Abstract
The development of a functional, adherent endothelium is one of the major factors limiting the successful development of tissue engineered vascular grafts (TEVGs). The adhesion and function of endothelial cells (ECs) on smooth muscle cells (SMCs) are poorly understood. The goal of this research was to optimize conditions for the direct culture of endothelium on SMCs, and to develop an initial assessment of co-culture on EC function. The co-culture consisted of a culture substrate, a basal adhesion protein, a layer of porcine SMCs, a medial adhesion protein, and a layer of porcine ECs. Conditions that led to successful co-culture were: a polystyrene culture substrate, a quiescent state for SMCs, subconfluent density for SMC seeding and confluent density for EC seeding, and fibronectin (FN) for the basal adhesion protein. EC adhesion was not enhanced by addition of FN, collagen I, collagen IV or laminin (LN) to the medial layer. 3-D image reconstruction by confocal microscopy indicated that SMCs did not migrate over ECs and the cells were present in two distinct layers. Co-cultures could be consistently maintained for as long as 10 days. After exposure to 5 dyne/cm2 for 7.5 h, ECs remained adherent to SMCs. PECAM staining indicated junction formation between ECs, but at a lower level than that observed with EC monocultures. Co-culturing ECs with SMCs did not change the growth rate of ECs, but EC DiI-Ac-LDL uptake was increased. Thus, a confluent and adherent layer of endothelium can be directly cultured on quiescent SMCs.
Keywords: Co-culture, Smooth muscle cells, Endothelial cells
1. Introduction
Tissue engineering represents a promising approach to treat a number of cardiovascular problems including atherosclerosis, damaged valves and heart failure. Development of a functional, adherent endothelium is one of the major factors limiting the successful development of TEVG [1]. Attachment of endothelial cells (Ecs) on cultured blood vessels is often sub-optimal, and adherent ECs may be pro-coagulant [2].
The phenotype of smooth muscle cells (SMCs) ranges from the growth arrested contractile phenotype present in the normal vessel wall to the proliferating and synthetic phenotype seen in culture or during atherosclerosis [3,4]. After injury to the endothelium in vivo or culture in vitro with serum, SMCs undergo a phenotypic modulation characterized by increased cell replication, reduced levels of actin and myosin and cell contraction, increased extracellular matrix synthesis, and enrichment of rough endoplasmic reticulum [5]. In culture, growth can be arrested with serum-free media containing insulin, transferrin, selenium and ascorbate [6]. Replication of growth-arrested SMCs can be re-established by the addition of serum [7]. Surface substrate also affects SMC differentiation state [8]. In addition, in vivo and in vitro experiments show that ECs can influence the contractile and growth properties of vascular SMCs [9,10].
Several co-culture systems have been developed to study EC–SMC interactions. These include (1) culture of SMCs and ECs on opposite sides of membranes [11–15]; (2) culture of ECs on collagen gels containing SMCs [16,17]; (3) microcarrier/spheroid-bound ECs or SMCs [18,19]; (4) conditioned media [14,20]; and (5) culture of ECs directly on SMCs [21,22]. Co-culture of ECs and SMCs on opposite sides of a thin membrane stimulated SMC proliferation [14] and up-regulated VEGF, PDGF-AA, PDGF-BB, and TGF-β gene expression and down-regulated bFGF gene expression [23]. Cultured ECs with SMCs also changed ECs from the normal polygonal morphology in vitro to an elongated shape [16], increased EC gene expression of tissue factor [20], VEGF [23], adhesion molecules [11], growth-related oncogene-α and monocyte chemotactic protein-1 [12].
Generally, existing co-culture systems bring ECs and SMCs within 10–50 μm of each other. The relatively large separation distance between ECs and SMCs reduces the likelihood of gap junction formation that is reported to occur between ECs and SMCs [24], although SMCs can still make contact with ECs through pores present in thin membranes [14]. In addition, the distance significantly increases the diffusion time between the two cell types, which may limit the effectiveness of short-lived metabolites such as nitric oxide [25]. While these models have helped to elucidate many important interactions between the two cell types, a co-culture system in which both cell types are in close contact is needed. In addition, the presence of a synthetic non-degradable membrane interposed between the cells is not applicable to tissue engineering applications. A more biomimetic system is needed in order to better replicate the in vivo spatial arrangement, and to better understand tissue engineered vascular grafts (TEVGs).
While reports of some systems exist in the literature of the direct co-culture of ECs on SMCs [19,21–22,26], there has been little effort to optimize the culture conditions or assess the degree of confluency of ECs, which is essential for developing TEVG. In this study we examined the substrate material, culture media, cell seeding density, SMC phenotype, and adhesion proteins in the medial layer in an attempt to establish a stable co-culture with a confluent layer of both cell types. The effects of co-culture on EC function were also examined.
2. Materials and methods
2.1. Cells
Porcine SMCs were isolated from carotid artery and aortic explants of Yucatan miniature swine or farm pigs, as previously described [27]. ECs were scraped directly from the arterial intima. Since SMCs grew faster than EC did under the culture conditions, the passage numbers of EC and SMC differed in a given experiment. Passages up to 6 were used for SMCs and passages up to 5 used for ECs, while all cells were incubated at 37 °C and 5% CO2/95% air.
2.2. Culture media
Proliferative media (PM) that stimulated SMC growth [28] consisted of DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Gibco), 10% porcine serum (PS, Gibco), 0.05 g/L vitamin C, 3 × 10−6 g/L CuSO4, 0.05 m HEPES, 0.05 g/L proline, 0.05 g/L alanine, 0.02 g/L glycine, 10 × 10−9 g/L basic fibroblast growth factor, 10 × 10−9 g/L platelet-derived growth factor, and 1× antibiotic/antimycotic (Sigma, St. Louis, MO). To induce quiescence of SMCs, the quiescent media (QM) [29] consisted of DMEM/F12 with 0.05 g/L vitamin C, 3 × 10−6 g/L CuSO4, 0.05 m HEPES, 0.05 g/L proline, 0.05 g/L alanine, 0.02 g/L glycine, 1 × insulin, transferrin, and selenium supplement (ITS, Gibco), and 1 × antibiotic/antimycotic. Prior to co-culture, ECs were grown in PM similar to SMCs except with lower serum content (5% FBS and 5% PS). Since ECs would not grow in the absence of serum, co-culture media consisted of 1 part of PM and 5 part of QM (3.3% serum).
2.3. Co-culture model
The co-culture model consisted of a basal adhesion protein (FN at 5 μg/cm2, or LN at 2 μg/cm2) adsorbed onto a cell culture surface for 4–8 h at 37 °C or overnight at 4 °C. Next, subconfluent SMCs were seeded onto the coated protein and allowed to grow for several days. A medial adhesion protein was then added above the SMC layer for at least 4 h. Last, ECs were seeded over SMCs for 4 h at 37 °C, after which media removed and replaced with fresh co-culture media. The cells were co-cultured for at least 2 days before any tests were performed to allow recovery of cellular functions, such as LDL scavenger receptor activity.
2.4. Live cell staining for ECs and SMCs
To visualize ECs and SMCs in the co-culture, SMCs were nonspecifically incubated with 10 μm Cell Tracker Green (CTG, 5-chloromethylfluorescein diacetate; Molecular Probes, Eugene, OR) for 45 min at 37 °C before the addition of ECs. Media was changed to CTG-free media and cells were incubated for another 30 min. Two days post-EC seeding, DiI-Ac-LDL (Biomedical Technologies Inc., Stoughton, MA) was used to specifically label ECs (SMCs did not pick up appreciable DiI-Ac-LDL). 10 μg/mL DiI-Ac-LDL was added to co-culture media and incubated with cells for 4 h at 37 °C [30]. Cells were rinsed several times in phosphate buffered saline (PBS), and then covered with co-culture media. ECs and SMCs were visualized with standard rhodamine and FITC filter sets, respectively.
Fluorescent and phase contrast images were obtained on a Zeiss Axiovert S-100 inverted microscope (Carl Zeiss Inc., Thornwood, NY) connected to a digital camera (Carl Zeiss Inc.). The camera was connected via a frame grabber card (Pr-LG3-01 PCI, Scion Corp., Frederick, MD) to a Macintosh G3 computer (Apple Computer Inc., Cupertino, CA). Calculation of EC coverage on co-culture system based on fluorescence was accomplished by Matlab image processing toolbox (version 6.0, MathWork Inc., Natick, MA). Briefly, grayscale images were converted to binary thresholded images, and the coverage of ECs was given by the percentage of “1” in the tested image normalized to the percentage of “1” in the control EC image that was confluent, based on its corresponding phrase contrast image. To determine SMC coverage when no fluorophore was utilized, cells were detected using ImageJ (version 1.3p, National Institutes of Health) as described previously [31].
2.5. Confocal microscopy
To detect EC and SMC layers in co-culture, Z-sections of co-cultured cells were obtained using a Confocal Laser Scanning Microscope LSM 510 (Carl Zeiss) with an Archoplan 40×/0.8 water-immersion objective. ECs and SMCs were detected with 541 and 488 nm lasers, respectively. The multi-track mode was chosen in conjunction with the filter sets in order to exclude cross-talking between fluorescent signals. Average intensity of an image (green channel for SMCs and red channel for ECs) at each z plane was calculated and then plotted against z-direction. The resulting plot was deconvolved with the experimental Point Spread Function (PSF) using Richard–Lucy method with a default setting [32]. Analysis was conducted using Matlab Image Processsing Toolbox. To measure the PSF, green (505/515 nm) and red (540/560 nm) fluorescent microspheres (PS-Speck™, Molecular Probe) with a diameter of 0.175 μm were used as external fluorescent microscopy standards, and sectioned at the same configurations as the co-culture system.
2.6. Cell proliferation analysis
An In Situ Cell Proliferation Kit, FLUOS (Roche Diagnostics, Chicago, IL) was used to label and detect BrdU within the proliferating SMC and EC monolayers. Briefly, cells were incubated with the BrdU labeling solution for one hour, fixed with ethanol, denatured with HCl, and incubated with a fluorescein-conjugated anti-BrdU monoclonal antibody. To determine the total number of cells, the nuclei of the cells were counter-stained with 4′,6-diamindino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes). Ziess Axiovert S100 fluorescent microscope and an image-capturing program (Scion Image, Frederick, MD) were used to capture images of all of the nuclei (blue fluorescence) and the proliferating cells (green fluorescence). Ten images were captured per slide, and the percentage of proliferating cells were determined.
Proliferating ECs in monoculture and cells in co-culture were determined by using a Hoechst nuclear stain (Molecular Probes) in conjunction with flow cytometry. ECs were plated at confluence (50,000 cells/cm2) on FN-coated polystyrene or SMCs (day 0) and incubated at 37 °C and 5% CO2 for 2 days before beginning the proliferation assay. ECs were labeled with DiI-Ac-LDL, and EC and SMC nuclei were counter-stained with Hoechst. Cells on the culture system were removed by trypsin/EDTA, and fixed with 1% formalin in suspension. FACScan flow cytometry (BD Bioscience, San Jose, CA) was used to distinguish the ECs from the SMCs with the detection of the DiI-Ac-LDL and to measure the intensity of the nuclear stain. Based on the intensity of the nuclear stain, the percentage of ECs and SMCs in the S-phase of their cell cycle (proliferating) was determined. Trypsin treatment (0.25% or 0.5% for 8 min) of co-cultures could not remove the majority of the SMCs, but the SMCs that were detached were analyzed. EC density was measured by capturing 10 images per culture, counting the number of ECs as determined by DiI-Ac-LDL up-take, normalizing the data per cm2.
2.7. Immunoblotting and immunocytochemistry
To assess the SMC phenotype, calponin and smooth muscle myosin were examined using Western blots. SMCs were scraped in lysis buffer (20 mm Tris, 1% Triton X-100, 10% glycerol, 137 mm NaCl, 2 mm EDTA, 0.25 mm PMSF, 10 nm mycrocystin, 5 μg/ml leopeptin, 150 μm Na3VO4). The concentration of cell lysate was determined by the standard Bradford method, and equal amount of protein was loaded in each lane. The gels were run for 1 h at 350 mA using Mini-PROTEAN electrophoresis cell (Bio-Rad, Hercules, CA). Protein was transferred to nitrocellulose and blots were incubated with mouse anti-human monoclonal antibodies for calponin or smooth muscle myosin (Dako A/S, Denmark) for 1 h (dilution 1:500), and then were rinsed for five minutes in TBS-Tween (0.1%). The membranes were then incubated in anti-mouse IgG-HRP (Santa Cruz Biotechnology Inc., Santa Cruz, CA) with 0.1% bovine serum albumin (BSA) for 1 h (dilution 1:500). Next, the membrane was rinsed five times in TBS. Enhanced chemiluminescence (ECL, Amersham, Piscataway, NJ) was used to visualize the proteins on film. Each film was scanned using a GS 300 Transmittance/Reflectance Scanning Densitometer (Hoefer Scientific Instruments, San Francisco, CA).
To visualize PECAM, cells were rinsed twice with PBS containing Ca2+ and Mg2+, fixed with neutralized formalin buffer for 10 min, and permeabilized with 0.1% Triton X-100. Then cells were rinsed twice with PBS and incubated for 30 min with 10% goat serum in PBS. Next, 30 μl of anti-PECAM (CD-31, Antigenix-America, Huntington Sta., NY) was added to 15 μL to Zenon labeling reagent (Molecular Probes) and incubated for 5 min, after which 255 μL 10% goat serum (Vector, Burlingame, CA) in PBS was added to produce a final antibody concentration of 10 μg/mL. Labeled antibody was incubated with cells at room temperature for 60 min in the dark. After two rinses with PBS, cells were post-fixed for 15 min with neutralized formalin solution, mounted and viewed using confocal microscopy with excitation at 488 nm and emission between 505 and 550 nm.
2.8. Flow studies
A parallel plate flow chamber was used to assess adhesion and shape of ECs and SMCs exposed to laminar flow [33]. ECs, SMCs, or EC/SMC co-cultures were prepared on 1 inch × 3 inch polystyrene slides (Thermanox, Nunc) as described above. For a volumetric flow rate Q (cm3/s), and fluid viscosity μ (cP), the wall shear stress τw (dyne/cm2) is
| (1) |
where w is the channel width and h is the local channel height. The flow channel height was 178.5 μm and the width was 1.9 cm. For the assay media, the viscosity at 37 °C was 0.86 cP.
2.9. Statistical analysis
Results are expressed as mean±SEM. Statistical analysis was calculated using InStat software (v. 2.00; Graphpad Software, San Diego, CA). Analysis of variance (ANOVA) with repeated measures was utilized for data comparisons and a Tukey post-test was performed to determine significance between groups.
3. Results
3.1. Effect of surface on SMC morphology and growth
Growth of porcine SMCs was sensitive to the substrate. SMCs were plated at 5 × 103 cells/cm2 in PM on surfaces coated with 10 μg/cm2 collagen type I. After 5 days, the medium was replaced with QM and cells were examined after 10 days in culture. On Permanox™ and glass, the SMCs were spread and appeared fibroblast-like, whereas on polystyrene the cells were elongated. In addition, cells grown on polystyrene surface (6 well cell culture cluster, Corning Inc., Corning, NY; or SlideFlask, NUNC A/S, Denmark) were more uniformly distributed. SMC coverage was 76.1±5.6%, 59.3±0.4% and 31.6±1.7% on polystyrene, Permanox™ and glass, respectively (n = 2). Polystyrene was used in all co-culture experiments.
3.2. Effect of culture media on EC/SMC phenotype and proliferation
The serum and growth factor components of growth media modulated SMC phenotype. With serum, SMCs were polygonal in appearance and proliferated quickly with overgrowth (Fig. 1A). After shifting culture media to QM and culturing for 2 days, polygonal SMCs became elongated (Fig. 1B). This process could be reversed by addition of serum using PM, and was independent of cell density. Two different phenotypes (rhomboid and spindle-shape) were also observed by Hao et al. [34] in the cultured SMCs from porcine coronary artery. In order to validate the phenotypic change in QM, we used calponin and smooth muscle myosin, two differentiated cell markers, to immunoblot SMC cultures. The blotting indicated that protein levels of calponin and smooth muscle myosin increased 2 days after the medium was shifted from PM to QM (Fig. 2), confirming the change of SMC phenotype from proliferating state to quiescent state.
Fig. 1.
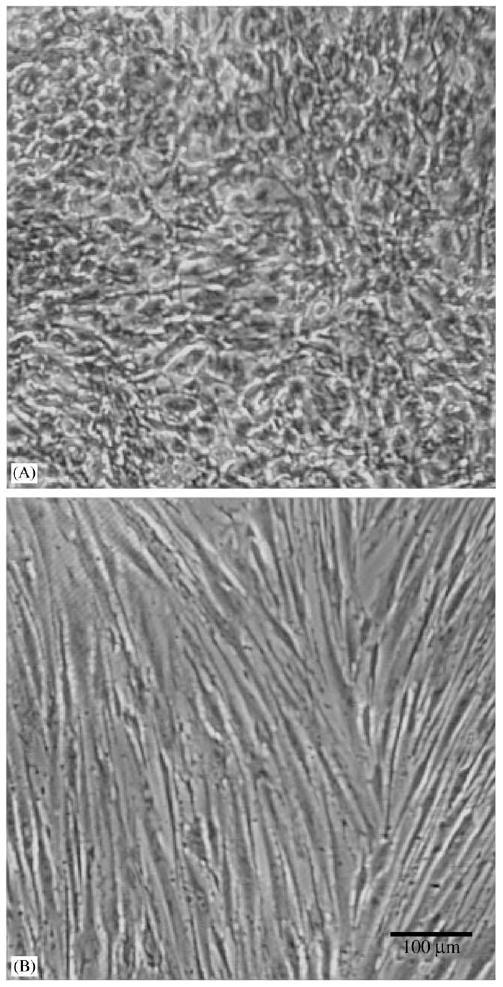
Effect of serum and growth factors on confluent SMC morphology. SMCs in PM (A, 20% total serum) and QM (B, no serum) after plating at 20 × 103 cells cm−2 and culturing for 5 days.
Fig. 2.
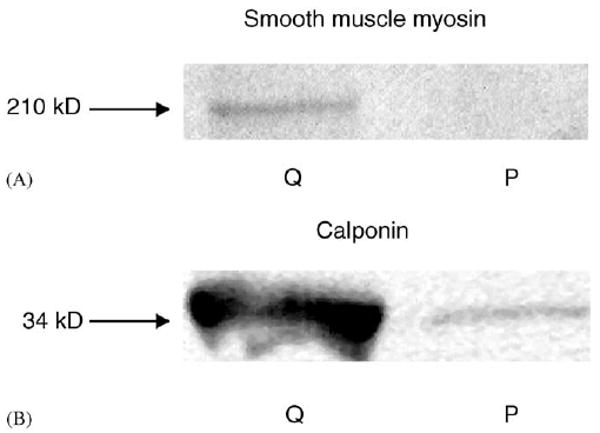
Western blots of SMC populations for calponin (A) and smooth muscle myosin (B). Blots are from quiescent SMCs (Q lanes) and proliferative SMCs (P lanes). For the Q lanes, SMCs were plated at 5 × 103 cells cm−2 and cultured with PM for 1 day, and then PM was changed to QM. SMCs were lysed 2 days later. For the P lanes, SMCs were plated at same seeding density with PM and lysed 2 days later.
ECs required serum to maintain a normal morphology. The absence of serum in the culture medium led to unhealthy looking ECs characterized by poor spreading, the inability to become confluent, no cobblestone morphology, and thin appendages. Therefore, 3.3% serum was used for the co-culture.
To quantitatively measure cell proliferation, we labeled the cells in S-phase with BrdU. The SMC proliferation stopped 2 days after the medium was changed from PM to QM (dashed line in Fig. 3A). When 3.3% serum was added on day 2 there was a transient change in SMC proliferation, but the cells remained in the quiescent phenotype until the end of the experiment. EC proliferation in monoculture (Fig. 3A) was also measured with BrdU. The results indicate a decrease in cell proliferation over time, which is probably due to contact inhibition. Co-culture experiments were preformed with BrdU, but since the cell types could not be distinguished, another method to measure proliferation was attempted.
Fig. 3.
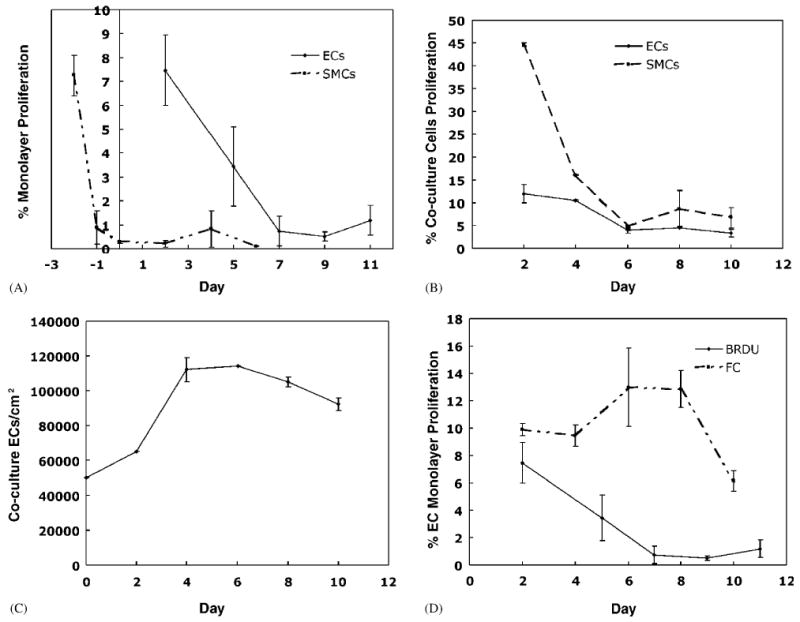
EC and SMC proliferation. (A) EC and SMC proliferation in monoculture detected by BrdU. (B) EC proliferation in co-culture system. ECs were separated from SMCs by incubation with 0.25% trypsin for 8 min and proliferation was determined by FC of Hoechst nuclear staining of cells.
Flow cytometry (FC) was used to separate the two cell types and to measure the percentage of cells proliferating in EC monolayers and ECs and SMCs in co-culture. EC and SMC proliferation decrease with time in co-culture (Fig. 3B). The percent of ECs proliferating in the co-culture decreased steadily until quiescence was reached on day 6. The steady drop indicates that the ECs did not attach at confluent density and continued to double to reach confluence. The ECs then became contact inhibited and quiescent. The results from measuring EC density in co-culture (Fig. 3C) agree with the proliferation results.
The EC and SMC proliferation in co-culture, measured by FC, are higher than values obtained for EC and SMC monoculture under identical culture conditions and measured using BrdU. With the SMCs, we noted that the trypsin treatment failed to detach a number of the SMCs from the surface and the SMCs that were removed formed clumps. Since proliferating cells are less adherent than non-proliferating cells, problems with cell detachment could explain the higher SMC proliferation levels. To examine other differences between FC and BrdU labeling, FC and BrdU measurements of EC proliferation were compared (Fig. 3D). The higher level of EC proliferation in the FC experiment is likely due to difficulties in discriminating and gating the population of non-proliferating cells by FC. In support of this we found that after 2 days of co-culture the total level of cell proliferations was 1.6% when measured with BrdU. Further, we did not observe overgrowth of EC population by SMCs. These results suggest that EC and SMC proliferation in co-culture was low.
3.3. Effect of SMC phenotype on EC attachment
ECs seeded on proliferating SMCs (PSMCs) attach with lower efficiency than ECs seeded on quiescent SMCs (QSMCs)(Fig. 4). Two days after attachment of ECs to SMCs, ECs were stained with DiI-Ac-LDL to distinguish them from SMCs. The coverage of ECs on PSMC was only 58±2%, whereas the coverage of ECs on QSMC was 93.6±1.4%. In addition, ECs did not bind uniformly to proliferating SMCs, rather they formed in patches and induced a contractile response in proliferating SMCs (Fig. 4A). EC coverage on proliferative SMCs continuously declined with time, and at day 6, there were few ECs left. ECs seeded onto QSMC exhibited a uniform binding (Fig. 4B). EC confluence was maintained for as long as 10 days.
Fig. 4.
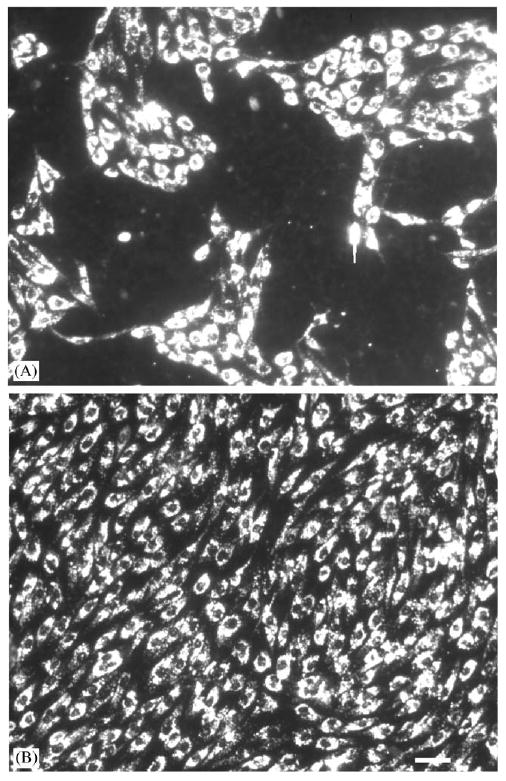
Effect of SMC growth state on EC attachment. ECs (passage 4) were stained with DiI-Ac-LDL, detached by trypsinization and attached to either proliferating SMCs (passage 6) (A) or quiescent SMCs (passage 6) (B), and examined 2 days later under epifluorescence. Scale bar is 50 μm.
3.4. Effect of adhesion proteins on EC coverage
FN, LN, collagen I, and collagen IV are natural components in the extracellular matrix, and play an important role in cell proliferation and differentiation. The effect of these medial proteins (FN at 5 μg/cm2, LN at 2 μg/cm2, collagen I at 10 μg/cm2, collagen IV at 10 μg/cm2) upon EC coverage on QSMCs was examined 2 days after attachment. In the absence of medial proteins the coverage was 85.2±13.4% (n = 4). Collagen I and IV did not affect EC coverage on SMCs (n = 4). FN and LN caused a small increase in cell coverage to 95.5±9.5% (n = 4) and 104±10% (n = 2), respectively. After 5 days of co-culture, coverage was 100% for all cases.
3.5. EC growth on quiescent and proliferative SMCs
To examine EC growth in the co-culture, ECs were seeded at a subconfluent density of 30,000 cells/cm2, EC growth on quiescent SMCs was no different than EC growth alone, indicating that co-culture with quiescent SMCs did not affect EC growth. Similar results were obtained by Ziegler et al. [17]. However, on proliferative SMCs EC number did not increase, suggesting that proliferative SMCs inhibited EC growth. When ECs were seeded at a superconfluent density of 100,000 cell/cm2, cell density decreased with time in co-culture on both proliferative SMCs and quiescent SMCs and monocultures until a density of approximately 60,000 cells/cm2, probably representing a stable confluent density for EC cultures (Fig. 5). It should be noted that although EC densities on the proliferative SMCs and quiescent SMCs were similar, ECs retracted on proliferative SMCs and formed patches with a coverage of 50%, as shown in Fig. 4.
Fig. 5.
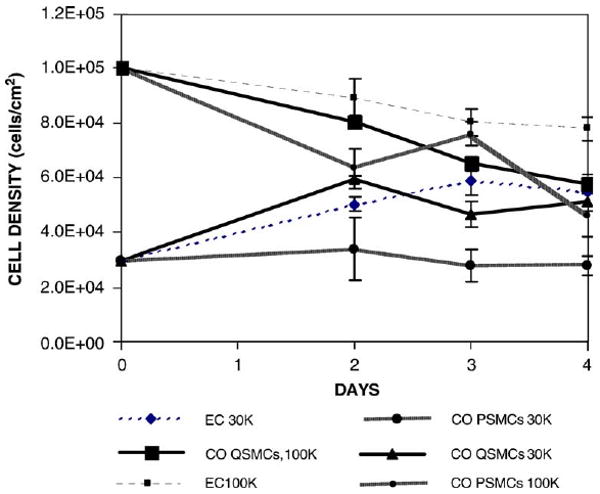
Growth of ECs on co-culture system vs. mono culture. ECs (passage 4) were seeded at densities of 30 × 103 or 100 × 103 cells/cm2 onto FN-coated SMCs (passage 6) construct or FN-coated 6-well-plate (polystyrene). ECs were labeled with DiI-Ac-LDL for visualization purpose. The number of cells on the area was counted manually to get cell density. 2-way ANOVA (treatment and time) was analyzed to determine the significant difference between groups. At seeding density of 30 × 103 cells/cm2, EC growth on proliferative SMCs are significantly different from EC cultured alone, whereas EC growth on quiescent SMCs showed no difference from EC cultured alone.
Based on these experiments, the optimal conditions for co-culture system are summarized in Table 1. To get a confluent EC monolayer on SMCs, it is critical to use quiescent SMCs. The final ratio of EC to SMC cell densities ranged between 1:1 and 1:2. These conditions were used in subsequent experiments.
Table 1. Summary of preferred conditions found for co-culture.
| Factor | Preferred condition |
|---|---|
| Substrate material | Polystyrene (tissue-culture treated) |
| SMC growth medium | DMEM with 10% FBS, 10% PS, and growth factors (20% total serum) |
| SMC quiescent medium | Serum-free DMEM/F12 with ITS supplement |
| EC growth medium | Same as SMC growth medium except with 5% FBS, 5% PS (10% total serum) |
| Co-culture medium | 1 part growth medium: 5 parts quiescent medium (3.3% total serum) |
| SMC seeding density | 25 × 103−50 × 103 cells/cm2 a |
| EC seeding density | 40 × 103−80 × 103 cells/cm2 a |
| SMC phenotype | Quiescent (contractile) |
| Basal adhesion protein | FN (5 μg/cm2) |
| Medial adhesion protein | No difference observed between collagen IV, FN, and no protein in terms of EC coverage and PECAM expression |
The range for the seeding density reflects the fact that size of EC and SMC from different source varies. SMCs are seeded at a subconfluent density, whereas ECs are seeded at a confluent density. The optimal seeding density has to be determined in each batch of cells.
3.6. ECs and SMCs form distinct layers in co-culture
To assess whether ECs and SMCs formed distinct layers, Z-sections of co-culture were obtained after two days of co-culture. ECs were labeled with DiI in red, and SMCs were labeled with CTG in green. After deconvolution of intensity profile (z-direction) of original images, the green and red intensity maxima are separated by a distance of 5 μm. The clear separation of these two curves indicates that neither migration nor growth of SMCs was detected above the EC layer, confirming the presence of two distinct layers of cells in the co-culture system. The maximum intensities for green and red fluorescences are normalized to unity to standardize the difference between these channels. (Fig. 6)
Fig. 6.
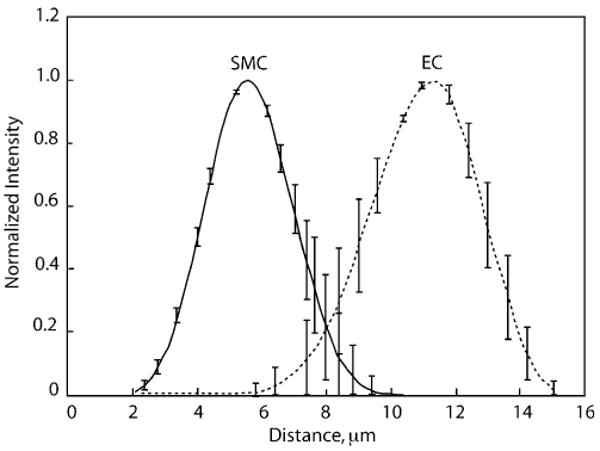
Distinct layer of ECs and SMCs on co-culture system. ECs (passage 2) and SMCs (passage 4) were labeled with DiI-Ac-LDL (red) and CTG (green), respectively. Z-section was performed using confocal microscopy. The original mean intensity vs. distance was deconvolved with experimental PSFs. Solid line is intensity of green fluorescence (SMCs), and dashed line is intensity of red fluorescence (ECs). Results are the average of two experiments. Four fields were examined in each experiment. Error bars represent SEM.
3.7. PECAM staining of ECs in co-culture
PECAM staining was used to verify the state of EC confluency. When ECs were cultured alone, PECAM stained the borders between cells. In subconfluent cultures, PECAM staining was absent from regions where ECs were not in contact with each other (not shown). ECs co-cultured with SMCs showed a decrease in PECAM staining relative to EC culture alone (Fig. 7A and B). In addition, ECs cultured alone displayed a typical polygonal morphology, whereas ECs in co-cultures showed an elongated morphology. Quantitative analysis of images showed that with FN and collagen IV, the decrease was 32% and 28% (n = 3), confirming that the reduction in PECAM staining was due to EC–SMC interactions, not due to specific medial adhesion molecules. Formation of mature cell junction may take days to complete, and the reduction of PECAM staining might be due to insufficient time to form junctions between cells. To test this, we stained co-culture system at a longer period (days 4 and 10) using FN in the medial layer. The result is shown in Fig. 7C. PECAM staining increased at day 4 post-EC seeding and then decreased slightly on day 10. The decline in PECAM intensity between days 4 and 10 was significant (p<0:05), but the difference in intensity between days 2 and 4 was not significant nor was the difference in intensity between days 2 and 10.
Fig. 7.
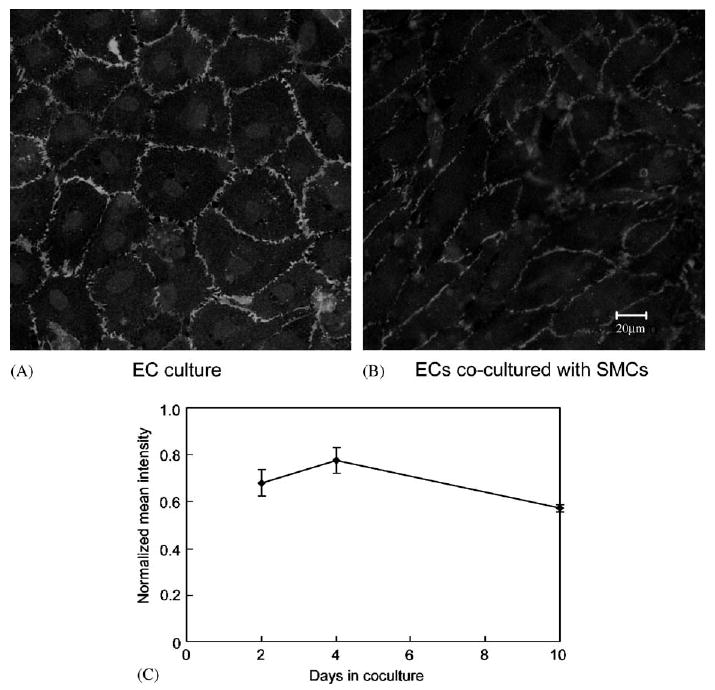
PECAM staining for ECs. Two days post-EC seeding, ECs were immunostained with anti-PECAM which was labeled with Alexa 488, and scanned using confocal microscopy. FN was used for the co-culture. (A)PECAM staining for EC culture alone (passage 5); (B) PECAM staining for ECs co-cultured with SMCs (passage 3). (C) Effect of days on PECAM staining. Intensity values were normalized to the value of PECAM staining in EC monoculture.
3.8. Increased endothelial cell DiI-Ac-LDL uptake in co-culture
ECs in co-culture accumulated more DiI-Ac-LDL than in monoculture. The average fluorescent intensity per cell when ECs were in co-culture was greater than that when ECs were cultured alone on polystyrene. ECs on proliferating SMCs showed the highest fluorescent intensity (74 in the scale from 0 to 255), followed by ECs on quiescent SMCs (45), and ECs cultured alone (30) (Fig. 8). All differences were significant (p<0:05). Various adhesion proteins had no effect on the uptake of DiI-Ac-LDL in the co-culture system. Table 2 shows that there is a 1.7–2.2 fold increase in fluorescent intensity using no protein, collagen I, collagen IV, FN, or LN as medial proteins, indicating that the increased DiI-Ac-LDL uptake is due to EC–SMC interactions, not due to adhesion protein. SMCs alone did not accumulate noticeable levels of DiI-Ac-LDL.
Fig. 8.
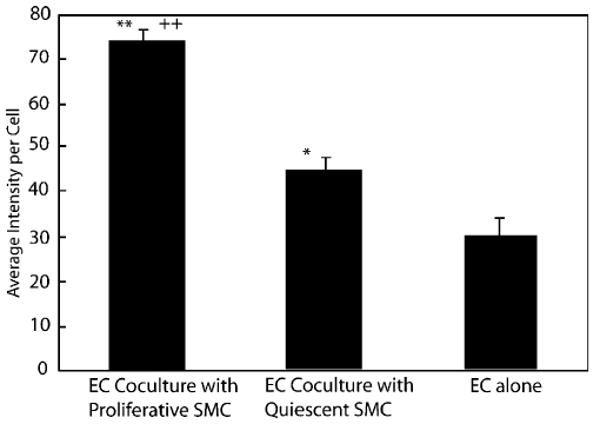
Effect of SMC phenotype on EC DiI-Ac-LDL uptake. ECs (passage 4) were seeded on proliferating SMCs (passage 6, SMCs cultured in PM), quiescent SMCs (passage 6, SMCs cultured in QM), or 6-well-plate. Two days post-EC seeding, ECs were stained with DiI-Ac-LDL and observed with fluorescent microscopy using standard rhodamine filter. The *p<0.01 and **p<0.001 relative to EC monoculture control. ++p<0.001 relative to quiescent SMCs.
Table 2. Effect of medial layer adhesion proteins on DiI-Ac-LDL uptake.
| Adhesion protein | None | Collagen I | Collagen IV | Fibronectin | Laminin |
|---|---|---|---|---|---|
| Normalized DiI-Ac-LDL uptake | 1.72±0.29 | 1.74±0.10 | 1.7±0.28 | 1.69±0.28 | 2.23±0.48 |
All the values are normalized with respect to DiI-Ac-LDL uptake by EC monoculture. DiI-Ac-LDL is greater in co-culture than monoculture but there is no significant difference produced by adhesion proteins.
3.9. EC adherence to SMCs after exposure to laminar flow
Co-cultures of porcine SMCs and ECs were created and exposed to 5 dyne/cm2 for 7.5 h in a parallel plate flow channel. Cell counts indicate a loss of 1.8±0.2% ECs after 7.5 h of flow (n = 2), which was not different than cell loss for ECs cultured on polystyrene.
When ECs cultured alone on FN-coated polystyrene were exposed to 5 dyne/cm2 for 7.5 h, the cells changed shape, as expected, but remained confluent and did not contract. In contrast, SMCs contracted when exposed to flow. This occurred both without and with ECs. SMC contraction also produced reorganization of ECs. In co-culture, SMC retracted a total of 34±6% whereas ECs retracted 44±7% after the 7.5 h of flow.
4. Discussion
We have characterized a system in which ECs are directly cultured on quiescent SMCs. While several reports of direct EC–SMC co-culture are in the literature, this study represents the first systematic examination of conditions necessary to maintain a confluent layer of ECs for as long as 10 days. The main finding of this paper is that ECs attached better on quiescent SMCs than on proliferating SMCs. Overgrowth of SMCs on ECs was not observed. SMC and EC proliferation rate were low. PECAM staining indicated that cell–cell contacts formed between ECs.
Several other systems of direct co-culture of ECs on SMCs are reported in the literature [13,19,21,26]. All appeared to use proliferating SMCs. Niwa et al. [21] developed a co-culture system in which bovine SMCs were plated at 50 × 103 cells/cm2 and allowed to proliferate for 4–12 days, at which time ECs were seeded at the same density. However, when we applied the same technique to porcine cells, there was cellular contraction within 24 h of EC seeding. Korff et al. [19] cultured ECs on SMC spheroids, but contraction was not examined. L'Heureux et al. [26] attached ECs to SMCs grown for eight weeks in a cylindrical geometry. Any contraction that occurred might have been uniform and would not have affected EC function. Proliferative SMC are known to produce more extracellular matrix components such as collagen, elastin, and proteoglycans [35–37]. Despite these additional proteins, porcine ECs bound better to porcine QSMC. The use of quiescent SMCs is more representative of the normal physiological state.
Based on reports in the literature, a possible mechanism for the differential effect of endothelium on proliferating and quiescent SMCs involves the activation of transforming growth factor-β (TGF-β) and endothelin release. In vivo, TGF-β production by quiescent SMCs is low [38]. In vitro, SMCs and ECs cultured alone produce TGF-β in an inactive form [39]. However, addition of EC conditioned medium to, or ECs co-cultured with SMCs results in activation of TGF-β through the action of plasmin produced by urokinase on the EC surface (reviewed in Sporn et al. [40]). TGF-β has been reported to be a potent inhibitor of proliferation (reviewed by Schusher and Krieglstein [41]) as well as an inducer of apoptosis (reviewed by [42]) in many cells in vitro including ECs. When ECs were seeded on proliferating SMCs, we also observed a significant decrease in the number of ECs over time, whereas ECs proliferated on quiescent SMCs as shown in Fig. 5. In direct culture, higher mRNA levels of TGF-β were found in human SMCs but no change in TGF-β levels was observed in ECs [43]. SMC conditioned medium produced a decrease in ECs TGF-β mRNA. Finally, TGF-β stimulates ET-1 release by SMCs which could induce SMC contraction [44].
Since ECs require serum and QSMC do not, an appropriate medium composition was identified. A 3.3% total serum concentration was sufficient to encourage EC attachment and binding yet not allow the high density SMCs to proliferate. Although confluent ECs in culture release a heparin-like inhibitor of SMC growth [45], the high density SMC culture was sufficient to inhibit SMC proliferation, even when FN was present. Low density SMCs bind to FN via the α5β1 integrin receptor which can initiate cell proliferation when the proper growth factors are present [46]. We did not observe an effect of FN on high density SMC culture proliferation. In low density cultures, however, we did observe that SMC spreading and growth better with FN.
In the current study we have made initial observations of the effect of co-culture on EC function. We found that direct culture of SMCs with ECs reduced PECAM localization to EC–EC cell junctions. PECAM was used as a marker for the junctions and is not necessarily an indicator of junctional integrity. Using electron microscopy, Korff et al. [19] found an increase in tight junctions in ECs co-cultured with SMCs versus ECs cultured alone.
Co-culture increased EC uptake of DiI-Ac-LDL and DiI-Ac-LDL uptake was greatest when ECs were cultured with PSMC. The increased DiI uptake with PSMC compared to that with QSMC was likely due to the artifact that EC retracted on PSMCs. The area of EC on QSMC was estimated to be 1244±78 μm2 based on fluorescent images, and was reduced to 889±131 μm2 on PSMCs. This reduction in area alone might account for 40% increase in DiI uptake. The increase in DiI fluoresence seen in co-culture versus monolayer indicates that the scavenger receptor on the ECs is more active. In addition, we observed that SMCs in co-culture accumulated more DiI when compared to SMCs culture alone, as shown by the increase in the brightness of the background in the fluorescent images. Therefore the increased DiI uptake by ECs might represent the extra burden for those cells to provide DiI-Ac-LDL to SMCs. The exact mechanism for increased DiI uptake in ECs and SMCs in the co-culture system is unknown. However, this increased activity does not appear to be modulated significantly by different medial adhesion proteins. Niwa et al. [21] observed a slight increase in DiI-Ac-LDL uptake by ECs after 6 days of co-culture and DiI-Ac-LDL uptake by ECs appeared to be sensitive to the length of time that the PSMC were cultured prior to attachment of ECs.
Under flow conditions, ECs remain attached to the SMCs after 7.5 h of flow and cell loss was minimal and comparable to levels observed for ECs alone. This is consistent with our observation that ECs could not be readily detached from SMCs without using 10 × the normal level of trypsin and increasing the period of incubation.
As observed previously [47], subconfluent rat QSMCs exposed to flow underwent contraction. This contraction was independent of intracellular calcium but did involve the Rho kinase pathway. We found that confluent QSMCs also underwent contraction following exposure to flow. ECs cultured on SMCs and exposed to flow, reorganized as a result of SMC contraction. EC movement was greater than when ECs alone were exposed to flow. The movement of the SMCs suggests that some flow may occur between junctions and that the EC response to flow induces a response by SMCs.
Surprisingly, the addition of exogenous medial proteins (FN, LN, collagen I, and IV) had no effect on EC coverage, PECAM staining, or DiI-Ac-LDL uptake. It is likely that SMCs have produced their own extracellular matrix, therefore, the addition of exogenous adhesion protein had minimal effect on EC and SMC function. However, these proteins may affect other EC functions, such as EC pro-coagulant properties, as observed in vitro [8]. Future studies will explore the interaction among ECs, SMCs and extracellular matrix as well as molecular mechanisms. The understanding of this co-culture model will be helpful for the development of TEVG.
We have not systemically examined the effect of cell passage on adhesion. However, we observed that up to passage 5, ECs attached firmly to SMCs, as indicated by the fact that it was very difficult to detach ECs from SMCs using normal level of Trypsin/EDTA. In addition, we did not observe any significant effect of passage number on EC function (PECAM staining, cell proliferation, DiI-Ac-LDL uptake). We speculate that the effect of cell passage might be overshadowed by the interactions between ECs and SMCs. For cells of passage higher than 6, we did observe reduced adhesion of EC to QSMC. Therefore, Experiments were restricted to cells in passages 2–5. The identical passage numbers for ECs and SMCs are desirable, however, it is extremely difficult to synchronize the growth of these cells.
5. Conclusion
We have established a co-culture model using EC seeding directly on quiescent SMCs, which reproduces the close physical proximity of ECs and SMCs observed in vivo and maintains SMCs in a quiescent state. The preliminary results demonstrate that such co-culture can alter EC function. The successful development of this co-culture model is of great importance in understanding EC/ SMC interaction in the TEVGs.
Acknowledgments
The authors are indebted to Amy Solan from Department of Biomedical Engineering, Duke University, for providing cells and technical assistance in Western blotting, Jeffery LaMack from Department of Biomedical Engineering, Duke University, for technical assistance in PECAM immunocytochemistry, Dr. Timothy Oliver from Department of Molecular Biology, Duke University Medical Center, for sharing of expertise in confocal microscopy. This work is supported by NIH grant R21HL 72189.
References
- 1.Mitchell SL, Niklason LE. Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 2.Remy M, Bareille R, Villars F, Rouais F, Gorodkov A, Bordenave L. Endothelial cells lining polyester fabric express pro-coagulant phenotype in vitro. Med Biol Eng Comput. 1998;36:256–7. doi: 10.1007/BF02510755. [DOI] [PubMed] [Google Scholar]
- 3.Dilley RJ, McGeachie JK, Prendergast FJ. A review of the proliferative behaviour, morphology and phenotypes of vascular smooth muscle. Atherosclerosis. 1987;63:99–107. doi: 10.1016/0021-9150(87)90109-2. [DOI] [PubMed] [Google Scholar]
- 4.Thyberg J, Hedin U, Sjolund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–90. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GR, Campbell JH. Development of the vessel wall. In: Schwartz SM, Mecham RP, editors. The vascular smooth muscle cell. San Diego: Academic Press; 1995. pp. 3–15. [Google Scholar]
- 6.Libby P, O'Brien KV. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983;115:217–23. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Sims S, Jiao Y, Chow LH, Pickering JG. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338–48. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- 8.Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239–46. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JH, Campbell GR. Endothelial cell influences on vascular smooth muscle phenotype. Annu Rev Physiol. 1986;48:295–306. doi: 10.1146/annurev.ph.48.030186.001455. [DOI] [PubMed] [Google Scholar]
- 10.Herman IM, Castellot JJ., Jr Regulation of vascular smooth muscle cell growth by endothelial-synthesized extracellular matrices. Arteriosclerosis. 1987;7:463–9. doi: 10.1161/01.atv.7.5.463. [DOI] [PubMed] [Google Scholar]
- 11.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667–74. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 12.Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech. 2004;37:531–9. doi: 10.1016/j.jbiomech.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Rainger GE, Stone P, Morland CM, Nash GB. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J Immunol Methods. 2001;255:73–82. doi: 10.1016/s0022-1759(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 14.Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res. 1997;67:169–78. doi: 10.1006/jsre.1996.4978. [DOI] [PubMed] [Google Scholar]
- 15.Nackman GB, Bech FR, Fillinger MF, Wagner RJ, Cronenwett JL. Endothelial cells modulate smooth muscle cell morphology by inhibition of transforming growth factor-beta 1 activation. Surgery. 1996;120:418–25. doi: 10.1016/s0039-6060(96)80318-7. discussion 425–16. [DOI] [PubMed] [Google Scholar]
- 16.van Buul-Wortelboer MF, Brinkman HJ, Dingemans KP, de Groot PG, van Aken WG, van Mourik JA. Reconstitution of the vascular wall in vitro. A novel model to study interactions between endothelial and smooth muscle cells. Exp Cell Res. 1986;162:151–8. doi: 10.1016/0014-4827(86)90433-7. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler T, Alexander RW, Nerem RM. An endothelial cell–smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann Biomed Eng. 1995;23:216–25. doi: 10.1007/BF02584424. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF, Truskey GA, Warren HB, O'Connor SE, Eisenhaure BH. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985;101:871–9. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–57. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JC, Ruan Q, Paucz L, Fabry A, Binder BR, Wojta J. Stimulation of tissue factor expression in human microvascular and macrovascular endothelial cells by cultured vascular smooth muscle cells in vitro. J Vasc Res. 1999;36:126–32. doi: 10.1159/000025635. [DOI] [PubMed] [Google Scholar]
- 21.Niwa K, Kado T, Sakai J, Karino T. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC–SMC coculture. Ann Biomed Eng. 2004;32:537–43. doi: 10.1023/b:abme.0000019173.79939.54. [DOI] [PubMed] [Google Scholar]
- 22.Wada Y, Sugiyama A, Kohro T, Kobayashi M, Takeya M, Naito M, Kodama T. In vitro model of atherosclerosis using coculture of arterial wall cells and macrophage. Yonsei Med J. 2000;41:740–55. doi: 10.3349/ymj.2000.41.6.740. [DOI] [PubMed] [Google Scholar]
- 23.Heydarkhan-Hagvall S, Helenius G, Johansson BR, Li JY, Mattsson E, Risberg B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem. 2003;89:1250–9. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 24.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–86. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705–1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 26.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Higgins SP, Solan AK, Niklason LE. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res. 2003;67A:295–302. doi: 10.1002/jbm.a.10599. [DOI] [PubMed] [Google Scholar]
- 28.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 29.Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- 30.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–40. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truskey GA, Proulx TL. Quantitation of cell area on glass and fibronectin-coated surfaces by digital image analysis. Biotechnol Prog. 1990;6:513–9. doi: 10.1021/bp00006a016. [DOI] [PubMed] [Google Scholar]
- 32.Biggs DSC, Andrews M. Acceleration of iterative image restoration algorithms. Appl Opt. 1997;36:36. doi: 10.1364/ao.36.001766. [DOI] [PubMed] [Google Scholar]
- 33.Olivier LA, Yen J, Reichert WM, Truskey GA. Short-term cell/substrate contact dynamics of subconfluent endothelial cells following exposure to laminar flow. Biotechnol Progr. 1999;15:33–42. doi: 10.1021/bp980107e. [DOI] [PubMed] [Google Scholar]
- 34.Hao H, Ropraz P, Verin V, Camenzind E, Geinoz A, Pepper MS, Gabbiani G, Bochaton-Piallat ML. Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arterioscler Thromb Vasc Biol. 2002;22:1093–9. doi: 10.1161/01.atv.0000022407.91111.e4. [DOI] [PubMed] [Google Scholar]
- 35.Merrilees MJ, Campbell JH, Spanidis E, Campbell GR. Glycosaminoglycan synthesis by smooth muscle cells of differing phenotype and their response to endothelial cell conditioned medium. Atherosclerosis. 1990;81:245–54. doi: 10.1016/0021-9150(90)90072-q. [DOI] [PubMed] [Google Scholar]
- 36.Ang AH, Tachas G, Campbell JH, Bateman JF, Campbell GR. Collagen synthesis by cultured rabbit aortic smooth-muscle cells. Alteration with phenotype. Biochem J. 1990;265:461–9. doi: 10.1042/bj2650461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjolund M, Madsen K, von der Mark K, Thyberg J. Phenotype modulation in primary cultures of smooth-muscle cells from rat aorta. Synthesis of collagen and elastin. Differentiation. 1986;32:173–80. doi: 10.1111/j.1432-0436.1986.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 38.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991;88:904–10. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–15. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–7. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- 41.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039–45. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Okada F, Abe M, Seguchi T, Kuwano M, Sato S, Furuya A, Hanai N, Tamaoki T. The mechanism for the activation of latent TGF-beta during co-culture of endothelial cells and smooth muscle cells: cell-type specific targeting of latent TGF-beta to smooth muscle cells. J Cell Biol. 1993;123:1249–54. doi: 10.1083/jcb.123.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Luozzo G, Bhargava J, Powell RJ. Vascular smooth muscle cell effect on endothelial cell endothelin-1 production. J Vasc Surg. 2000;31:781–9. doi: 10.1067/mva.2000.103788. [DOI] [PubMed] [Google Scholar]
- 45.Castellot JJ, Jr, Addonizio ML, Rosenberg R, Karnovsky MJ. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981;90:372–9. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 47.Civelek M, Ainslie K, Garanich JS, Tarbell JM. Smooth muscle cells contract in response to fluid flow via a Ca2+-independent signaling mechanism. J Appl Physiol. 2002;93:1907–17. doi: 10.1152/japplphysiol.00988.2001. [DOI] [PubMed] [Google Scholar]


