Abstract
Background
Bone marrow stem cells (BMSCs) may be a novel treatment modality for organ ischemia, possibly through beneficial paracrine mechanisms. However, stem cells from older hosts exhibit decreased function during stress. We therefore hypothesized that: 1) BMSCs derived from neonatal hosts would provide protection to ischemic myocardium; and 2) neonatal stem cells would enhance post-ischemic myocardial recovery above that seen with adult stem cell therapy.
Materials and Methods
Female adult Sprague-Dawley rat hearts were subjected to an ischemia/reperfusion protocol via Langendorff isolated heart preparation (15 minutes equilibration, 25 minutes ischemia, and 60 minutes reperfusion). BMSCs were harvested from adult and neonatal mice and cultured through several passages under normal conditions (37 C, 5% CO2/air). Immediately prior to ischemia, one million adult or neonatal BMSCs were infused into the coronary circulation. Cardiac functional parameters were continuously recorded.
Results
Pretreatment with adult BMSCs significantly increased post-ischemic myocardial recovery as noted by improved left ventricular developed pressure, end diastolic pressure, contractility, and rate of relaxation. Neonatal stem cells, however, did not cause any noticeable improvement in myocardial functional parameters following ischemia.
Conclusion
Neonatal and adult BMSCs are distinctly different in the degree of beneficial tissue protection that they can provide. The data herein suggests that a critical age exists as to when stem cells become fully activated to provide their beneficial protective properties. Defining the genes that initiate these protective properties may allow for genetic amplification of beneficial signals, and the generation of “super stem cells” that provide maximum protection to ischemic tissues.
Keywords: Injury, neonatal, adult, myocardial, VEGF, estrogen
INTRODUCTION
Coronary artery disease is a source of tremendous morbidity and mortality (1). Medical management of the more severe cases of cardiac ischemia is often inadequate, thereby warranting revascularization via percutaneous stenting or arterial bypass. The subsequent reperfusion injury associated with the ischemic episode is also detrimental, and results in significant aberrations to the inflammatory cascade. In this regard, bone marrow mesenchymal stem cells (BMSCs) represent a novel treatment modality with increasing therapeutic potential (2–6). In addition, stem cells have been shown to exhibit a variety of paracrine effects, including the release of protective growth factors and antiapoptotic signals, which may afford protection to injured cardiac tissue (7–9).
Indeed, we and others have previously demonstrated that BMSC differentiation is not required for stem cell mediated cardioprotection. The infusion of BMSCs into myocardium subjected to acute ischemia and reperfusion injury was noted to improve functional recovery, decrease proinflammatory cytokine production, and decrease activation of proapoptotic caspases (9, 10). Thus, concomitant pro- and anti- inflammatory properties of BMSCs may be an important aspect of their ability to heal injured tissue.
Although BMSCs from hosts of varying ages are able to show multipotent potential, increasing age of stem cells and their hosts has been associated with decreased functional capacity under conditions of stress (11, 12). Specifically, increasing age has been associated with telomere shortening and dysfunction, mesenchymal progenitor cell dysfunction, a reduced capacity of bone marrow stromal cells to maintain functional hematopoeitic stem cells, and noted changes in cytokine production (13). Furthermore, umbilical cord and adult derived dendritic cells were noted to express different chemokines which subsequently correlated with unique migratory responses (14). It therefore seems logical that stem cells from younger hosts may be better adapted to stress during states of ischemia, and may provide better functional recovery after injury. We therefore hypothesized that 1) intracoronary infusion of BMSCs derived from neonatal hosts would increase post-ischemic myocardial functional recovery, and 2) neonatal stem cell infusion would provide superior myocardial protection compared to stem cells derived from adults.
METHODS
Animals
Normal adult female Sprague-Dawley rats (200–250g, Harlan, Indianapolis, IN) and adult female C57/BL6J mice (The Jackson Laboratory, Bar Harbor, ME) were fed a standard diet and acclimated in a quiet quarantine room for 1 week before the experiments. Female neonatal C57/BL6J mice (2.5 weeks old, Jackson Laboratory, Bar Harbor, ME) were allowed to birth naturally, and were kept with their mothers until the time of sacrifice. The animal protocol was reviewed and approved by the Indiana Animal Care and Use Committee of the Indiana University. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 85-23, revised 1985).
Preparation of Mouse Bone Marrow Stromal Cells
A single-step stem cell purification method using adhesion to cell culture plastic was employed as previously described (15). Briefly, neonatal and adult mouse bone marrow stromal cells were collected from bilateral femurs and tibias after sacrifice by removing the epiphyses and flushing the shaft with complete media (Iscove’s Modified Dulbecco’s Medium (GIBCO Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (GIBCO Invitrogen, Carlsbad, CA)) using a syringe with a 26G needle. Cells were disaggregated by vigorous pipetting several times, and were passed through a 30-μm nylon mesh to remove remaining clumps of tissue. Cells were washed by adding complete media, centrifuging for 5 min at 300 g @ 24°C, and removing the supernatant. The cell pellet was then resuspended and cultured in 75 cm2 culture flasks with complete media at 37°C in 5%CO2 in air. BMSCs preferentially attached to the polystyrene surface; after 48 h, nonadherent cells in suspension were discarded. Fresh complete media was added and replaced every three or four days thereafter. When the cultures reached 90% of confluence, the stem cell culture was passaged; cells were recovered by the addition of a solution 0.25% trypsin-EDTA (GIBCO Invitrogen, Carlsbad, CA) and replated in flasks. Adult and neonatal stem cells maintained similar mesenchymal stem cell markers. Both cell lines were negative for the hematopoietic markers CD34, CD45, and CD117, and were positive for the mesenchymal stem cell marker CD44 (16). Cells were utilized for experimentation between passages 3–9.
Isolated heart preparation (Langendorff)
Rats were anesthetized (sodium pentobarbital, 60 mg/kg i.p.) and heparinized (500 U i.p.), and hearts were rapidly excised via median sternotomy and placed in 4 °C Krebs–Henseleit solution (119 mM NaCl, 20.8 mM NaHCO3, 11 mM Dextrose, 12 mM CaCl2(2H2O), 47 mM KCl, 11.7 mM MgSO4(7H2O), and 11.8 mM KH2PO4). The aorta was cannulated and the heart was perfused under constant pressure (mean 75 mmHg) with oxygenated (95% O2/5% CO2) Krebs–Henseleit solution (37 °C). A left atrial resection was performed prior to insertion of a water-filled latex balloon through the atrium into the ventricle. The balloon was initially adjusted to a desired mean end diastolic pressure (EDP) of 5 mmHg and hearts were allowed to equilibrate for 15 minutes. Pacing wires were fixed to the atrium and hearts were paced at approximately 6 Hz, 3V, 2 ms (350 bpm) during equilibration and reperfusion to ensure a standard heart rate between groups. A three-way stopcock above the aortic root was used to create warm global ischemia, during which time the heart was placed in a 37 °C degassed organ bath. After 25 minutes of ischemia, hearts were reperfused for 60 minutes. Data were continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments Inc., Milford, MA) and an Apple G4 PowerPC computer (Apple Computer Inc., Cupertino, CA). The developed pressure, which can be considered a surrogate for cardiac output (17), as well as +dp/dt (cardiac contractility), −dp/dt (rate of myocardial relaxation), and end diastolic pressures were recorded.
Experimental isolated heart groups
Rat hearts were divided into the following groups (N=5 per group): 1) control, 2) adult stem cell infusion, and 3) neonatal stem cell infusion. Stem cells were recovered by the addition of a solution 0.25% trypsin-EDTA (GIBCO Invitrogen, Carlsbad, CA) to culture flasks. Cells were counted with the aid of a hemocytometer, and one million cells were isolated. Cells were centrifuged at 300g, media was removed, and cells were resuspended in 1 ml of Krebs–Henseleit solution (37 C). Over the course of one minute prior to ischemia, the BMSC solution was infused into the coronary circulation.
Presentation of data and statistical analysis
All reported values are mean ± SEM at end reperfusion and p< 0.05 was considered statistically significant. Developed pressure, contractility (+dp/dt), and rate of relaxation (−dp/dt) are presented as a percentage of baseline during equilibration. Data were compared using Student’s t test or two-way analysis of variance (ANOVA) with post-hoc Bonferroni when appropriate.
RESULTS
Adult Stem Cells increase myocardial functional recovery after ischemic injury
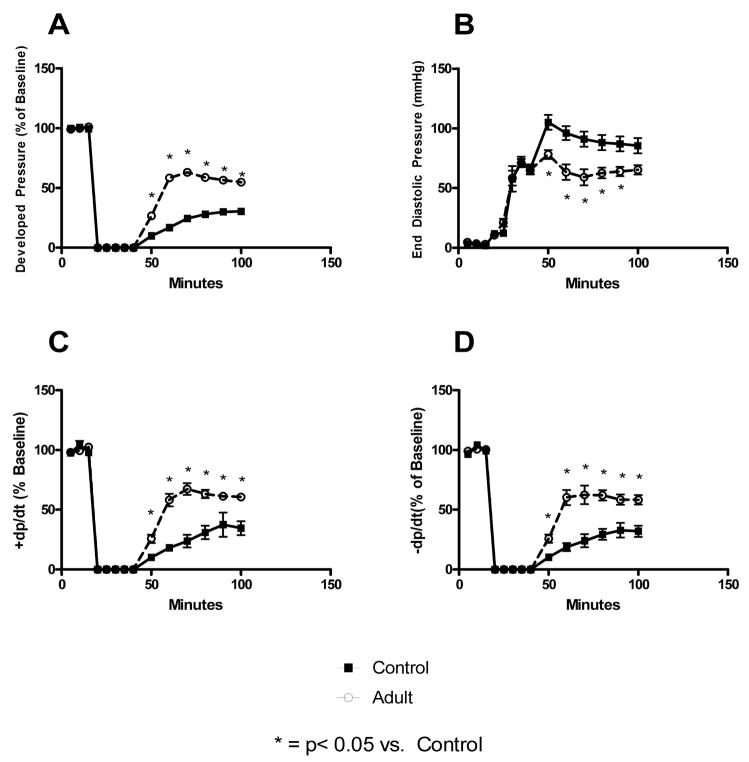
Pretreatment of myocardium with adult BMSCs prior to ischemia resulted in significantly improved post-ischemic functional recovery as compared to controls. This was noted in improved developed pressure (Control: 30.44 +/− 4.99 % of baseline vs. Adult BMSC: 54.81 +/− 3.65 % of baseline), +dp/dt (Control: 34.63 +/− 5.82 % of baseline vs. Adult BMSC: 60.64 +/− 2.57 % of baseline), −dp/dt (Control: 32.02 +/− 4.72 % of baseline vs. Adult BMSC: 58.31 +/− 4.05 % of baseline), and EDP (Control: 85.52 +/− 6.39 mmHg vs. Adult BMSC: 58.31 +/− 4.05 mmHg) within the adult BMSC infused groups (Figure 1). Although EDP was significantly higher throughout reperfusion in the adult BMSC group compared to controls, there was no significant difference in EDP at end reperfusion.
Figure 1. Adult stem cells protect ischemic myocardium.
Pretreatment of myocardium with adult BMSCs prior to ischemia significantly improved post-ischemic myocardial recovery. This was noted by increased developed pressure (A), decreased end diastolic pressure, increased contractility (C), and increased rate of relaxation (D) during reperfusion in the adult stem cell infusion groups. * = p<0.05 vs. control.
Neonatal Stem Cells do not increase myocardial functional recovery after ischemic injury
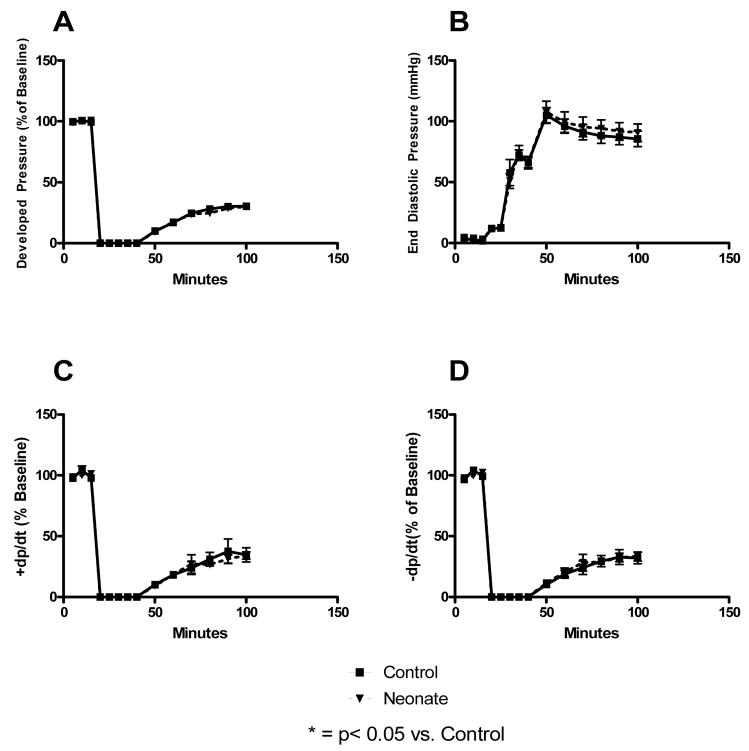
Infusion of neonatal stem cells prior to ischemia did not improve post-ischemic protection of injured myocardium. This was denoted by similar myocardial functional indices between control hearts and those infused with neonatal BMSCs (Figure 2).
Figure 2. Neonatal stem cells do not protect ischemic myocardium.
Pretreatment of myocardium with BMSCs derived from 2.5 week old neonates did not improve post-ischemic myocardial recovery. This was noted by similar developed pressures (A), end diastolic pressures (B), contractilities (C), and rates of relaxation (D) within neonatal stem cell infusion groups and controls.
An Age Threshold Exists in Mesenchymal Stem Cell Ischemic Protection
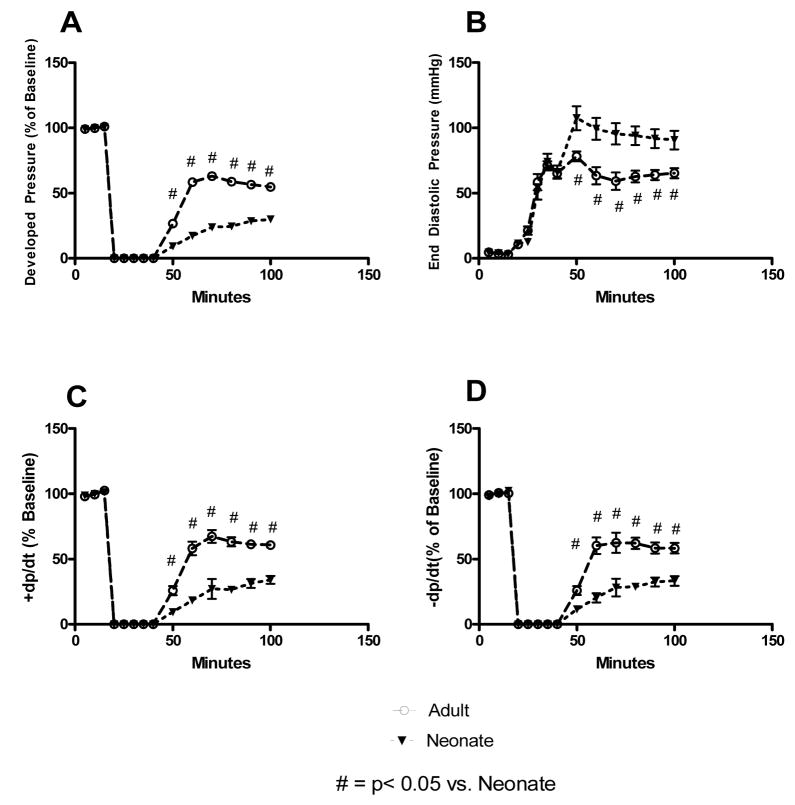
Neonatal and adult BMSCs were distinctly different in the degree of beneficial tissue protection that they provided. Adult BMSCs provided significantly better protection to ischemic myocardium than did neonatal BMSCs. This was seen in developed pressure (Adult: 54.81 +/− 3.65 % of baseline vs. Neonatal: 29.98 +/− 2.27% of baseline), EDP (Adult: 65.32 +/− 3.87 mmHg vs. Neonatal: 90.67 +/− 7.15 mmHg), +dp/dt (Adult: 60.64 +/− 2.57 % of baseline vs. Neonatal: 34.05 +/− 3.01 % of baseline), −dp/dt (Adult: 58.31 +/− 3.05 % of baseline vs. Neonatal: 33.26 +/− 3.67 % of baseline) (Figure 3).
Figure 3. Neonatal stem cells are distinctly different from adult stem cells.
Pretreatment of myocardium with adult BMSCs facilitated significantly higher developed pressure (A), decreased end diastolic pressure, increased contractility (C), and increased rate of relaxation (D) during reperfusion as compared to neonatal BMSCs. A difference in the degree of myocardial protection provided by these cell lines confirms that neonatal and adult BMSCs are distinctly different from one another. # = p<0.05 vs. neonates.
DISCUSSION
Adult stem cells have previously been shown to improve myocardial functional recovery following ischemic injury. However, emerging evidence suggests that younger stem cells may improve post-ischemic recovery beyond that seen with adult stem cell therapy. Herein we confirmed that adult BMSC infusion increased post-ischemic myocardial functional recovery. In addition, we showed that: 1) neonatal BMSCs did not increase post-ischemic myocardial function beyond that seen in controls; and 2) a critical age threshold likely exists as to when stem cells are able confer post-ischemic myocardial protection.
The potential for BMSCs to serve as a beneficial treatment modality for multiple types of ischemic diseases continues to rise (3). However, an apparent criticism of stem cell therapy is that the beneficial effects of stem cells may not persist into the chronic period of disease (18, 19). As such, it becomes important to understand and determine the optimum stem cell source for providing maximum protection during therapy. An ideal situation for therapy would entail autologous BMSC harvest, rapid ex vivo manipulation and expansion, and therapeutic application to ischemic boarder zones surrounding areas of central infarction. However, adult stem cells may not be the best cell source for this therapy. Multiple studies suggest that younger patients are relatively protected from injury and the multi-organ failure associated with trauma (20). In this regard, stem cells from younger hosts or even embryos may be more apt to provide maximum post-ischemic recovery of native tissues.
Previous studies examining the utility of embryonic stem cells (ESCs) in tissue protection have suggested that ESCs possess the ability to protect ischemic myocardium (21, 22). Because neonatal stem cells fall between ESCs and adult BMSCs in terms of “maturity”, it stood to reason that neonatal stem cells might also provide protection to ischemic tissues. However, in this study we saw that neonatal BMSCs did not confer acute protection to ischemic myocardium. This was indeed perplexing as both ESCs and adult BMSCs have facilitated cardioprotection following injury. ESCs though, have a higher degree of pluripotency, and as such, likely possess different intracellular mechanisms that are capable of providing protection. However, it is highly plausible that ESCs lose some of the beneficial signaling cascades that provide ischemic protection as they develop and mature into more differentiated cells.
Stem cell studies focusing on the mechanisms of tissue protection have shown that greater than 95% of cells infused into the coronary circulation do not become engrafted into the myocardium. This has led to the important recognition that stem cells may produce a number of paracrine factors during their transit through the heart that mediate post-ischemic tissue protection (23). Our group has shown that adult and neonatal stem cells exhibit distinct differences in the amount of protective growth factors that they produce in vitro. Specifically, neonatal stem cells produced lower levels of IL-6 and VEGF compared to adult BMSCs (16). IL-6 has potent anti-inflammatory properties, including the ability to prevent apoptosis and tissue damage via the inhibition of metalloproteinases, which work to degrade the extracellular matrix. IL-6 may also serve to increase production of the IL-1 receptor antagonist, thereby limiting the degree of inflammation (24–26). Other work has suggested that VEGF is critical to the post-ischemic recovery of myocardial tissue (27–29), and that adult BMSCs facilitate enhanced cardioprotection via the upregulation of VEGF expression during ischemia (30–32). In light of these previous studies, it is quite possible that the lower IL-6 and VEGF levels produced by neonatal stem cells could have limited the protective capacities of these cells during therapy.
Furthermore, the presence of estrogen has also been shown to increase tissue protection in a number of acute injury models (33–35). This protection is thought to be due to the down-regulation of proinflammatory cytokines by endogenous estrogen (36). In this regard, sex hormones play a role in stem cell function, intracellular signaling, and cardioprotection (8, 37). Because the adult stem cell hosts had achieved sexual maturity, their level of endogenous estrogen was higher than the neonatal hosts prior to stem cell harvest. Therefore, chronic in vivo exposure to estrogen in the adult stem cell population may have enhanced protective signaling cascades within these cells, thereby providing them with a greater potential for therapeutic protection. It is therefore highly likely that the activation of intracellular signaling cascades associated with adolescence has a positive effect on stem cells, and may work to increase the protective properties of stem cells during therapeutic intervention.
In conclusion, the results of this study confirm that neonatal and adult BMSCs are distinctly different, and suggest that a critical age exists as to when stem cells may become fully activated to provide their beneficial protective properties. Further studies are certainly necessary to understand the mechanisms associated with the increased degree of myocardial protection seen with the application of adult stem cell therapy. Defining the genes that initiate protective signaling mechanisms within neonatal stem cells may allow for the genetic amplification of vital genes, as well as the generation of “super stem cells” that provide maximum protection to ischemic tissues.
Acknowledgments
This work was supported in part by NIH R01GM070628, NIH R01HL085595, NIH K99/R00 HL0876077-01, NIH F32 HL085982, AHA Grant in aid, and AHA Post-doctoral Fellowship 0526008Z.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Nagy RD, Tsai BM, Wang M, Markel TA, Brown JW, Meldrum DR. Stem cell transplantation as a therapeutic approach to organ failure. J Surg Res. 2005;129:152–160. doi: 10.1016/j.jss.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089–1099. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]
- 5.Haider H, Ashraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol. 2005;38:225–235. doi: 10.1016/j.yjmcc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Raeburn CD, Zimmerman MA, Arya J, Banerjee A, Harken AH. Stem cells and myocardial repair. J Am Coll Surg. 2002;195:686–693. doi: 10.1016/s1072-7515(02)01309-1. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL–6 expression: role of the 55 kDa TNF receptor (TNFR1) J Mol Cell Cardiol. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with Adult Progenitor Cells Improves Recovery and Decreases Native Myocardial Proinflammatory Signaling after Ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 11.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 12.Ballard VL, Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–1127. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- 13.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Basu S, Han MK, Kim YJ, Broxmeyer HE. Influence of ERK activation on decreased chemotaxis of mature human cord blood monocyte-derived dendritic cells to CCL19 and CXCL12. Blood. 2007;109:3173–3176. doi: 10.1182/blood-2006-04-014753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 16.Markel TA, Wang M, Crisostomo PR, Poynter JN, Manukyan MC, Meldrum DR. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling which distinguish them from their adult counterparts. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00031.2008. In press. [DOI] [PubMed] [Google Scholar]
- 17.Pantos C, Mourouzis I, Markakis K, Dimopoulos A, Xinaris C, Kokkinos AD, Panagiotou M, Cokkinos DV. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur J Cardiothorac Surg. 2007;32:333–339. doi: 10.1016/j.ejcts.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 19.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 20.Calkins CM, Bensard DD, Moore EE, McIntyre RC, Silliman CC, Biffl W, Harken AH, Partrick DA, Offner PJ. The injured child is resistant to multiple organ failure: a different inflammatory response? J Trauma. 2002;53:1058–1063. doi: 10.1097/00005373-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 24.Jordan M, Otterness IG, Ng R, Gessner A, Rollinghoff M, Beuscher HU. Neutralization of endogenous IL-6 suppresses induction of IL-1 receptor antagonist. J Immunol. 1995;154:4081–4090. [PubMed] [Google Scholar]
- 25.Shingu M, Miyauchi S, Nagai Y, Yasutake C, Horie K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Br J Rheumatol. 1995;34:101–106. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]
- 26.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 27.Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 28.Guzman MJ, Crisostomo PR, Wang Y, Wang M, Markel TA, Erwin GE, Meldrum DR. Vascular Endothelial Growth Factor Improves Myocardial Functional Recovery Following Ischemia/Reperfusion Injury. J Surg Res. 2007 doi: 10.1016/j.jss.2007.12.772. In press. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Zhu SJ, Wang W, Wei YJ, Hu SS. Transplantation of microencapsulated genetically modified xenogeneic cells augments angiogenesis and improves heart function. Gene Ther. 2008;15:40–48. doi: 10.1038/sj.gt.3303049. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–745. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Hiasa K, Egashira K, Kitamoto S, Ishibashi M, Inoue S, Ni W, Zhao Q, Nagata S, Katoh M, Sata M, Takeshita A. Bone marrow mononuclear cell therapy limits myocardial infarct size through vascular endothelial growth factor. Basic Res Cardiol. 2004;99:165–172. doi: 10.1007/s00395-004-0456-9. [DOI] [PubMed] [Google Scholar]
- 32.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh YC, Frink M, Thobe BM, Hsu JT, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. 17Beta-estradiol downregulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Mol Immunol. 2007;44:2165–2172. doi: 10.1016/j.molimm.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuebler JFJD, Toth B, Bland KI, Rue L, 3rd, Wang P, Chaudry IH. Estradiol administration improves splanchnic perfusion following trauma-hemorrhage and sepsis. Archives of Surgery. 2002;137:74–79. doi: 10.1001/archsurg.137.1.74. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2005;288:E321–326. doi: 10.1152/ajpendo.00278.2004. [DOI] [PubMed] [Google Scholar]
- 36.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 37.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26:571–574. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]