Abstract
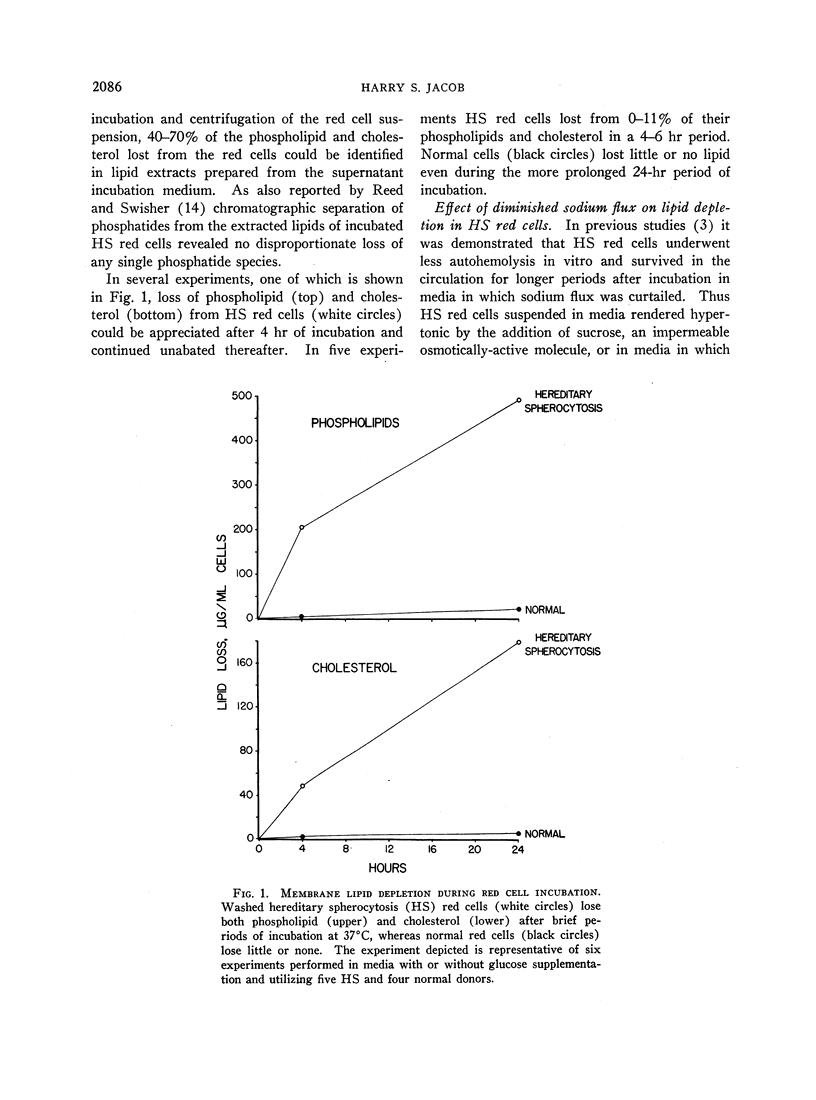
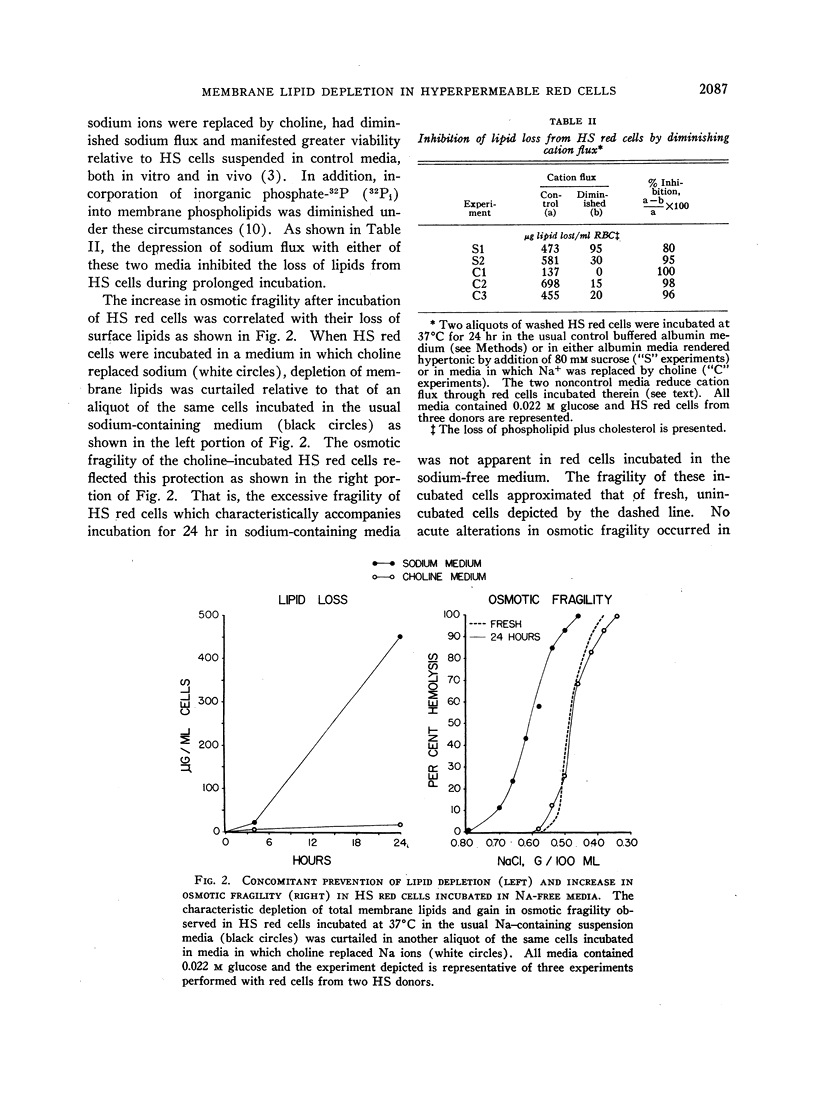
Hereditary spherocytosis (HS) red cells lose membrane lipids excessively during incubation in vitro. Individual phosphatides as well as cholesterol are lost in proportion to their content in membranes, suggesting that fragments of membrane are removed. Supplementation of HS red cells with glucose during incubation has no consistent protective effect, whereas diminishing the excessive sodium flux through these cells by suspending them in either sodium-free or hypertonic media prevents membrane fragmentation. The characteristic excessive increase in osmotic fragility which occurs in incubated HS red cells results both from inordinate accumulation of intracellular sodium ions which produces osmotic swelling, and from depletion of surface material which generates microspherocytosis. Inhibiting both of these processes by incubating HS red cells in sodium-free media completely prevents increases in osmotic fragility despite prolonged incubation.
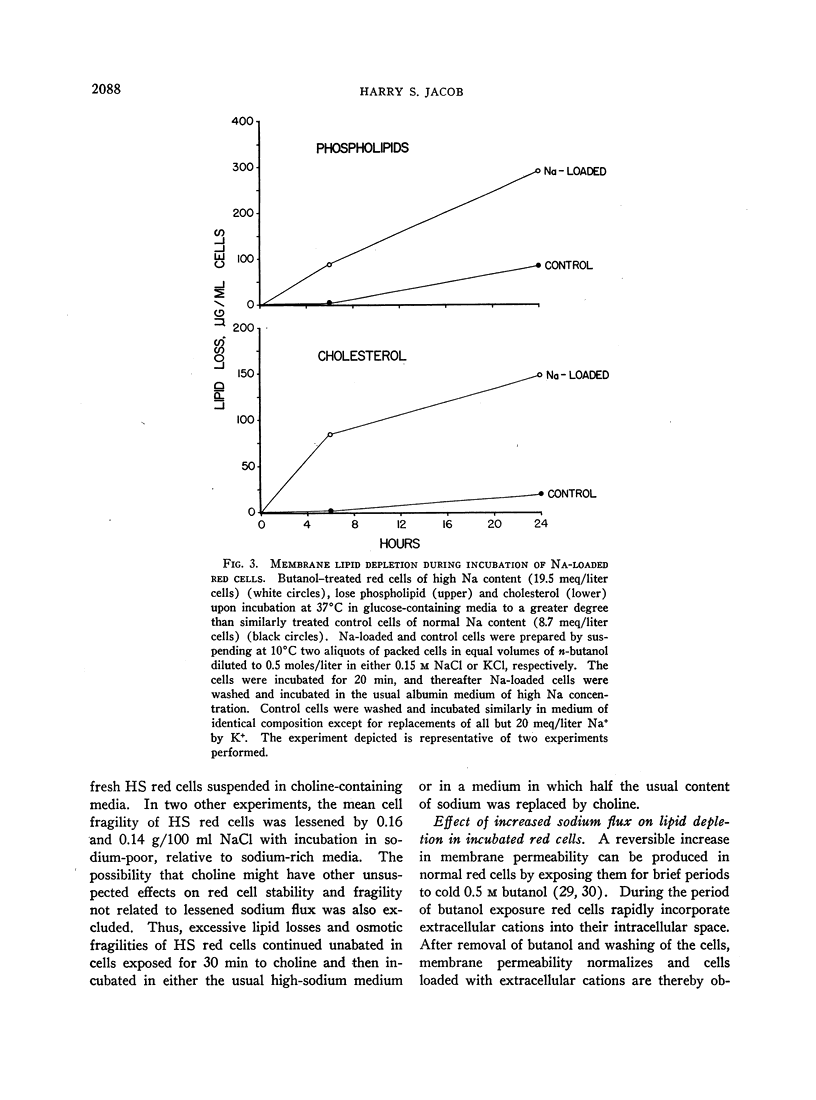
Normal red cells rendered hyperpermeable to cations by exposure either to n-butanol or to inhibitors of membrane sulfhydryl groups, lose membrane lipid upon incubation in a similar fashion to untreated HS red cells; perfectly smooth microspherocytes, akin to those seen in HS, are thereby generated.
I conclude that depletion of membrane lipids in HS which leads to microspherocytosis is correlatable with the excessive cation flux and possibly to the stimulated metabolism of acidic phosphatides in these red cells. It is suggested that this relation is derived from the fact that these phosphatides are in some way involved in maintaining the proper alignment of repeating membrane lipoprotein units, and that this function is adversely affected when these molecules are turning over more rapidly in response to increased cation flux.
Full text
PDF


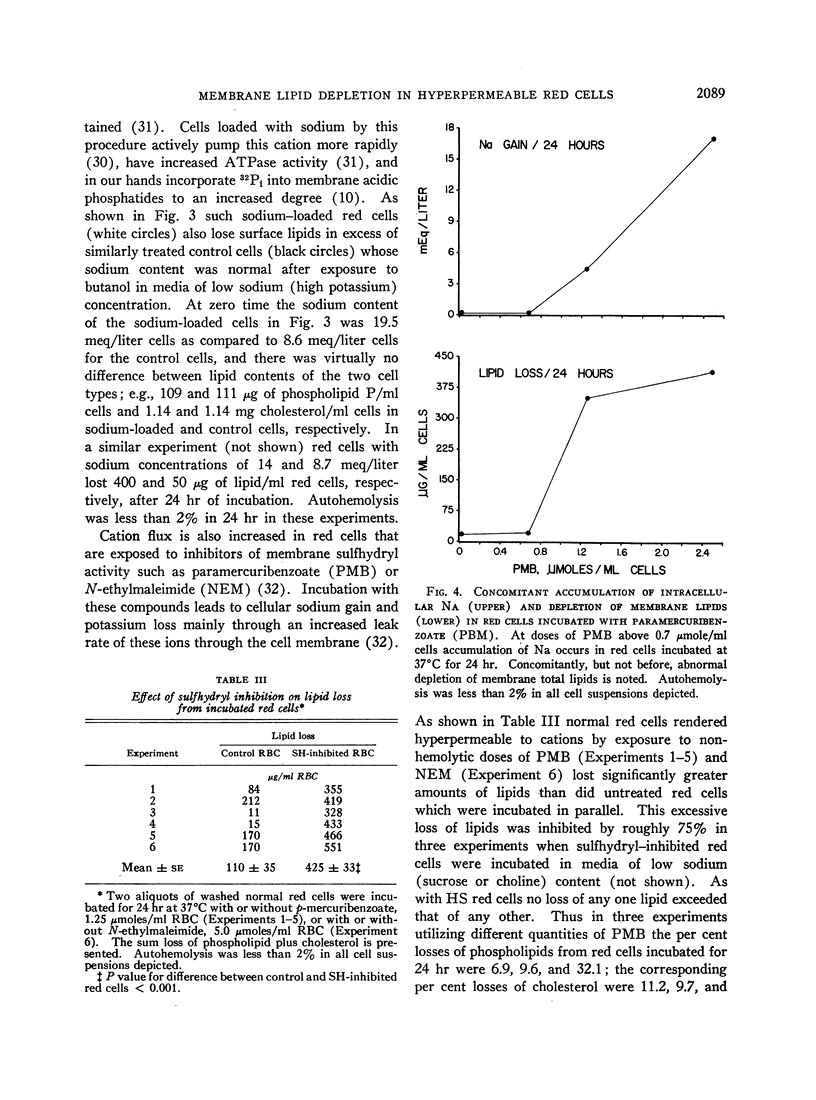
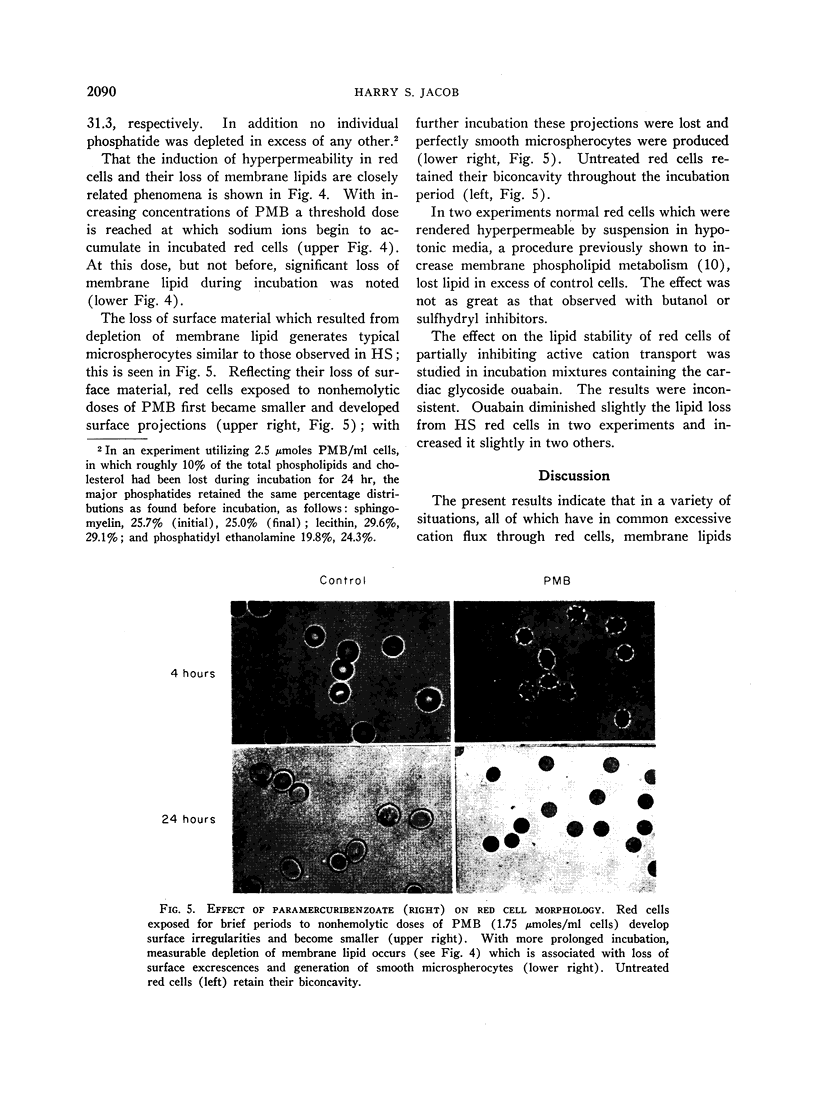




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., WILSON C. E., GREGOR H. P. IONIC PROPERTIES OF AQUEOUS DISPERSIONS OF PHOSPHATIDIC ACID. J Biol Chem. 1964 Dec;239:4066–4072. [PubMed] [Google Scholar]
- ALTMAN K. I., IZZO M. J., SWISHER S. N., YOUNG L. E. Studies on spontaneous in vitro autohemolysis in hemolytic disorders. Blood. 1956 Nov;11(11):977–997. [PubMed] [Google Scholar]
- BERTLES J. F. Sodium transport across the surface membrane of red blood cells in hereditary spherocytosis. J Clin Invest. 1957 Jun;36(6 Pt 1):816–824. doi: 10.1172/JCI103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERSON C. P. The influence of the spleen on the osmotic behavior and the longevity of red cells in hereditary spherocytosis (congenital hemolytic jaundice); a case study. BMQ. 1954 Sep;5(3):65–76. [PubMed] [Google Scholar]
- GARDOS G. Akkumulation der Kaliumionen durch menschliche Blutkörperchen. Acta Physiol Acad Sci Hung. 1954;6(2-3):191–199. [PubMed] [Google Scholar]
- Green D. E., Allmann D. W., Bachmann E., Baum H., Kopaczyk K., Korman E. F., Lipton S., MacLennan D. H., McConnell D. G., Perdue J. F. Formation of membranes by repeating units. Arch Biochem Biophys. 1967 Mar;119(1):312–335. doi: 10.1016/0003-9861(67)90461-4. [DOI] [PubMed] [Google Scholar]
- Grossman C. M., Horky J., Kohn R. In vitro incorporation of 32P-orthophosphate into phosphatidyl ethanolamine and other phosphatides by mature human erythrocyte ghosts. Arch Biochem Biophys. 1966 Oct;117(1):18–27. doi: 10.1016/0003-9861(66)90120-2. [DOI] [PubMed] [Google Scholar]
- HARRIS E. J., PRANKERD T. A. The rate of sodium extrusion from human erythrocytes. J Physiol. 1953 Sep;121(3):470–486. doi: 10.1113/jphysiol.1953.sp004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. BIOLOGICAL TRANSPORT. Annu Rev Biochem. 1963;32:553–578. doi: 10.1146/annurev.bi.32.070163.003005. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L., CASTLE W. B. Red cell filtration and the pathogenesis of certain hemolytic anemias. Blood. 1961 Aug;18:133–148. [PubMed] [Google Scholar]
- Jacob H. S. Abnormalities in the physiology of the erythrocyte membrane in hereditary spherocytosis. Am J Med. 1966 Nov;41(5):734–743. doi: 10.1016/0002-9343(66)90034-9. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Karnovsky M. L. Concomitant alterations of sodium flux and membrane phospholipid metabolism in red blood cells: studies in hereditary spherocytosis. J Clin Invest. 1967 Feb;46(2):173–185. doi: 10.1172/JCI105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONSEK J., BISHOP C. Relationship between adenosine triphosphate and cation transport in the human red cell. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:813–817. doi: 10.3181/00379727-110-27660. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- PARPART A. K., GREEN J. W. Potassium and sodium exchanges in rabbit red cells with n-butyl alcohol. J Cell Physiol. 1951 Dec;38(3):347–360. doi: 10.1002/jcp.1030380304. [DOI] [PubMed] [Google Scholar]
- PRANKERD T. A. Studies on the pathogenesis of haemolysis in hereditary spherocytosis. Q J Med. 1960 Apr;29:199–208. [PubMed] [Google Scholar]
- Reed C. F., Swisher S. N. Erythrocyte lipid loss in hereditary spherocytosis. J Clin Invest. 1966 May;45(5):777–781. doi: 10.1172/JCI105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAGAMI T., MINARI O., ORII T. BEHAVIOR OF PLASMA LIPOPROTEINS DURING EXCHANGE OF PHOSPHOLIPIDS BETWEEN PLASMA AND ERYTHROCYTES. Biochim Biophys Acta. 1965 Feb 1;98:111–116. [PubMed] [Google Scholar]
- SALEM L. The role of long-range forces in the cohesion of lipoproteins. Can J Biochem Physiol. 1962 Sep;40:1287–1298. [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEITEL P. RELATION OF ALTERED PLASTICITY OF THE ERYTHROCYTES TO THEIR SHORTENED LIFE SPAN. Sangre (Barc) 1964;41:421–426. [PubMed] [Google Scholar]
- VAN GASTEL, VAN DEN BERG D., DE GIER J., VAN DEENEN L. SOME LIPID CHARACTERISTICS OF NORMAL RED BLOOD CELLS OF DIFFERENT AGE. Br J Haematol. 1965 Mar;11:193–199. doi: 10.1111/j.1365-2141.1965.tb06577.x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. Potassium movements and ATP in human red cells. J Physiol. 1958 Mar 11;140(3):479–497. [PMC free article] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]
- Weed R. I., Bowdler A. J. Metabolic dependence of the critical hemolytic volume of human erythrocytes: relationship to osmotic fragility and autohemolysis in hereditary spherocytosis and normal red cells. J Clin Invest. 1966 Jul;45(7):1137–1149. doi: 10.1172/JCI105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]