IL-6−/− mice (×200) as described in (A). Insets show expanded image. (E) Quantification of lung inflammation in A.f.-exposed wild type (n=5) and IL-6−/− (n=4) mice as indicated by H&E staining (p=0.34). (F) IL-5 expression in total lung tissue from A.f.-exposed wild type (n=5) and IL-6−/− (n=4) mice was determined by real time RT-PCR and normalized to 18S RNA (p<0.05). (G) BALF eotaxin levels from A.f.-exposed wild type (n=9) and IL-6−/− (n=8) mice were quantified by ELISA (p<0.05). (H) Wild type (n=5) and IL-6−/− (n=4) mice were exposed to A.f. extracts as described in (A) and IL-17 expression in CD4+ T cells purified from wild type and IL-6−/− mice was determined by real time RT-PCR and normalized to β2m RNA (p<0.05). Data are representative of two or three independent experiments.
Since IL-5 is one of the major factors that promotes airway eosinophilia following allergen exposure (33), it was possible that IL-6 deficiency did not affect the levels of this cytokine. However, a reduction of IL-5 expression was detected in the IL-6−/− mice (Fig. 2F), suggesting that some other factors could compensate for the impaired IL-5 production. Eotaxin is also a potent chemotactic factor for eosinophils (34). Surprisingly, eotaxin levels in the BALF were significantly higher in IL-6−/− mice compared with wild type mice (Fig. 2G). Thus, increased levels of eotaxin could compensate for the impaired IL-5 production and support eosinophil recruitment in IL-6 deficient mice.
Eotaxin is primarily produced by epithelial cells (34) and there is no evidence suggesting that IL-6 has a direct negative effect on eotaxin production by these cells. However, recent studies have shown that the neutrophil chemoattractant IL-17, interferes with eosinophil recruitment by inhibiting the production of eotaxin by epithelial cells (16). IL-6 together with TGFβ is a major promoting factor for the differentiation of Th17 cells and it has been shown to be required for the development of Th17 cells in autoimmune disease (5-7). Thus, increased eotaxin in IL-6−/− mice could be due to impaired IL-17 production. We therefore examined whether A.f.-induced allergic airway inflammation leads to increased IL-17 production and whether loss of IL-6 reduces the Th17 response. IL-17 was clearly expressed in CD4+ T cells from A.f.-exposed wild type mice (Fig. 2H), but it was almost undetectable in CD4+ T cells from IL-6−/− mice (Fig. 2H). Thus, IL-6 deficiency also impairs development of Th17 cells in A.f.-induced allergic airway inflammation. Reduced IL-17 levels could be responsible for the upregulation of eotaxin in these mice.
Increased IgE levels in A.f.-exposed IL-6−/− mice correlate with the requirement of IL-6 for IL-21 production
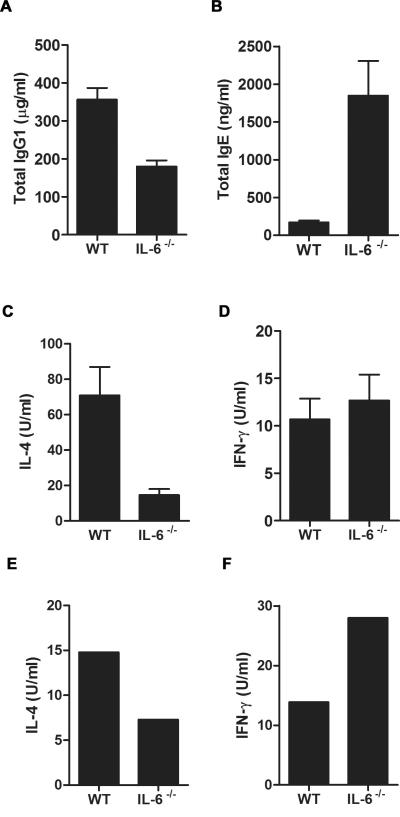
Since another hallmark feature of allergic airway inflammation is the production of the antibody isotypes IgE and IgG1 (35), we measured serum antibody levels from A.f.-exposed mice. IL-6−/− mice showed significantly lower circulating levels of IgG1 than wild type mice in response to A.f. (Fig. 3A). However, serum IgE levels in A.f.-exposed IL-6−/− mice were elevated compared with the levels in exposed wild type mice (Fig. 3B). IL-6 therefore contributes to IgG1 production during the induction of allergic airway inflammation, while it seems to have a negative effect on IgE production.
Figure 3.
IL-6 is required for IgG1, but negatively regulates IgE production in A.f.-induced allergic airway inflammation. (A) and (B) Wild type and IL-6−/− mice (n=4) were exposed to A.f. extracts as described in Fig. 2. (A) Serum IgG1 (p<0.05) and (B) IgE levels (p<0.05) were determined by ELISA. (C) and (D) Splenic CD4+ T cells from A.f.-exposed wild type (n=7) and IL-6−/− (n=4) mice were restimulated ex vivo for 24 h with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) mAbs. Cell supernatant was analyzed for (C) IL-4 (p<0.05) and (D) IFNγ (p=0.51) by ELISA. (E) and (F) FACS-sorted lung CD4+ T cells pooled from three wild type mice and three IL-6−/− mice exposed to A.f. extracts were restimulated ex vivo with A.f. (5 μg/ml) for 72h. Cell supernatant was analyzed for (E) IL-4 and (F) IFNγ by ELISA. Data are representative of two or three independent experiments.
The Th2 cytokine IL-4 is a critical factor that drives antibody production in allergic airway inflammation because it promotes B cell Ig class switching to IgE, IgG1 and IgG2b (36, 37). Since IL-6 induces IL-4 production by CD4+ T cells (38, 39), we examined IL-4 levels in splenic CD4+ T cells isolated from A.f.-exposed wild type and IL-6−/− mice upon ex vivo restimulation. Decreased levels of IL-4 by CD4+ T cells from IL-6−/− mice were found compared with cells from wild type mice (Fig. 3C). The levels of IFNγ were also examined in these cells. Although very low, levels were comparable between IL-6−/− and wild type cells (Fig. 3D). In addition, we purified lung-specific CD4+ T cells from A.f.-exposed wild type and IL-6−/− mice and restimulated them ex vivo with antigen for 72h. Lung CD4+ T cells from IL-6−/− mice also produce less IL-4 (Fig. 3E), but more IFNγ (Fig. 3F) than CD4+ T cells from than wild type mice, further supporting the role of IL-6 in promoting Th2, while inhibiting Th1 CD4+ T cell differentiation.
While the reduced production of IL-4 could explain the decreased IgG1 production, it could not account for the increased IgE levels observed in the IL-6−/− mice. Similar to IL-4, IL-21 induces plasma cell differentiation and regulates antibody production by promoting IgG1, IgG2b, and IgG3 isotype switching (40, 41). However, it negatively regulates IgE production (40-42) by inducing the inhibitor of differentiation 2 (Id2) gene expression in B cells (43). We have recently shown that IL-6 alone, is a highly specific and effective inducer of IL-21 in naïve and memory CD4+ T cells and it is required for IL-21 production in vivo (44). In order to test whether enhanced IgE levels in A.f.-exposed IL-6−/− mice were due to impaired IL-21 production, IL-21 levels in CD4+ T cells purified from A.f.-exposed wild type and IL-6−/− mice were measured. IL-21 was expressed in CD4+ T cells from A.f.-exposed wild type mice (Fig. 4A), but it was almost undetectable in CD4+ T cells from IL-6−/− mice (Fig. 4A). Thus, the effect of IL-6 deficiency on IgE production during A.f.-induced allergic airway inflammation is likely an indirect effect caused by insufficient IL-21 production from CD4+ T cells.
Figure 4.
IL-6 is necessary for IL-21 production by CD4+ T cells during allergic airway inflammation. (A) IL-21 expression levels in CD4+ T cells from A.f.-exposed wild type (n=5) and IL-6−/− (n=4) mice were examined by real time RT-PCR (p<0.05). (B) Serum IgE levels were analyzed in non-exposed wild type (n=5), IL-21−/− (n=7), and IL-6−/− (n=5) mice (*, p<0.05). Data are representative of two or three independent experiments.
IL-21R deficient mice have been shown to have increased baseline IgE levels due to the lack of IL-21 signaling (40). Similarly, analysis of serum IgE levels in unexposed IL-21−/− mice showed increased baseline IgE levels (Fig. 4B). To determine whether IL-6 deficiency could also lead to increased IgE secretion, we examined the basal levels of IgE in IL-6−/− mice. Similar to IL-21−/− mice, the IgE levels were significantly elevated in naive IL-6−/− mice (Fig. 4B). Together these data indicate that both IL-21 and IL-6 deficiency result in systemic IgE hypersecretion and thereby increased IgE levels during allergic airway inflammation.
IL-6 is essential for mucus production by lung epithelial cells
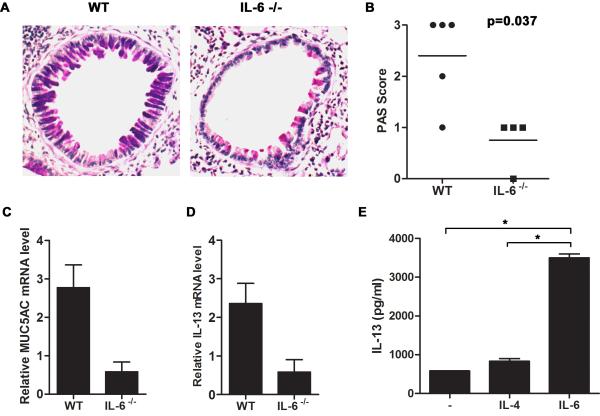
A major feature of allergic airways disease is mucus metaplasia and mucus hypersecretion by airway epithelium. Excessive mucus production can lead to physical obstruction of smaller caliber airways (45). Goblet cell hyperplasia and increased mucus production requires the adaptive immune response. Although, mucus hypersecretion and eosinophilia in the lung are normally associated, they are two independent pathological events. We therefore examined the role of IL-6 in mucus production in the airway during allergic airway inflammation. Lung sections from A.f.-exposed wild type and IL-6 −/− mice were stained for PAS in order to visualize mucus production by airway epithelial cells. The PAS score was based on the intensity of PAS staining per airway as well as the total number of positive airways. Both the number of airways with mucus producing cells as well as the number of mucus producing cells per airway were strongly reduced in IL-6−/− mice relative to wild type mice (Fig. 5A and 5B), indicating that IL-6−/− mice were more resistant to developing mucus metaplasia. The impaired mucus production was further confirmed by examining the expression of the mucus glycoprotein Muc5AC in the lung by real time RT-PCR. High levels of Muc5AC were present in A.f.-exposed wild type mice while only marginal Muc5AC levels were detected in IL-6−/− mice (Fig. 5C). Thus, IL-6 is essential for mucus production in the airway.
Figure 5.
IL-6 mediates mucus production by lung epithelium through the induction of IL-13 in CD4+ T cells in allergic airway inflammation. (A-D) Wild type (n=5) and IL-6−/− (n=4) mice were exposed to A.f. extracts as described in Fig. 2. (A) Mucus production in the lung was visualized by PAS staining of paraffin embedded lung sections. Photomicrographs from A.f.-challenged wild type (left) and IL-6−/− mice (right) are original magnification, ×200. (B) Histological score indicating intensity of PAS staining for each group (p<0.05). (C) Muc5AC (p<0.05) and (D) IL-13 (p<0.05) expression in the lung were determined by real time RT-PCR and normalized to 18S RNA. (E) Naïve CD4+ T cells from wild type mice were activated in vitro with anti-CD3 and anti-CD28 mAbs in the presence or absence of IL-6 (100 ng/ml) or IL-4 (1×103 U/ml). IL-13 was measured in the culture supernatant after 48 h by ELISA in triplicate (*, p<0.05 CD4+ T cells treated with IL-6 compared with cells activated in the presence of medium alone or IL-4).
Since IL-13 is the major cytokine involved in mucus production by lung epithelial cells (12, 13, 46), we investigated the effect of IL-6 deficiency on IL-13 expression during allergic airway inflammation. Correlating with the inability to produce mucus in the airway, a profound reduction in IL-13 expression in the lung was observed in IL-6−/− mice relative to wild type mice (Fig. 5D). IL-13 is a Th2 type of cytokine that is primarily induced by IL-4 (47). To determine whether IL-6 could also directly promote IL-13 production by CD4+ T cells, naïve CD4+ T cells were activated in vitro with anti-CD3 and anti-CD28 mAbs in the presence or absence of IL-6 or IL-4. IL-13 in the culture supernatant was measured after 48 h. CD4+ T cells activated in the absence of exogenous cytokine produced marginal levels of IL-13. While IL-4 had a minimal effect on IL-13 levels at this time of activation (Fig. 5E). In contrast, IL-6 strongly upregulated IL-13 production (Fig. 5E). Thus, IL-6 directly induces IL-13 production by CD4+ T cells during activation, providing a potential mechanism by which IL-6 enhances mucus production in the airways following allergen exposure.
Discussion
Although IL-6 has been shown to be elevated in allergic asthma in humans, its exact contribution to the hallmark features of this disease remains unclear. Correlative studies have provided evidence that IL-6 is upregulated in the airway and systemic circulation of asthmatic patients (18, 19, 48-52). However, the traditional view of IL-6 as a proinflammatory biomarker together with TNFα and IL-1β, suggested that increased IL-6 levels in asthma could merely be a result an ongoing inflammatory response, rather than serving a functional role. Recently, we have shown that there is a selective increase in IL-6, but not TNF• and IL-1• , in the large central airways of mild allergic asthmatic patients compared with healthy control subjects (Neveu et al., submitted). This increase in IL-6 inversely associates with airflow obstruction. Similar observations were made in a patient cohort that included asthmatics with more severe disease (53). Together these data suggest that IL-6 influences pathological changes within the airway that results in increased airflow resistance. In this study, using null IL-6 deficient mice, we show for the first time that indeed IL-6 is essential for mucus production during allergic airway inflammation.
Increased mucus production from lung epithelium in chronic inflammatory diseases such as asthma can physically obstruct the airway, resulting in an increase in airflow resistance (45, 54). The lung utilizes a homeostatic mechanism that balances mucus production with airway clearance (55). However, if either of these components are perturbed, this can lead to lung dysfunction. Thus, the presence of IL-6 in the asthmatic airway could contribute to lung function through induction of mucus hypersecretion. The effect of IL-6 on mucus production may be a consequence of IL-6 regulating IL-13 expression in the lung and IL-13 inducing mucus cell metaplasia in the airway. Alternatively, since in vitro studies have shown that IL-6 can directly induce the mucin genes Muc5AC and Muc5B in human lung epithelial cell lines, we cannot exclude the possibility that IL-6 may have a direct effect on mucus production by lung epithelial cells during allergic airway inflammation (56).
The role of IL-6 in the development of allergen-induced airway eosinophilia remains unclear. In vivo blockade of IL-6 signaling by the administration of an anti-IL-6R Ab during the challenge phase with the OVA antigen seems to decrease the accumulation of eosinophils in the lung (23). However, previous studies using the OVA model in IL-6 KO mice showed increased eosinophil accumulation in the lungs of these mice (21, 22). In our study, using the A.f. exposure system we show no significant difference in eosinophilia between IL-6 deficient mice and wild type mice. This apparent discrepancy could be due to the different experimental models (OVA v.s. A.f.). Alternatively, it could also be due to the use of IL-6 targeted mice that were generated by different genetic approaches. In our study, we used IL-6 deficient mice that were previously generated by deletion of Exons I, II and III of the IL-6 gene (32). The IL-6 KO mice used in the previous studies were generated by insertion of the neomycin-resistance cassette in Exon 2 in the same orientation as the IL-6 gene (57), leading to increased levels of IL-6 mRNA that may encode for a potential recombinant IL-6. We have also examined the response to A.f. exposure in those IL-6 KO mice and found significantly higher number of eosinophils in the airway in these mice than in wild type mice (data not shown), corroborating the observations that were previously published using the OVA model (21, 22). Thus, the apparent difference in the two studies is likely due to the difference in the genetically manipulated mouse models used to disrupt IL-6 gene expression.
We also describe here for the first time that IL-6 deficiency leads to hyper-IgE production, and it is more pronounced upon induction of allergic airway inflammation. This phenotype resembles that of mice deficient for IL-21. Considering the suppressive effect of IL-21 on IgE production, partly mediated by Id2 activity (43), and the selective induction of IL-21 expression by IL-6 (58), we propose that increased IgE production in IL-6 deficient mice is caused by insufficient IL-21 production by CD4+ T cells. The strong reduction of IL-21 levels in IL-6 deficient mice during allergic airway inflammation support this model. Although the production of IL-4 is also impaired in these mice, remaining IL-4 in the absence of the suppressive effect of IL-21 could be sufficient to trigger an enhanced IgE production. Alternatively, it is also possible that both IL-6 and IL-21 use similar signaling pathways to repress IgE production independent of one another. Interestingly, the primary immunodeficiency hyper-IgE syndrome (HIES) has been genetically linked to mutations that lead to the generation of a dominant negative mutant of the transcription factor STAT3 (59, 60). Since STAT3 is activated by either IL-6 or IL-21, deficiency of one these cytokine may be sufficient to prevent STAT3 activation and, thereby, lead to hyper IgE production. Interestingly, we show here that both IL-21 and IL-6 deficiencies lead to an increase in basal IgE levels in the serum that is similar to the phenotype of HIES.
In summary, the results from this study show that IL-6 is rapidly produced in the lung upon allergen exposure and that it plays a key role in dictating the immune response against allergens, as well as in specific pathological features of allergic airway inflammation, primarily mucus production.
MATERIALS AND METHODS
Mice
C57Bl/6J mice were purchased from Jackson Laboratories. IL-6 deficient mice (IL-6−/−) generated by deletion of Exon I, II, and III have been previously described (32) and obtained from Dr. G. Ciliberto. IL-21 deficient mice, as previously described (44). All mice were housed under sterile conditions at the animal care facility at the University of Vermont. All procedures performed on the mice were approved by the University of Vermont Institutional Animal Care and Use Committee. Mice were administered o.p. 5 μg of A.f. extracts in PBS as previously described (27). For quantification of early cytokine/chemokine production, BAL was collected 6 or 24 h after A.f. dose. For analysis of allergic airway inflammation, mice were administered A.f. extracts on day 0, 7, and 14, and 48 h after the last challenge mice were euthanized and tissue was processed for analysis.
IL-6 levels in serum were also measured after 6 h of i.p. immunizations of wild type mice with OVA (25 μg) (Sigma Aldrich) and either alum (2.25 mg) (Thermo Scientific) or A.f. extracts (15 μg).
BALF collection, cell count, and cell differential
Cold PBS (1 ml) was instilled into the lungs as previously described (27). Cells were centrifuged and counted on the Advia Cell Counter (Bayer). Cells (5 × 104) were cytospun and stained with Hema-3 (Biochemical Sciences). Two hundred cells per high power field were counted and classified as macrophages, neutrophils, lymphocytes, or eosinophils by cell morphology and staining.
CD4+ T cell purification and activation
Splenic CD4+ T from A.f.-exposed animals were negatively selected as previously described (3). Briefly, cells expressing CD8, CD11b, NK1.1, and MHC II were depleted using specific mAb antibodies (BD Pharmingen) and Ig-coated magnetic beads (Qiagen). Isolated CD4+ T cells (1 × 106) were activated with plate-bound anti-CD3 (2C11) (5 μg/ml) and soluble anti-CD28 (1 μg/ml; BD Pharmingen) mAbs for 24 h.
Lung CD4+ T cells were purified from lung cell homogenates by sorting on a FACS-Aria (BD) for CD4 (CD4+ T cells) and CD1d-tetramer (CD1d tetramer negative to discard NKT cells) expression. Cells were restimulated with A.f. extracts (5 μg/ml) in the presence of APCs (T cell-depleted spleen cells) for 72 h.
Cytokine and immunoglobulin detection
Detection of IL-6 and eotaxin in BALF was performed using the mouse IL-6 and eotaxin Duoset according to manufacturer’s instructions (R&D Systems). Multiple cytokines and chemokines were detected in BALF by using the Mouse 23-plex Panel (Bio-Rad). Samples were analyzed using the Bio-Plex Manager Sofware (Bio-Rad). Detection of IL-13 (R&D Systems), IL-4 and IFNγ (BD Pharmingen) in cell supernatants by ELISA was conducted as recommended by the manufacturer using the corresponding antibodies. Serum from anesthetized mice was collected by right heart puncture. Total IgG1 and IgE levels were determined by ELISA using capture and detection antibodies according to manufacturer’s instructions (BD Pharmingen).
Lung Histology
Formalin-fixed, paraffin embedded sections were prepared as previously described (61). Sections were stained for H&E and PAS according to routine procedures. Three investigators blinded to each experimental group scored airway inflammation and mucus production in the following way: 0, no inflammation or mucus production; 1, mild inflammation or mucus production; 2, moderate inflammation or mucus production with multiple airway involvement; 3, severe inflammation or mucus production with majority of airways involved. Images were obtained by the Olympus BX50 light microscope with an Optronics Magnafire digital camera.
RNA analysis
Total RNA was extracted from whole lung tissue and CD4+ T cells by using the RNAeasy kit (Qiagen) as recommended by the manufacturer. First-strand cDNA synthesis was performed as previously described (27). Quantitative PCR was performed on cDNA using Assay on Demand probe/primer sets for IL-5, IL-13, IL-17, IL-21, Muc5AC, 18S and β2-microglobulin (Applied Biosystems). Gene amplification was performed on an ABIPrism® 7700 instrument from Applied Biosystems. Expression of target genes in the lung and CD4+ T cells was normalized to 18S and β2-microglobulin levels, respectively. Relative values were determined by the comparative Ct method.
Statistical analysis
The data are presented as the mean ± SEM. The significance of differences between two groups was determined by the Mann-Whitney test with GraphPad Prism (v. 5.0). For all analyses, p<0.05 is considered statistically significant and anything greater than this value is not significant (n.s.).
ACKNOWLEDGMENTS
We thank Kieren Marr (Fred Hutchinson Cancer Center, University of Washington, Seattle, WA) for providing us with the A.f. extracts. We thank Timothy Hunter and Mary Lou Shane (DNA Sequencing Facility, University of Vermont, Burlington, VT) for assistance with real time RT-PCR analysis. We also thank Charlie Irvin, Matthew Poynter, Elizabeth Bonney, Janice Bunn, and Juan Anguita for helpful discussion.
Footnotes
This research was supported by the National Institute of Health R01HL69136 (M.R.) grant and the Center for Biomedical Research Excellence Program of the National Center for Research Resources Grant P20RR15557 (L.W.).
REFERENCES
- 1.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 2.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:804–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 3.Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincon M. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 2002;196:39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 9.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science (New York, N.Y. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 10.Berton MT, Uhr JW, Vitetta ES. Synthesis of germ-line gamma 1 immunoglobulin heavy-chain transcripts in resting B cells: induction by interleukin 4 and inhibition by interferon gamma. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2829–2833. doi: 10.1073/pnas.86.8.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, Cohn L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol. 2002;27:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 13.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science (New York, N.Y. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 14.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 18.Virchow JC, Jr., Walker C, Hafner D, Kortsik C, Werner P, Matthys H, Kroegel C. T cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–968. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, Ishida S, Hiwada K. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995;151:1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 20.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of potent inflammatory cytokines, granulocytic-macrophage-colony-stimulating factor and interleukin-6 in bronchial epithelial cells of patients with asthma. J. Allergy Clin. Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Homer RJ, Chen Q, Elias JA. Endogenous and exogenous IL-6 inhibit aeroallergen-induced Th2 inflammation. J Immunol. 2000;165:4051–4061. doi: 10.4049/jimmunol.165.7.4051. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Z, Fujimura M, Kurashima K, Nakao S, Mukaida N. Enhanced airway inflammation and decreased subepithelial fibrosis in interleukin 6-deficient mice following chronic exposure to aerosolized antigen. Clin Exp Allergy. 2004;34:1321–1328. doi: 10.1111/j.1365-2222.2004.02013.x. [DOI] [PubMed] [Google Scholar]
- 23.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latge JP. Aspergillus fumigatus and aspergillosis. Clinical microbiology reviews. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettiol J, Sele J, Henket M, Louis E, Malaise M, Bartsch P, Louis R. Cytokine production from sputum cells after allergenic challenge in IgE-mediated asthma. Allergy. 2002;57:1145–1150. doi: 10.1034/j.1398-9995.2002.23586.x. [DOI] [PubMed] [Google Scholar]
- 26.Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol. 2003;111:285–289. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- 27.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 28.Bao L, Zhu Y, Elhassan AM, Wu Q, Xiao B, Zhu J, Lindgren JU. Adjuvant-induced arthritis: IL-1 beta, IL-6 and TNF-alpha are up-regulated in the spinal cord. Neuroreport. 2001;12:3905–3908. doi: 10.1097/00001756-200112210-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mihara M, Nishimoto N, Yoshizaki K, Suzuki T. Influences of anti-mouse interleukin-6 receptor antibody on immune responses in mice. Immunology letters. 2002;84:223–229. doi: 10.1016/s0165-2478(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venge J, Lampinen M, Hakansson L, Rak S, Venge P. Identification of IL-5 and RANTES as the major eosinophil chemoattractants in the asthmatic lung. J Allergy Clin Immunol. 1996;97:1110–1115. doi: 10.1016/s0091-6749(96)70265-8. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 35.Herz U, Braun A, Ruckert R, Renz H. Various immunological phenotypes are associated with increased airway responsiveness. Clin Exp Allergy. 1998;28:625–634. doi: 10.1046/j.1365-2222.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 36.Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986;136:4538–4541. [PubMed] [Google Scholar]
- 37.Vitetta ES, Ohara J, Myers CD, Layton JE, Krammer PH, Paul WE. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985;162:1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diehl S, Krahl T, Rinaldi L, Norton R, Irvin CG, Rincon M. Inhibition of NFAT specifically in T cells prevents allergic pulmonary inflammation. J Immunol. 2004;172:3597–3603. doi: 10.4049/jimmunol.172.6.3597. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science (New York, N.Y. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 41.Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron JC, Yssel H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 42.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 43.Kishida T, Hiromura Y, Shin-Ya M, Asada H, Kuriyama H, Sugai M, Shimizu A, Yokota Y, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 induces inhibitor of differentiation 2 and leads to complete abrogation of anaphylaxis in mice. J Immunol. 2007;179:8554–8561. doi: 10.4049/jimmunol.179.12.8554. [DOI] [PubMed] [Google Scholar]
- 44.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Current opinion in pharmacology. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 48.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 49.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174:1110–1118. doi: 10.1164/rccm.200603-392OC. [DOI] [PubMed] [Google Scholar]
- 50.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 51.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel AB, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 52.Stankiewicz W, Dabrowski MP, Chcialowski A, Plusa T. Cellular and cytokine immunoregulation in patients with chronic obstructive pulmonary disease and bronchial asthma. Mediators of inflammation. 2002;11:307–312. doi: 10.1080/09629350210000015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon AE, Raymond DM, Suratt BT, Bourassa LM, Irvin CG. Lower Airway Disease in Asthmatics with and without Rhinitis. Lung. 2008;186:361–368. doi: 10.1007/s00408-008-9119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol. 2007;102:399–405. doi: 10.1152/japplphysiol.00630.2006. [DOI] [PubMed] [Google Scholar]
- 55.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 57.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 58.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clinical immunology (Orlando, Fla. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 60.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 61.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]