Abstract
We compared outcomes of 916 diffuse large B cell lymphoma (DLBCL) patients age ≥ 18 years undergoing first autologous (n=837) or myeloablative allogeneic hematopoietic cell transplant (HCT) (n=79) between 1995–2003 reported to the CIBMTR. Median follow-up was 81 months for allogeneic HCT vs. 60 months for autologous. Allogeneic HCT recipients were more likely to have high risk disease features including higher stage, more prior chemotherapy regimens and resistant disease. Allogeneic HCT was associated with a higher 1 year treatment-related mortality (TRM) (RR 4.88, 95% CI, 3.21–7.40, p<0.001), treatment failure (RR 2.06, 95% CI, 1.54–2.75, p<0.001) and mortality (RR 2.75, 95% CI, 2.03–3.72, p<0.001). Risk of disease progression was similar in the 2 groups (RR 1.12, 95% CI, 0.73–1.72, p=0.59). In fact, for 1 year survivors, no significant differences were observed for TRM, progression, progression-free or overall survival. Increased risks of TRM and mortality were associated with older age (>50 years), lower performance score, chemoresistance and earlier year of transplant. In a cohort of mainly high risk DLBCL patients, upfront myeloablative allogeneic HCT while associated with increased early mortality was associated with a similar risk of disease progression compared to lower risk patients receiving autologous HCT.
Key words or short phrases: unrelated, allogeneic transplantation, Hodgkin Lymphoma
INTRODUCTION
One-third of all non-Hodgkin lymphoma (NHL) are of the Diffuse Large B-Cell Lymphoma (DLBCL) subtype and despite the addition of rituximab to conventional therapy, many patients still relapse and die of disease (1–3). Autologous hematopoietic stem cell transplantation (HCT) potentially is curative and leads to long-term disease-free survival (DFS) in nearly 50% of patients with chemotherapy-sensitive relapsed DLBCL (4–6). The vast majority of autologous HCT procedures use peripheral blood rather than marrow as the graft source as this modality is superior in terms of faster hematopoietic recovery from myelosuppression, easier to perform, cheaper and less hazardous (7–9). Autologous HCT, however, is less effective in patients with chemoresistant relapse (10–13). This observation often is explained by an increase in relapse risk due to a lack of graft-versus-lymphoma effect and because of re-infusion of malignant cells (4, 5, 14–16). Allogeneic HCT, usually employing bone marrow as the stem cell source, is a potential therapeutic option especially for patients with matched sibling donors and higher risk disease. Potential advantages of allogeneic HCT include the use of a tumor-free graft and a graft-versus-lymphoma (GVL) effect that may reduce the risk of relapse in addition to a reduction of the risk of secondary leukaemia by hematopoietic stem cell replacement (16–21). Acute or chronic graft-versus-host disease (GVHD) and high rates of opportunistic infection, however, may lead to high treatment-related mortality (TRM) and morbidity and offset the benefits of this approach (16–21). While there are many reports describing outcomes after autologous HCT for DLBCL patients, there are fewer publications that describe the results using myeloablative allogeneic HCT (22–29).
To date, there are few prospective, randomized reports comparing autologous versus allogeneic HCT for DLBCL. The Ontario BMT Network reported the results of a large trial (17). Most other reports that compare autologous versus allogeneic HCT are small, retrospective and single institution trials comprising heterogeneous histologic NHL subtypes (17, 18, 30–33). The main objectives of this study were to compare the clinical outcomes between patients with DLBCL receiving autologous versus allogeneic matched sibling donor HCT and to determine patient-, disease- and transplant- related variables associated with favorable outcomes.
PATIENTS AND METHODS
Data Sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of over 500 transplant centers worldwide. Participating centers register basic information on consecutive transplants to a Statistical Center at the Medical College of Wisconsin. Detailed demographic and clinical data are collected on a representative sample of patients in the registry using a weighted randomization scheme. Participating centers are required to report all consecutive transplant data; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow up.
The CIBMTR collects data at two levels: Registration and Research. Registration data includes disease type, age, sex, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow, peripheral blood and cord blood derived hematopoietic stem cells), conditioning regimen, post transplant disease progression and survival, development of secondary cancers and cause of death. Requests for data on progression or death for registered subjects are at six-month intervals. All CIBMTR teams contribute registration data. Research data are collected on subsets of registered subjects and includes comprehensive pre- and post transplant clinical data. Computerized checks for errors, physician reviews of submitted data and on-site audits of participating centers ensure the quality of data.
Patients
The outcomes of 916 adult DLBCL patients between the ages of 18 and 60 years, receiving autologous or matched sibling allogeneic HCT reported to the CIBMTR between January 1, 1995 and December 31, 2003 were analyzed. Patients receiving reduced-intensity conditioning or T cell depleted grafts were excluded. Patients receiving allogeneic HCT after a prior autologous transplant also were excluded. Patients were reported to the CIBMTR by 156 centers in 17 different countries.
Transplant types were categorized as autologous (n=837) or HLA-identical sibling allogeneic transplants (n=79). Median follow-up was 60 (range, 1–130) months for autologous HCT vs 81 (range, 14–120) months for allogeneic HCT.
Study Endpoints
Outcomes included TRM, progression, progression-free survival (PFS), and overall survival (OS). TRM was defined as death within 28 days post transplant or death without lymphoma progression. Progression was defined as progressive lymphoma post transplant (>28 days) or lymphoma recurrence and could follow a period of “stable” disease post transplant, or a partial or complete remission. For PFS, subjects were considered treatment failures at the time of lymphoma progression or death from any cause. OS was defined as time from the date of transplant to the date of death or last contact. Other outcomes analyzed included acute- and chronic graft-versus-host disease (aGVHD and cGVHD) and cause of death (COD). aGVHD was defined and graded using established criteria. cGVHD was defined as the development of any cGVHD based on clinical criteria.
Statistical Analysis
Patient-, disease- and transplant-related variables for the two study groups were compared using the chi-square statistic for categorical and the Kruskal-Wallis test for continuous variables. Univariate probabilities of PFS and OS were calculated using the Kaplan-Meier estimator. Probabilities of acute and chronic GVHD, TRM and relapse/progression were calculated using cumulative incidence curves to accommodate competing risks.
Cox Proportional Hazards Model
Assessment of potential risk factors for outcomes of interest was evaluated in multivariate analyses using Cox proportional hazards regression. Variables considered in multivariable analysis are listed in Table 1. A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for donor type. Factors significant at a 5% level were kept in the final model. The potential interactions between main effect and all significant risk factors were tested. The proportionality assumption was tested by adding a time-dependent covariate for each factor. When test indicated differential effects over time (non-proportional hazards), models were constructed breaking the post-transplant course into two time periods, using the maximized partial likelihood method to find the most appropriate breakpoint. The proportionality assumptions were further tested. After the above modeling of time varying effects, the final multivariate model was built. Adjusted probabilities of progression free survival (PFS) and overall survival were generated from the final Cox models stratified on treatment of donor type and weighted by the pooled sample proportion value for each prognostic factor. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors.
Table 1.
Variables tested in Cox proportional hazards regression models.
| Main effect variablea |
| Transplant type: Autologous vs HLA-identical siblingAllogeneic |
| Patient-related variables: |
| Age at transplant: 18–30, 31–50, > 50 years |
| Karnofsky performance status at transplant: ≥90% vs <90% |
| Sex: male vs female |
| Disease-related: |
| Disease stage at diagnosis: I or II vs. III or IV |
| Chemosensitive disease at transplant: Sensitive vs resistant |
| B symptoms at diagnosis: present vs absent |
| Time from diagnosis to transplant: continuous |
| Extranodal disease or splenic involvement at diagnosis: yes vs no |
| Marrow involvement at diagnosis: yes vs no |
| Treatment-related: |
| Source of stem cells: bone marrow vs peripheral blood |
| Year of transplant: 1995–2000 vs. 2001–2003 |
| HLA-identical sibling only |
| Donor-recipient gender match: F-M vs others |
| GVHD prophylaxis: MTX + CsA ± other vs MTX ± other vs CsA ± other vs FK506 ± other vs none |
| Donor/Recipient CMV status: −/− vs others |
| Autologous only |
| Purging: yes vs no |
Included in all models.
Matched Pair Analysis
Clinical characteristics of the patient and disease lead to selection of patients for an allogeneic transplant as opposed to autologous HCT. In a retrospective dataset, this selection bias results in significant pretransplant differences between the autologous and allogeneic cohorts. In order to validate the findings based on multivariate analysis of Cox model, we performed an additional matched pair comparison of the allogeneic HCT group with a subset of closely matched autologous HCT patients selected based on propensity score matching.
The propensity score is the probability of receiving an allogeneic transplant, which was calculated based on fitting a logistic-regression model (34). We fit a logistic-regression with key risk factors of age, sex, Karnofsky performance score (KPS) pre-transplant, disease stage at diagnosis, B symptoms at diagnosis, extranodal disease at diagnosis, marrow involvement at diagnosis, number of prior chemotherapy regimens, sensitivity to chemotherapy prior to transplant, time from diagnosis to transplant, graft source and year of transplant (Table A of supplemental data). The median propensity score for the combined sample was 0.042 (range: 0.002 – 0.895; sd=0.1228). For each allogeneic transplant (case) patient, any autologous transplant (control) patient with a difference in the propensity score of less than sd=0.1228 was considered a potential matched control. The matched control with the smallest difference in propensity score among all potential matched controls was selected. These steps were repeated among the remaining cases until four possible matched controls were identified for each of the cases. Allogeneic recipients (69 cases) were then matched in random order to autotransplant (232 controls) recipients with similar propensity scores. The final matched cohorts included 69 allogeneic transplant recipients and 232 autotransplant recipients (49 cases were found with 4 matches, 2 cases were found with 3 matches, 12 cases were found 2 matches and 6 cases were found 1 to 1 matches). Multivariate analysis was again performed by fitting a Cox model stratified on matched-pairs.
RESULTS
Patient, disease, and transplant characteristics of the autologous and allogeneic cohorts are summarized in Table 2. As expected, there were significant differences between the cohorts receiving autologous HCT vs. allogeneic HCT. Recipients of autologous HCT had lower disease stage, lower age-adjusted International Prognostic Index (aaIPI) and a lower likelihood of B symptoms, extra nodal disease or marrow involvement. At transplantation, autologous HCT patients were more likely to have chemosensitive disease or be in a complete remission (CR). They also were less likely to have received prior radiation and were transplanted later in their disease course. Matched sibling allograft recipients were more likely to be in primary induction failure or with relapsed lymphoma not having achieved a CR. The supplemental matched pair analysis consisted of a cohort of 69 allogeneic sibling transplant recipients and 232 autologous HCT recipients with no differences in age, sex, KPS, lymphoma stage, chemotherapy sensitivity, time from diagnosis to transplant or year of transplant (Table B of supplemental data).
Table 2.
Patient-, disease -, and transplant characteristics
| Autologous | Allogeneic | ||||
|---|---|---|---|---|---|
| Variable | N eval | N (%) | N eval | N (%) | P-valueb |
| Patient related | |||||
| Number of patients | 837 | 79 | |||
| Age, median (range), years | 837 | 48 (18 – 60) | 79 | 46 (21 – 59) | 0.05 |
| Male sex | 837 | 483 (58) | 79 | 49 (62) | 0.46 |
| Karnofsky score pre-transplant | 817 | 77 | 0.18 | ||
| <90 | 298 (36) | 34 (44) | |||
| Disease related | |||||
| Disease stage at diagnosis | 822 | 79 | 0.003 | ||
| I | 90 (11) | 8 (10) | |||
| II | 185 (23) | 8 (10) | |||
| III | 219 (27) | 14 (18) | |||
| IV | 326 (39) | 49 (62) | |||
| Age adjusted IPIc | 391 | 38 | 0.02 | ||
| Low | 50 (13) | 0 | |||
| Low-Intermediate | 137 (35) | 12 (32) | |||
| High-Intermediate | 180 (46) | 20 (55) | |||
| High | 24 (6) | 6 (16) | |||
| Missinge | 446 | 41 | |||
| B Symptoms at diagnosis | 775 | 355 (46) | 76 | 44 (58) | 0.04 |
| Extranodal disease or splenic involvement at diagnosis | 800 | 452 (57) | 77 | 54 (70) | 0.02 |
| Marrow involvement at diagnosis | 800 | 139 (17) | 77 | 32 (42) | <0.001 |
| TBI at conditioning | 833 | 151 (18) | 79 | 46 (58) | <0.001 |
| Conditioning regimen for autologous | 837 | NA | --- | ||
| TBI-based | 151 (18) | ||||
| BEAM and similar | 515 (61) | ||||
| CBV or similar | 82 (10) | ||||
| Othersf | 89 (11) | ||||
| Conditioning regimen for allogeneic | NA | 79 | --- | ||
| TBI+CY | 41 (52) | ||||
| BU+CY | 24 (30) | ||||
| Othersg | 14 (18) | ||||
| Autologous | Allogeneic | ||||
| Variable | N eval | N (%) | N eval | N (%) | P-valuea |
| Number of prior chemotherapy regimens | 836 | 79 | 0.12 | ||
| 1 | 133 (16) | 7 (9) | |||
| 2 | 360 (43) | 32 (40) | |||
| 3 | 232 (28) | 22 (28) | |||
| 4 | 83 (10) | 15 (19) | |||
| 5 | 23 (3) | 3 (4) | |||
| Best response to 1st line of chemotherapy | 836 | 79 | 0.007 | ||
| CR | 344 (41) | 24 (30) | |||
| CRU/nodal PR | 55 (7) | 4 (5) | |||
| PR | 265 (32) | 28 (36) | |||
| No response/stable disease | 45 (5) | 13 (17) | |||
| Progression | 70 (8) | 8 (10) | |||
| Not Evaluable / Unknown | 57 (7) | 2 (2) | |||
| Interval from diagnosis to transplant, median (range), months | 837 | 13 (2 – 287) | 79 | 11 (2 – 156) | 0.03 |
| Chemosensitive disease at transplant | 837 | 79 | <0.001 | ||
| Sensitive | 709 (85) | 46 (58) | |||
| Resistant | 128 (15) | 33 (42) | |||
| Disease status at transplant | 830 | 76 | <0.001 | ||
| PIF-sensitive | 180 (21) | 19 (25) | |||
| PIF-resistant | 66 (8) | 20 (26) | |||
| CR1 | 149 (18) | 5 (7) | |||
| CR2+ | 138 (17) | 6 (8) | |||
| REL-sensitive | 238 (29) | 14 (18) | |||
| REL-resistant | 59 (7) | 12 (16) | |||
| Transplant related | |||||
| Donor/Recipient CMV status | NA | 78 | --- | ||
| −/− | 24 (31) | ||||
| Donor-recipient gender match | NA | 79 | --- | ||
| F-M | 26 (33) | ||||
| Source of stem cells | 837 | 79 | <0.001 | ||
| BM | 76 (9) | 29 (37) | |||
| Year of transplant | 837 | 79 | 0.04 | ||
| 1995–1997 | 386 (46) | 35 (44) | |||
| 1998–2000 | 300 (36) | 21 (27) | |||
| 2001–2003 | 151 (18) | 23 (29) | |||
| Autologous | Allogeneic | ||||
| Variable | N eval | N (%) | N eval | N (%) | P-valuea |
| GVHD prophylaxis | NA | 79 | --- | ||
| MTX + CsA ± other | 46 (58) | ||||
| CsA ± other | 18 (23) | ||||
| FK506 ± other | 13 (17) | ||||
| None | 2 (2) | ||||
| Median follow-up of survivors, months | 423 | 60 (1 – 130) | 19 | 81 (14 – 120) | |
Abbreviations: N = Number; EVAL = evaluable; IPI = International Prognostic Index; TBI = total body irradiation; BEAM = BCNU+etoposite+Ara-C+melphalan; CBV = cyclosphamide+BCNU+VP16 = etoposide; CY = cyclosphosphamide; BU = busulfan; CR = complete remission; CRU = complete remission undetermined; PR = partial remission; PIF = primary induction failure; REL = relapse; F = female; M = male; BM = bone marrow; MTX = methotrexate; CsA = cyclosporine; FK506 = tacrolimus.
- First autologous or HLA-identical sibling allogeneic transplant for diffuse large B-cell lymphoma transplanted between 1995–2003 included.
- 53 patients with reduced-intensity regimens excluded
- 16 patients with T-cell depletion excluded
- 367 patients with age <18 or >60 were excluded
- 9 patients with untreated and 2 with not evaluable chemosensitive disease were excluded
The chi-square test was used for discrete covariates; the Kruskal-Wallis test was used for continuous covariates.
- Ara-C+carboplatin(n=1)
- BU alone (n=3)
- Carboplatin±other (n=6)
- CY+BU (n=38)
- CY+carboplatin (n=4)
- L-PAM only (n=20)
- BU-MEL (n=7)
- L-PAM±other (n=8)
- VP16+BU (n=1)
- VP16+Carboplatin (n=1)
- BU±other (No CY) (n=2)
- Carboplatin+thiotepa (n=1)
- CY±other (No BU) (n=5)
- L-PAM±other (n=1)
- TBI±other (No CY) (n=5)
Follow-up completeness index = 80% (Overall); 80% (Auto); 90% (Allo). Overall = 96% @ 1 year; 86% @ 3 years; 73% @ 5 years.
Table 3 summarizes univariate probabilities of all outcomes of interest after transplantation.
Table 3.
Outcomes after autologous and HLA-identical sibling Allogeneic HCTs for diffuse large B-cell lymphoma
| Autologous | Allogeneic | |
|---|---|---|
| Outcome event | Prob (95% CI) | Prob (95% CI) |
| Acute GVHD @ 100 days, grades (2–4) | NA | 42 (31 – 52) |
| Chronic GVHD | NA | |
| @ 1 year | 23 (15 – 33) | |
| @ 3 years | 26 (17 – 36) | |
| @ 5 years | 26 (17 – 36) | |
| TRM | ||
| @ 1 year | 12 (9 – 14) | 41 (30 – 51) |
| @ 3 years | 16 (14 – 19) | 43 (32 – 54) |
| @ 5 years | 18 (15 – 20) | 45 (34 – 56) |
| Progression/Relapse | ||
| @ 1 year | 33 (29 – 36) | 30 (21 – 41) |
| @ 3 years | 37 (34 – 41) | 33 (23 – 43) |
| @ 5 years | 40 (36 – 43) | 33 (23 – 43) |
| PFS | ||
| @ 1 year | 56 (53 – 59) | 29 (20 – 39) |
| @ 3 years | 47 (43 – 50) | 24 (15 – 34) |
| @ 5 years | 43 (39 – 46) | 22 (13 – 32) |
| Overall survival | ||
| @ 1 year | 66 (63 – 70) | 33 (23 – 43) |
| @ 3 years | 53 (49 – 56) | 26 (17 – 36) |
| @ 5 years | 49 (46 – 53) | 22 (14 – 33) |
Abbreviations: TRM = treatment-related mortality; PFS = progression-free survival; PROB = probability; CI = confidence interval.
Probabilities of acute GVHD, chronic GVHD, treatment-related mortality and progression/relapse were calculated using the cumulative incidence estimate. Progression-free survival and overall survival was calculated using the Kaplan-Meier product limit estimate.
Transplant Related Mortality
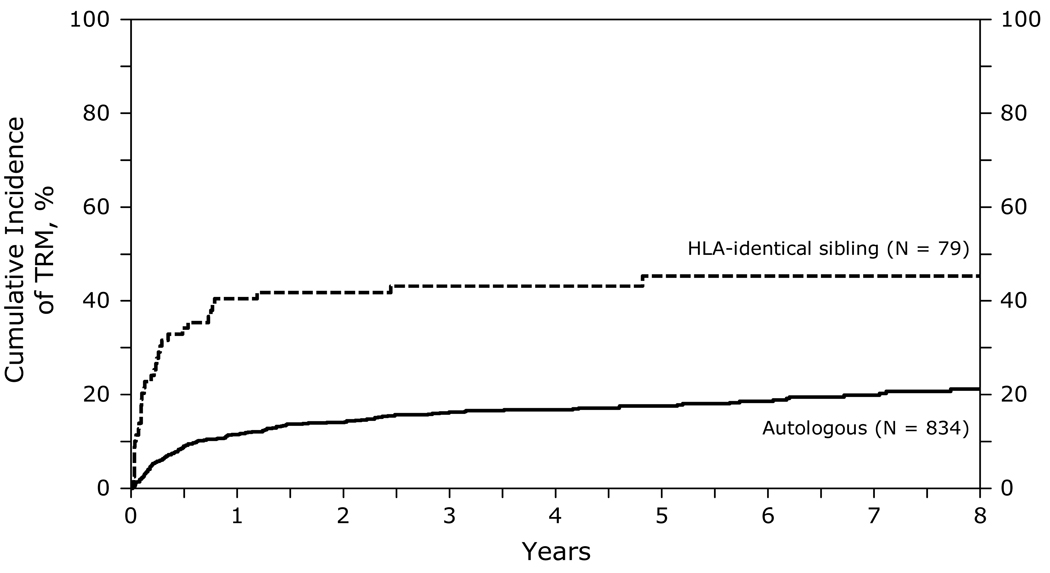
At 1, 3 and 5 years after transplant, TRM was higher after HLA-identical sibling HCT (41, 43 and 45% respectively) than after autologous HCT (12, 16 and 18% respectively) (Figure 1). TRM was significantly higher in the first 12 months after HLA identical transplants compared to autologous HCT [relative risk (RR) 4.88, 95% CI 3.21–7.40, p<0.001]. In the subsequent period beyond 12 months, the risk of TRM was not different. Other significant covariates associated with a higher TRM included greater age at transplant, lower KPS (<90), chemotherapy resistant disease and transplants performed prior to 2001. Table C of supplemental data.
Figure 1.
Cumulative incidence of treatment-related mortality after autologous and HLA-identical sibling HCTs for diffuse large B-cell lymphoma
Relapse/Progression
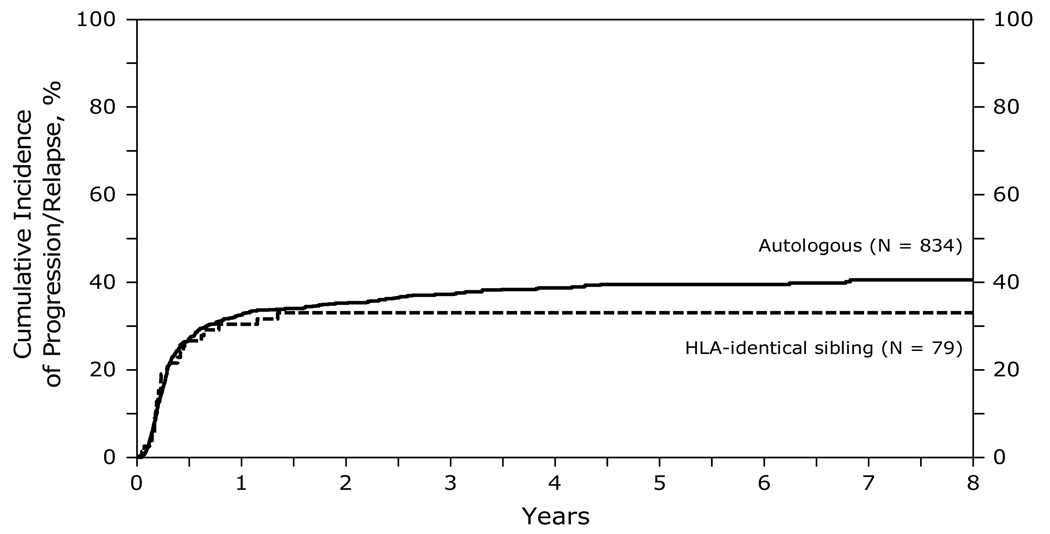
Figure 2 summarizes the cumulative incidence of relapse at 1, 3 and 5 years after transplant. There were no significant differences in the risk of relapse/progression after allogeneic transplants compared to autologous HCT. Significant covariates associated with a higher risk of relapse/progression included greater age at transplant (>50 years) and chemotherapy resistant disease. Table D of supplemental data.
Figure 2.
Cumulative incidence of progression/relapse after autologous and HLA-identical sibling HCTs for diffuse large B-cell lymphoma
Progression Free Survival and Treatment Failure
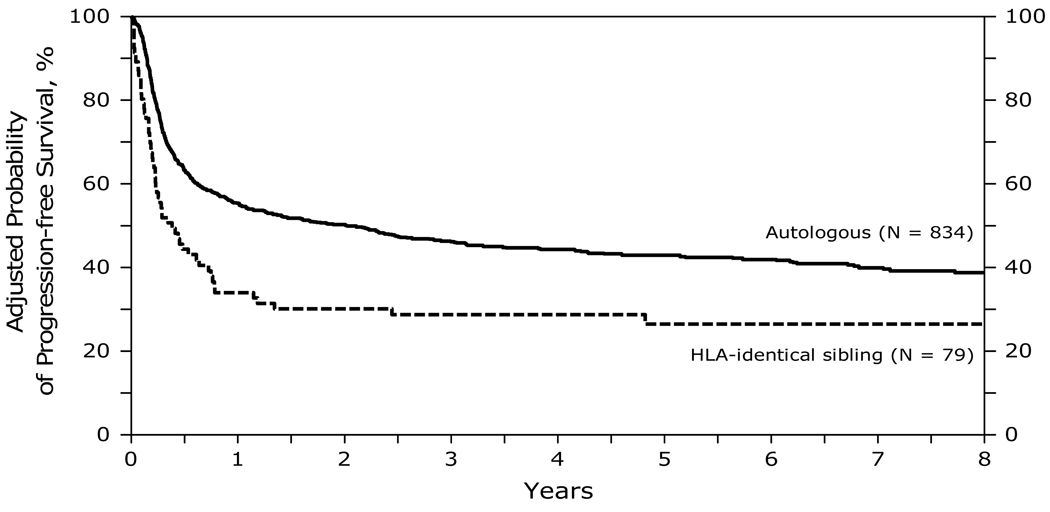
At 1, 3 and 5 years after transplant, PFS was lower after allogeneic HCT (29, 24 and 22% respectively) than after autologous HCT (56, 47 and 43% respectively) (Figure 3). PFS was significantly worse in the first 12 months after HLA identical transplants compared to autologous HCT. In the subsequent period beyond 12 months, the risk was no different between the 2 groups. Other significant covariates associated with a lower PFS included greater age at transplant (>50 years), chemotherapy resistant disease and transplants performed prior to 2001. Table E of supplemental data.
Figure 3.
Probability of progression-free survival after autologous and HLA-identical sibling HCTs for diffuse large B-cell lymphoma
Survival
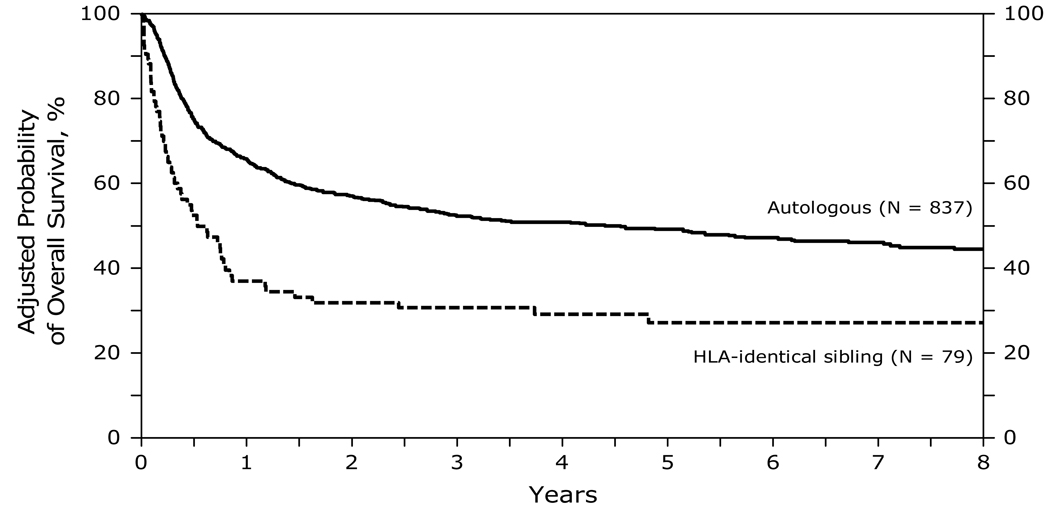
At 1, 3 and 5 years after transplant, survival was lower after HLA-identical sibling HCT (33, 26 and 22% respectively) than after autologous HCT (66, 53 and 49% respectively) (Figure 4). Survival was significantly lower in the first 12 months after HLA-identical transplants compared to autologous HCT. In the subsequent period beyond 12 months, the risk of mortality was not different. Other significant covariates associated with a higher mortality and lower survival included greater age (>50 years) at transplant, lower KPS (<90), chemotherapy resistant disease and transplants performed prior to 2001. Table F of supplemental data.
Figure 4.
Probability of overall survival after autologous and HLA-identical sibling HCTs for diffuse large B-cell lymphoma
Secondary Outcomes
The incidence of grade 2–4 acute GVHD was 42% while the incidence of chronic GVHD was 26% at five years after allogeneic transplant.
Matched Pair Analysis
The matched pair analysis comparing HLA-identical sibling-matched allogeneic HCT versus matched autologous HCT recipients confirmed the results of the multivariate Cox model (Table 4). Lymphoma relapse/progression rates did not differ between the two groups. However, within the first 12 months after transplant, allogeneic HCT was associated with higher TRM, lower PFS and higher risk of treatment failure. The risk of mortality was also higher in the allogeneic cohort within the first 12 months of transplant. There were no differences beyond 12 months.
Table 4.
Summary of Outcomes from Matched Pair comparison
| Outcome: | Relative Risk (95% CI) | P-value |
|---|---|---|
| TRM: | ||
| (1) Allogeneic vs autologous (overall) | 3.91 (2.16 – 7.08) | < 0.0001 |
| (2) Within first 12 months after transplant | 5.11 (2.63 – 9.94) | < 0.0001 |
| Beyond first 12 months after transplant | 1.05 (0.24 – 4.56) | 0.9499 |
| Progression/Relapse: | ||
| (1) Allogeneic vs autologous (overall) | 1.18 (0.70 – 1.98) | 0.5347 |
| (2) Within first 12 months after transplant | 1.16 (0.68 – 1.97) | 0.5842 |
| Beyond first 12 months after transplant | 2.00 (0.11 – 37.83) | 0.6440 |
| Treatment Failure (PFS): | ||
| (1) Allogeneic vs autologous (overall) | 1.95 (1.34 – 2.83) | 0.0005 |
| (2) Within first 12 months after transplant | 2.04 (1.38 – 3.01) | 0.0003 |
| Beyond first 12 months after transplant | 1.19 (0.32 – 4.40) | 0.7948 |
| Mortality (Survival): | ||
| (1) Allogeneic vs autologous (overall) | 2.38 (1.68 – 3.53) | < 0.0001 |
| (2) Within first 12 months after transplant | 2.77 (1.81 – 4.25) | < 0.0001 |
| Beyond first 12 months after transplant | 1.05 (0.38 – 2.93) | 0.9232 |
TRM: Overall test (2 d.f.): P < 0.0001; Test early effect = late effect: P = 0.0542
Progression/Relapse: Overall test (2 d.f.): P = 0.7737; Test early effect = late effect: P = 0.7204
PFS: Overall test (2 d.f.): P = 0.0015; Test early effect = late effect: P = 0.4384
Survival: Overall test (2 d.f.): P < 0.0001; Test early effect = late effect: P = 0.0865
Cause of Death
The main cause of death among allogeneic vs. autologous HCT was primary disease in both groups (48% vs 73%, respectively). Other causes of death were interstitial pneumonia (7% vs 6%), infection (15% vs 3%), organ failure (8% vs 6%), GVHD (10% vs 0%) and secondary malignancy (0% vs 2%).
DISCUSSION
We compared the outcomes of 916 DLBCL patients receiving an initial autologous (n=837) or myeloablative HLA-identical sibling allogeneic (n=79) HCT from 1995 to 2003. Factors considered when recommending an autologous versus allogeneic transplantation for DLBCL include potential differences in TRM, concerns over tumor contamination in an autograft, inability to mobilize hematopoietic progenitor cells and the expected benefits of a GVL effect from an allograft. Allogeneic transplantation therefore is likely to be offered to patients perceived to be at lower risk for TRM and higher risk for disease relapse/progression. Although non-myeloablative and reduced intensity conditioning regimens are increasingly used in allogeneic HCT for NHL, approximately two-thirds of allografts for DLBCL reported to the CIBMTR utilized myeloablative regimens demonstrating the wide prevalence of this approach (35).
The patient-, disease- and transplant-related differences observed between the cohorts reflect a clear effect of patient selection with the allotransplant cohort having lower median age, higher incidence of extra nodal and marrow involvement and more resistant, higher risk disease. The differences between the groups in terms of graft source and the greater use of total body radiation in conditioning are intrinsic to the myeloablative transplant approach.
In this analysis, we controlled the pre transplant imbalances between the cohorts in 2 separate statistical analyses that yielded very similar results. In multivariate Cox model comparing all the autograft recipients to the allograft cohort, overall TRM after allogeneic transplant was significantly higher than after autologous HCT. This was especially driven by a higher TRM in the first 12 months after allogeneic transplant with no difference in survivors beyond 12 months. In their prospective study, the Johns Hopkins group (18) reported 100-day TRM in 183 relapsed DLBCL patients as 33.3% for the allogeneic HCT recipients versus 17.4% for the autologous HCT recipients (p=0.03). After 100 days, TRM remained significantly higher for the allograft HCT recipients (17.8% vs 6.5%, p<0.001) (13). Ratanatharathorn and associates (32) reported in their prospective comparison that 12 of 16 deaths in the allogeneic HCT group were not related to NHL compared to only 4 of 22 in the autologous HCT population. These results are similar to our data with 31 of the 60 deaths in the allogeneic group were unrelated to lymphoma compared to 110 out of 414 deaths in the autologous group. Other studies which compared autologous versus allogeneic HCT for NHL that included low- as well as aggressive-grade NHL also found TRM after myeloablative allogeneic HCT was a significant factor for early death (17, 19, 20, 36).
Many authors have noted that relapse rates after allogeneic transplant for NHL are lower than after autologous transplant (17, 20, 32, 37–39). Our study shows that the relapse/progression rate after allogeneic transplant was similar to that after autologous despite higher risk disease (higher stage, aaIPI and chemotherapy resistant disease) in the allograft group. The prospective comparative trial reported by Ratanatharathorn and associates (32) reported similar rates of PFS, but a higher rate of disease progression after autologous transplant in NHL, suggesting a GVL effect. The prospective 100 patient trial reported by Goldstein et al (33) that also included Hodgkin disease patients showed an improved freedom from progression in the recipients of allografts compared to the subjects treated using autologous HCT but there were no statistical differences in PFS or OS between the two populations. Other evidence for GVL has included reports of remissions after donor leukocyte infusion or reduced-intensity allotransplant for NHL, particularly in low-grade NHL (40–43). On the other hand, an analysis of syngeneic versus allogeneic HCT showed no significant difference in NHL relapse rates (19). This study suggested that tumor contamination, rather than a GVL effect, may contribute to differences in relapse between allogeneic and autologous procedures.
The five-year PFS and OS were superior in those DLBCL patients undergoing autologous HCT compared to myeloablative allogeneic sibling-matched HCT, (43% vs 22% and 49% vs 22%, respectively). Most relapse events occurred in the early post-transplant period and any potential benefit in relapse after allogeneic HCT was offset by a higher early TRM in the first 12 months. Our results differ from the matched comparison reported by Schimmer et al (17) in which TRM and OS were similar. Unlike our series that was restricted to DLBCL, their heterogeneous population included about one-third indolent NHL as well as other histologic subtypes. The European Blood and Marrow Transplant Registry (EBMTR) matched analysis included 1185 allogeneic HCT patients but only 147 intermediate-grade NHL recipients (38). While the 4-year actuarial survival of 38% for allogeneic HCT was better than we noted, the matched pair analysis data still demonstrated superiority of the autologous HCT procedure.
A clinically driven patient selection bias for allogeneic HCT explains some of our observations. For example, Schimmer and co-workers (17) reported that those patients with bone marrow involvement or in whom the stem cell harvest was inadequate were offered an allogeneic HCT if they had a related donor and had chemosensitive tumor. Further, they comment that in some cases, patient, physician or a combination modified the HCT prescription. Advancing age often is a reason not to offer a myeloablative allogeneic HCT to a NHL patient. In recent years the use of reduced-intensity conditioning has increased in the older patient subset although this approach is suggested to be significantly more efficacious for indolent NHL rather than DLBCL (42, 44, 45). These clinical preferences and patient or disease features may have led to the selection of allogeneic transplants in a lower age, higher risk lymphoma subset. These patterns of patient selection are reflective of practices across a large number of centers and provide an opportunity to analyze the efficacy of the allogeneic approach. Therefore it is notable that despite the significantly higher proportion of high risk patients in the allogeneic group attributable to patient selection, the overall risk of relapse or progression of DLBCL was similar to the autologous group. The efficacy of the allogeneic approach in preventing lymphoma progression could be attributable to greater use of TBI, lack of tumor contamination in the allograft and a GVL effect operating in the allogeneic group.
Relapse remains the biggest drawback for successful autologous HCT and in this series the 5-year relapse rate was 39%. Attempts to induce autologous GVHD and corresponding GVL have not been successful (46). Other investigators have incorporated post-HCT treatment as well as implementation of targeted therapies such as radioimmunoconjugates into the preparative regimens in order to lower relapse rates after autologous HCT (47, 48). The results of such trials, including a 131I- Tositumomab and BEAM-containing Blood & Marrow Transplant Clinical Trials Network (BMT CTN) study, are on-going. The increasing use of Rituximab has likely changed the profile of patients currently receiving autologous and allogeneic HCT for B cell lymphoma especially follicular lymphoma. However, the impact of this agent on the utilization of and outcomes after HCT for DLBCL is not clear at this time.
In summary, for DLBCL patients, autologous HCT was associated with superior survival compared to myeloablative HLA-identical, sibling-matched allogeneic HCT. For a cohort of high risk DLBCL patients receiving myeloablative matched sibling allogeneic transplants, relapse risk was similar to the autologous group despite the differences in disease characteristics between the groups. The high incidence of early TRM after allogeneic transplants reduces the overall efficacy of this modality.
Supplementary Material
Acknowledgments
CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
We would also like to acknowledge the following for their contributions to this manuscript: Christopher N. Bredeson, Mitchell S. Cairo, John Gibson, Roger H. Herzig, Osman Ilhan, Willis H. Navarro and Peter H. Wiernik.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 4.Blay J, Gomez F, Sebban C, et al. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Parma Group. Blood. 1998;92:3562–3568. [PubMed] [Google Scholar]
- 5.Nademanee A, Molina A, Dagis A, et al. Autologous stem-cell transplantation for poor-risk and relapsed intermediate- and high-grade non-Hodgkin's lymphoma. Clin Lymphoma. 2000;1:46–54. doi: 10.3816/clm.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 6.Lerner RE, Thomas W, Defor TE, Weisdorf DJ, Burns LJ. The International Prognostic Index assessed at relapse predicts outcomes of autologous transplantation for diffuse large-cell non-Hodgkin's lymphoma in second complete or partial remission. Biol Blood Marrow Transplant. 2007;13:486–492. doi: 10.1016/j.bbmt.2006.12.452. [DOI] [PubMed] [Google Scholar]
- 7.Kessinger A, Sharp JG. The whys and hows of hematopoietic progenitor and stem cell mobilization. Bone Marrow Transplant. 2003;31:319–329. doi: 10.1038/sj.bmt.1703837. [DOI] [PubMed] [Google Scholar]
- 8.Vellenga E, van Agthoven M, Croockewit AJ, et al. Autologous peripheral blood stem cell transplantation in patients with relapsed lymphoma results in accelerated haematopoietic reconstitution, improved quality of life and cost reduction compared with bone marrow transplantation: the Hovon 22 study. Br J Haematol. 2001;114:319–326. doi: 10.1046/j.1365-2141.2001.02926.x. [DOI] [PubMed] [Google Scholar]
- 9.Glaspy JA. Economic considerations in the use of peripheral blood progenitor cells to support high-dose chemotherapy. Bone Marrow Transplant. 1999;23 Suppl 2:S21–S27. doi: 10.1038/sj.bmt.1701670. [DOI] [PubMed] [Google Scholar]
- 10.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 11.Kewalramani T, Nimer SD, Zelenetz AD, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin's disease or aggressive non-Hodgkin's lymphoma. Bone Marrow Transplant. 2003;32:673–679. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
- 12.Prince HM, Imrie K, Crump M, et al. The role of intensive therapy and autologous blood and marrow transplantation for chemotherapy-sensitive relapsed and primary refractory non-Hodgkin's lymphoma: identification of major prognostic groups. Br J Haematol. 1996;92:880–889. doi: 10.1046/j.1365-2141.1996.437976.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Hoffknecht M, Hanel M, et al. Busulfan, cyclophosphamide and etoposide as high-dose conditioning therapy in patients with malignant lymphoma and prior dose-limiting radiation therapy. Bone Marrow Transplant. 1998;21:1171–1175. doi: 10.1038/sj.bmt.1701245. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport AP, Lifton R, Constine LS, et al. Autotransplantation for relapsed or refractory non-Hodgkin's lymphoma (NHL): long-term follow-up and analysis of prognostic factors. Bone Marrow Transplant. 1997;19:883–890. doi: 10.1038/sj.bmt.1700772. [DOI] [PubMed] [Google Scholar]
- 16.Mollee P, Lazarus HM, Lipton J. Why aren't we performing more allografts for aggressive non-Hodgkin's lymphoma? Bone Marrow Transplant. 2003;31:953–960. doi: 10.1038/sj.bmt.1704040. [DOI] [PubMed] [Google Scholar]
- 17.Schimmer AD, Jamal S, Messner H, et al. Allogeneic or autologous bone marrow transplantation (BMT) for non-Hodgkin's lymphoma (NHL): results of a provincial strategy. Ontario BMT Network, Canada. Bone Marrow Transplant. 2000;26:859–864. doi: 10.1038/sj.bmt.1702625. [DOI] [PubMed] [Google Scholar]
- 18.Aksentijevich I, Jones RJ, Ambinder RF, Garrett-Mayer E, Flinn IW. Clinical outcome following autologous and allogeneic blood and marrow transplantation for relapsed diffuse large-cell non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:965–972. doi: 10.1016/j.bbmt.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Bierman PJ, Sweetenham JW, Loberiza FR, Jr, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 20.Verdonck LF. Allogeneic versus autologous bone marrow transplantation for refractory and recurrent low-grade non-Hodgkin's lymphoma: updated results of the Utrecht experience. Leuk Lymphoma. 1999;34:129–136. doi: 10.3109/10428199909083388. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Grinblatt D, van Besien K. Autologous and allogeneic transplantation for aggressive NHL. Cytotherapy. 2002;4:223–240. doi: 10.1080/146532402320219745. [DOI] [PubMed] [Google Scholar]
- 22.Dhedin N, Giraudier S, Gaulard P, et al. Allogeneic bone marrow transplantation in aggressive non-Hodgkin's lymphoma (excluding Burkitt and lymphoblastic lymphoma): a series of 73 patients from the SFGM database. Societ Francaise de Greffe de Moelle. Br J Haematol. 1999;107:154–161. doi: 10.1046/j.1365-2141.1999.01666.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitterbauer M, Neumeister P, Kalhs P, et al. Long-term clinical and molecular remission after allogeneic stem cell transplantation (SCT) in patients with poor prognosis non-Hodgkin's lymphoma. Leukemia. 2001;15:635–641. doi: 10.1038/sj.leu.2402053. [DOI] [PubMed] [Google Scholar]
- 24.Soiffer RJ, Freedman AS, Neuberg D, et al. CD6+ T cell-depleted allogeneic bone marrow transplantation for non-Hodgkin's lymphoma. Bone Marrow Transplant. 1998;21:1177–1181. doi: 10.1038/sj.bmt.1701271. [DOI] [PubMed] [Google Scholar]
- 25.van Besien K, Thall P, Korbling M, et al. Allogeneic transplantation for recurrent or refractory non-Hodgkin's lymphoma with poor prognostic features after conditioning with thiotepa, busulfan, and cyclophosphamide: experience in 44 consecutive patients. Biol Blood Marrow Transplant. 1997;3:150–156. [PubMed] [Google Scholar]
- 26.Stein RS, Greer JP, Goodman S, et al. Intensified preparative regimens and allogeneic transplantation in refractory or relapsed intermediate and high grade non-Hodgkin's lymphoma. Leuk Lymphoma. 2001;41:343–352. doi: 10.3109/10428190109057989. [DOI] [PubMed] [Google Scholar]
- 27.Przepiorka D, van Besien K, Khouri I, et al. Carmustine, etoposide, cytarabine and melphalan as a preparative regimen for allogeneic transplantation for high-risk malignant lymphoma. Ann Oncol. 1999;10:527–532. doi: 10.1093/oxfordjournals.annonc.a010369. [DOI] [PubMed] [Google Scholar]
- 28.Juckett M, Rowlings P, Hessner M, et al. T cell-depleted allogeneic bone marrow transplantation for high-risk non-Hodgkin's lymphoma: clinical and molecular follow-up. Bone Marrow Transplant. 1998;21:893–899. doi: 10.1038/sj.bmt.1701209. [DOI] [PubMed] [Google Scholar]
- 29.Doocey RT, Toze CL, Connors JM, et al. Allogeneic haematopoietic stem-cell transplantation for relapsed and refractory aggressive histology non-Hodgkin lymphoma. Br J Haematol. 2005;131:223–230. doi: 10.1111/j.1365-2141.2005.05755.x. [DOI] [PubMed] [Google Scholar]
- 30.Vaishampayan U, Karanes C, Du W, Varterasian M, al-Katib A. Outcome of relapsed non-Hodgkin's lymphoma patients after allogeneic and autologous transplantation. Cancer Invest. 2002;20:303–310. doi: 10.1081/cnv-120001174. [DOI] [PubMed] [Google Scholar]
- 31.Nachbaur D, Oberaigner W, Fritsch E, Nussbaumer W, Gastl G. Allogeneic or autologous stem cell transplantation (SCT) for relapsed and refractory Hodgkin's disease and non-Hodgkin's lymphoma: a single-centre experience. Eur J Haematol. 2001;66:43–49. doi: 10.1034/j.1600-0609.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 32.Ratanatharathorn V, Uberti J, Karanes C, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin's lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- 33.Goldstein SC, Perkins J, Janssen WE, et al. A prospective, comparative trial of high dose therapy followed by allogeneic versus autologous stem cell transplantation in 100 patients with high risk lymphoma. Blood. 2001:98. [abstract #1754] [Google Scholar]
- 34.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Pasquini MC, Wang Z, Schneider L. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2007;13:5–9. Available at: http://www.cibmtr.org/PUBLICATIONS/newsletter/index.html.
- 36.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 37.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77:649–653. [PubMed] [Google Scholar]
- 38.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 39.Chopra R, Goldstone AH, Pearce R, et al. Autologous versus allogeneic bone marrow transplantation for non-Hodgkin's lymphoma: a case-controlled analysis of the European Bone Marrow Transplant Group Registry data. J Clin Oncol. 1992;10:1690–1695. doi: 10.1200/JCO.1992.10.11.1690. [DOI] [PubMed] [Google Scholar]
- 40.van Besien KW, de Lima M, Giralt SA, et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graft-versus-lymphoma effect. Bone Marrow Transplant. 1997;19:977–982. doi: 10.1038/sj.bmt.1700781. [DOI] [PubMed] [Google Scholar]
- 41.Mandigers CM, Meijerink JP, Raemaekers JM, Schattenberg AV, Mensink EJ. Graft-versus-lymphoma effect of donor leucocyte infusion shown by real-time quantitative PCR analysis of t(14;18) Lancet. 1998;352:1522–1523. doi: 10.1016/S0140-6736(05)60328-5. [DOI] [PubMed] [Google Scholar]
- 42.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakou CA, Milligan D, al CRe. Outcome of non-myeloablative stem cell transplantation for NHL is dependent on histology: good for patients with low grade disease and poor for those with high grade lymphoma. Blood. 2001:98. [abstract #1737] [Google Scholar]
- 45.Spitzer TR, McAfee SL, Dey BR, et al. Durable progression-free survival (PFS) following non-myeloablative bone marrow transplantation (BMT) for chemorefractory diffuse large B-cell lymphoma (B-LCL) Blood. 2001:98. [abstract #2813] [Google Scholar]
- 46.Kline J, Subbiah S, Lazarus HM, van Besien K. Autologous graft-versus-host disease: harnessing anti-tumor immunity through impaired self-tolerance. Bone Marrow Transplant. 2008;41:505–513. doi: 10.1038/sj.bmt.1705931. [DOI] [PubMed] [Google Scholar]
- 47.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 48.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or = 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25:1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.