Abstract
Early disruption of steroids affects the development of mammalian neural circuits underlying affective processes. In humans, patients with classic Congenital Adrenal Hyperplasia (CAH) can serve as a natural model to study early hormonal alterations on functional brain development. CAH is characterized by congenital glucocorticoid insufficiency, leading to altered hypothalamic-pituitary-adrenal (HPA) function, and hyperandrogenism. Using fMRI, we compared fourteen adolescents with CAH to 14 healthy controls on amygdala response to a face viewing task. In response to negative facial emotions, CAH females activated the amygdala significantly more than healthy females, whereas CAH males did not differ from control males. Furthermore, females with CAH showed a similar pattern of amygdala activation to control males, suggesting virilized amygdala function in females with CAH. These findings suggest a prominent effect of early hyperandrogenism on the development and function of the amygdala in females with CAH, whereas no effects were detected in males with CAH. This study provides data that can be further tested in a model of the neurobiological mechanisms underlying early androgen organizational effect on amygdala function.
Keywords: development, corticosteroid, androgen, affective processing, fMRI, stress hormones
Steroids have been associated with behavioral deficits, corticosteroids with mood and anxiety (Charney 2004; de Kloet, Joels, & Holsboer 2005), and androgens with aggression and social dominance (Rowe, Maughan, Worthman, Costello, & Angold 2004; Zitzmann & Nieschlag 2001). The precise neural mechanisms underlying these associations remain unclear. Effects of steroids on brain and behavior could involve organizational neural mechanisms, i.e., permanent changes in the structure or function of the brain following steroid exposure in utero or during critical periods of development. These effects have been delineated in animal models (see review Negri-Cesi, Colciago, Celotti, & Motta 2004), but human studies in children are lacking.
The examination of brain function in patients with classic (severe) congenital adrenal hyperplasia (CAH) provides a natural model for examining such influences. Classic CAH is an autosomal recessive genetic disorder with a prevalence rate estimated at one in 15,000 live births (Merke & Bornstein 2005). It is characterized by impaired cortisol biosynthesis, with severe androgen excess. The deficit of adrenal cortisol, by removing its negative feedback, stimulates the secretion of pituitary adrenocorticotropic hormone (ACTH) and hypothalamic corticotropin-releasing hormone (CRH). Overstimulation of the adrenals by ACTH results in excessive androgen production.
Levels of testosterone in utero in the female fetus affected by classic CAH are comparable to that of an unaffected healthy male fetus. Because of the exposure to high levels of testosterone in utero, females with classic CAH are born with ambiguous genitalia. Although rare, classic CAH is the most common cause of ambiguous genitalia in the newborn, and, in some cases, females can be so virilized at birth that they are incorrectly thought to be healthy males. Because these abnormalities occur in utero, they may influence brain development and organization. Behavioral studies suggest that females with CAH tend to manifest male-like behaviors in their play and interests (Berenbaum 1999; Berenbaum & Resnick 1997; Hall, Jones, Meyer-Bahlburg, Dolezal, Coleman, Foster, Price, & Clayton 2004; Meyer-Bahlburg 2001), women with CAH report themselves as being more aggressive than their healthy counterparts (Berenbaum, Duck, & Bryk 2000; Cohen-Bendahan, van de, & Berenbaum 2005), supporting the notion that prenatal androgens may masculinize the brain.
To our knowledge there are no data available on social function in males. However, some studies suggest that those patients with the most severe form of CAH and who have experienced salt-wasting adrenal crises with abnormal electrolytes as neonates are at risk for cognitive impairment (reported by Johannsen 2006 in a sample of 35 women with CAH, and no males). But this affects both males and females similarly. In addition, surprisingly given this suggestion, an IQ advantage has been reported in a number of studies of CAH (discussed by Nass and Baker 1991), possibly due to socioeconomic, genetic, or hormonal factors. Overall, studies of cognitive function have reported inconsistent results.
The amygdala plays a central role in processing emotion, and androgens have been proposed to influence memory and emotional learning (Naghdi & Asadollahi 2004) through their action on limbic function (Vazquez-Pereyra, Rivas-Arancibia, Loaeza-Del Castillo, & Schneider-Rivas 1995) In an initial study using structural MRI, we reported reduced amygdala size in adolescents with CAH compared to controls (Merke, Fields, Keil, Vaituzis, Chrousos, & Giedd 2003). Reduced amygdala size was found in both males and females. This finding suggested that this structural alteration was not the sole consequence of the female-selective testosterone disturbance, which would have affected primarily females, but might have resulted from corticosteroid perturbations, which is of similar severity in both sexes. However, the absence of significant sex-related differences in amygdala structural alterations does not preclude the presence of functional differences. The present study extends this work by comparing amygdala function between adolescents with CAH and matched controls on response to social threat-related stimuli (evocative faces). To this purpose, we conducted a functional magnetic resonance imaging (fMRI) study paired with a well-validated face-viewing paradigm that reliably engages amygdala function (McClure, Monk, Nelson, Zarahn, Leibenluft, Bilder, Charney, Ernst, & Pine 2004; Monk, McClure, Nelson, Zarahn, Bilder, Leibenluft, Charney, Ernst, & Pine 2003; Nelson, McClure, Monk, Zarahn, Leibenluft, Pine, & Ernst 2003).
We predicted that CAH patients would have altered amygdala function in response to negative stimuli (angry and fearful facial expressions). In addition, because of the different involvement of sex steroid abnormalities with respect to gender, we also examined males and females separately to evaluate possible androgen-specific effects which would affect females more than males. Previous neuropsychological studies have focused on females with CAH as a model of the effects of excess androgen on the brain in females because of their sex-atypical exposure to androgens prenatally,. This is the reason why it is important to study gender specific effects. With regard to males, there is very little data on cognitive and behavioral effects. Levels of androgen in males with CAH are similar to those in healthy males. However, males with CAH are exposed to excessive adrenocorticotropic hormone (ACTH) and CRH, and diminished cortisol (similarly to females with CAH). Thus, findings suggesting differences in brain function between males with CAH and healthy males would be more likely to reflect abnormalities in corticosteroids than sex steroids.
MATERIALS AND METHODS
Sample
Participants were recruited through fliers and newspaper advertisements inviting healthy youth and adults to participate in studies of emotion at the National Institute of Mental Health (NIMH). Patients with CAH being followed at the NIH Clinical Center in Bethesda, Maryland enrolled in endocrine studies and those aged 9 to 18 years were invited to participate. The study was approved by the institutional review boards at the National Institute of Mental Health and the National Institute of Child Health and Human Development. Each parent gave written informed consent, and children gave written assent. Eight patients with CAH (4 females) participated in our previous structural MRI study (Merke et al., 2003).
Twenty-eight adolescents completed the study. The sample included 14 patients with classic CAH due to 21-hydroxylase deficiency (7 female, mean ± SD: age 13.5 ± 2.10, bone age 13.9 ± 2.4, IQ 103.7 ± 13.4) and 14 controls (7 female, mean ± SD: age 13.6 ± 2.53, IQ 110.7 ± 14.4). All subjects underwent physical, neurological and psychiatric (Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson, & Ryan 1997) examinations. Full scale IQ scores were prorated based on the Vocabulary and Block Design subtests of the Wechsler Intelligence Scales for Children. Age and IQ did not differ between groups (age: t(26)= 0.27, p=0.8; IQ: t(26)= 1.25, p=0.2). Demographic and clinical characteristics for both groups are presented in Table 1.
Table 1.
Demographic and clinical characteristics in patients with CAH and healthy control children.
| CAH patients (n=14) | Healthy children (n=14) | p | |
|---|---|---|---|
| Age (yr) | |||
| Group: | 13.9 ± 2.1 | 13.6 ± 2.5 | 0.787 |
| Boys: | 15.0 ± 1.1 | 14.9 ± 1.2 | 0.776 |
| Girls: | 12.7 ± 2.2 | 12.4 ± 3 | 0.836 |
| Bone Age (yr) | |||
| Group: | 14.1 ± 2.7 | ||
| Boys: | 16.0 ± 1.7 | ||
| Girls: | 12.2 ± 1.9 | ||
| Height (inches) | |||
| Group: | 62.4 ± 4.3 | 62.6 ± 7.2 | 0.930 |
| Boys: | 65.3 ± 2.6 | 66.8 ± 4.2 | 0.431 |
| Girls: | 59.4 ± 3.7 | 58.3 ± 7.2 | 0.716 |
| Weight (lbs) | |||
| Group: | 130.8 ± 45.5 | 118.5 ± 37.9 | 0.442 |
| Boys: | 151.1 ± 30.4 | 141.8 ± 35.4 | 0.608 |
| Girls: | 110.5 ± 51 | 95.1 ± 24.3 | 0.489 |
| BMI (kg/m2) | |||
| Group: | 23.4 ± 7.6 | 20.7 ± 3.3 | 0.242 |
| Boys: | 25.1 ± 5.9 | 22 ± 3.8 | 0.261 |
| Girls: | 21.7 ± 9.2 | 19.4 ± 2.4 | 0.552 |
| BMI SDS2 | |||
| Group: | 0.695 ± 1.2 | 0.159 ± 1.09 | 0.249 |
| Boys: | 1.01 ± 1.14 | 0.32 ± 1.29 | 0.312 |
| Girls: | 0.38 ± 1.38 | −0.03 ± 0.867 | 0.542 |
| Tanner stage | |||
| Group: | 3.2 ± 1.3 | 3.0 ± 1.5 (n=13)1 | 0.963 |
| Boys: | 3.4 ± 1.7 | 4.0± 1.1 | 0.485 |
| Girls: | 3.0 ± 0.8 | 2.1 ± 1.2 | 0.147 |
| Wechsler IQ | |||
| Group: | 103.8 ± 13.4 (n=12)1 | 110.6 ± 14.4 | 0.222 |
| Boys: | 107.7 ± 14.3 | 111.7 ± 10.2 | 0.559 |
| Girls: | 98.2 ± 11.1 | 109.6 ± 18.5 | 0.252 |
All values are mean ± SD. Reduced n indicate missing data.
Reduced n because of data lost.
BMI standard-deviation score (SDS) was determined using anthropometric reference data for US children (Center for Disease Control. Epi Info™, version 3.3.2. 2000)
One girl with CAH had been treated in utero with dexamethasone. However, she showed signs of virilization at birth, which necessitated corrective surgery, and suggested that the in-utero treatment had been insufficient to protect her against early exposure to excess androgens. Twelve of the 14 patients were salt wasters by genotype, but only two had experienced a salt-wasting adrenal crisis in the neonatal period. All females were diagnosed at birth, and males within the first year of age, except for one boy who was diagnosed at 4 years (males, mean ± SD age at diagnosis: 0.64 ± 1.7 years). All patients were treated with hydrocortisone (mean ± SD dose: 12.1 ± 5.3 mg/m2·d) and fludrocortisone 120 ± 48 mcg/day) and had a normal 24-hour urinary free cortisol measurement within 1 to 2 days of the testing (42.8 ± 5.1 μg/m2 ·24 hr; normal < 68). Testosterone was within the normal range for age, sex and pubertal stage for all but one patient (10.5 yo female: 171 ng/dl; normal < 74 ng/dl, Mayo Laboratories). Because she did not have clinical signs of virilization and none of the findings were altered by the removal of her data, she was retained in the final analysis.
Face viewing paradigm
Stimuli were selected from three standardized sets of gray-scale photographs depicting different facial expressions constructed by Ekman and Friesen (1976), Gur (www.uphs.upenn.edu/bbl/pubs/downloads/nptasks.shtml), and Tottenham and Nelson (www.macbrain.org/faces/index.htm). The photographs comprised 32 adult actors selected randomly from a larger pool of 56 actors. The actors displayed three emotional expressions (happy, angry, and fear) and a neutral expression. Thus, eight different actors were viewed for each expression (8 × 4 = 32). Facial expressions for a given actor varied randomly across participants so that each participant viewed different actors displaying different expressions to control for variability in non-emotional aspects of the actors (e.g., ethnicity, hair texture). For example, a given actor might be randomly selected to portray “anger” across the four viewings for one subject, whereas this same actor might be randomly chosen to portray “fear” for another subject and “happiness” for yet another subject. Female actors were used in half of the photographs to control for stimulus gender.
While in the scanner, participants viewed the series of 32 adult faces (8 happy, 8 angry, 8 fearful, 8 neutral) under four conditions. Three conditions required participants to attend to different aspects of the face stimuli. Using a five-key response box, participants rated the faces from 1 (not very) to 5 (extremely) according to their subjective fear (“How afraid are you?”), perceived hostility (“How hostile is the face?”), and a non-emotional physical feature (“How wide is the nose?”). In the fourth condition, participants’ attention was unconstrained, such that participants viewed the faces passively without making any ratings. Because we were interested in the reactivity of the threat system (amygdala) to the exposure to negative stimuli across attention conditions, we pooled the four attention conditions together and compared neural responses to negative expressions (angry and fearful) with responses to neutral expressions. This strategy also increased the power of this study to detect group differences based on the larger number of stimuli. For baseline comparison, 32 trials of fixation crosses were presented randomly within each condition.
The four conditions were presented as a 160-trial (8 actors × 5 stimuli –4 emotions and fixation– × 4 conditions) 14-minute single run. Each facial expression was presented a total of four times, once during each of the four conditions. Trials within a given condition were blocked together, and presentation order of condition and facial expressions was randomized across participants. Rating instructions appeared for 3 s before each condition block. Facial expression and fixation trials were shown for 4 s each. Each face and fixation trial was followed by an inter-trial interval showing a blank screen that varied randomly from 750–1250 ms.
Stimuli were displayed using Avotec Silent Vision Glasses (Stuart, FL) and responses were recorded by a five-key button box developed by MRI Devices (Waukesha, WI). Participants were trained in an MRI simulator prior to entering the scanner to become familiar with the actual MRI environment and response device. Participants were also administered a practice version of the task to ensure understanding of the task. The practice version contained pictures of only neutral facial expressions that were not shown in the MRI scanner.
fMRI data acquisition
Whole-brain blood oxygen level dependent (BOLD) functional MRI data were acquired on a General Electric Signa 3-Tesla magnet (Waukesha, WI). Following sagittal localization and a manual shim procedure, functional T2*-weighted images were gathered using an echo-planar single-shot gradient echo pulse sequence with a matrix size of 64 × 64 mm, repetition time (TR) of 2000 ms, echo time (TE) of 40 ms, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 5 mm, providing whole brain coverage. Echo-planar images (EPI) were acquired in 23 contiguous 5-mm axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC-PC) line. All functional data were obtained in a single, 14-minute run per subject. Following EPI data collection, a high-resolution T1-weighted anatomical image was acquired for each subject using a standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV = 256, number of excitations [NEX] = 1, TR = 11.4 ms, TE = 4.4 ms, matrix size of 256 × 256, time to inversion [TI] = 300 ms, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels) to facilitate spatial normalization.
STATISTICAL ANALYSIS
Sample characteristics analysis
Sample characteristics were compared between CAH and healthy groups using two- sample two-sided Student’s t test for continuous measures or chi-square test for nominal measures.
Performance task analysis
Two three-way ANOVAs were used with Diagnosis and Sex as the between-subjects factor and Emotion (negative, neutral) as the within-subjects factor. One of these analyses assessed the factors of Diagnosis, Sex and Emotion on the ratings of aversiveness made by the subjects. The other analysis assessed the reaction time to make these ratings. The Emotion factor included two levels, negative and neutral. The negative Emotion variables comprised scores related to angry and afraid. In addition, the ratings for ‘how hostile the face is’ and ‘how afraid does the face make you feel’ were pooled together as they were highly correlated (r > 0.5, P<0.005), and represented an index of how aversive the stimuli were. The ratings were made on a 5-point likert scale (1: not very; 5: extremely).
FMRI data analysis
Preprocessing data procedures and fMRI data analyses were performed using the Statistical Parametric Mapping (SPM99b, Wellcome Department of Cognitive Neurology, London, England, 1999) software and supplemental routines written in Matlab 5.3 (The Mathworks Inc., Natick, MA, 1999). Imaging data were included for participants who moved less than 2.0 mm in any plane as assessed with MedX software (Medical Numerics, Sterling, VA). Preprocessing procedures included corrections for slice timing and motion, co-registration to the anatomical data, and spatial normalization to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99b. Correction for scanner drift over the whole scanning session was not performed to prevent the filtering out task-related effects. This decision to not perform filtering may increase the possibility of false-negative findings, but it is unlikely to increase the possibility of false-positive findings..
Individual subject level event-related response amplitudes were estimated using a General Linear Model (GLM) for each event type. Two event-types were specified based on (1) angry & fearful and (2) neutral emotions. Fixation trials served as an implicit baseline. The waveform used to model event-related responses was a rectangular pulse (4 s duration) convolved with the hemodynamic response function specified in SPM99. The contrast of interest consisted in the comparison of BOLD signal changes during all events of exposure to negative facial expressions (angry & fearful) vs. BOLD signal changes during all events of exposure to neutral facial expressions. This contrast was selected to specifically examine regional neural responses to exposure to negative facial emotions, independently of attention state.
Contrast images were created for each subject using pair-wise comparisons of the event-related BOLD response amplitudes across conditions. Before performing group-level analyses, each contrast image was divided by the subject-specific voxel time series mean, generating values proportional to percentage fMRI signal change (Zarahn, Aguirre, & D’Esposito 1997). These normalized contrast images were then smoothed with an isotropic Gaussian kernel (FWHM = 11.4) to reduce non-stationarity in the spatial autocorrelation structure produced by the previous step (Friston, Mechelli, Turner, & Price 2000).
To examine the neural effects of diagnosis and diagnosis-by-sex interaction, the contrast images produced from each participant were fit to second-level random effects analyses of diagnosis (CAH, controls) and sex. Based on the question targeted to the function of the amygdala, we conducted a region of interest analysis centered on this structure. Left and right amygdalae were ascertained from standard anatomical criteria on a single MNI template and applied to all normalized brains at the group level (Szeszko, Robinson, Alvir, Bilder, Lencz, Ashtari, Wu, & Bogerts 1999; Szeszko, Strous, Goldman, Ashtari, Knuth, Lieberman, & Bilder 2002). Manual tracing methods were used to delineate these regions of interest (ROIs), as traced on the MNI template brain and then applied to the normalized brains, at the group level.. The boundaries of the amygdala were defined anteriorly by the white matter of the parahippocampal gyrus (which provided the anterior, lateral and inferior borders of the anterior part of the amygdala), and posteriorily by the temporal horn of the lateral ventricle (inferior border). We performed voxel-wise tests in these anatomically defined volumes of interest. Consistent with the current standard (Hariri, Tessitore, Mattay, Fera, & Weinberger 2002; Winston, Strange, O’Doherty, & Dolan 2002), we utilized the Gaussian random field threshold (alpha = .05, corrected) with small volume correction (SVC) implemented in SPM99 (right and left amygdalae) (Worsley, Marrett, Neelin, Vandal, Friston, & Evans 1996). Statistical significance of activation in regions of interest was set to Pcorrected <0.05 and cluster size > 30 voxels.
Significant findings were further assessed using SPSS-14.0 after extracting the individual values of percent BOLD signal changes at the peak activation of significant clusters. The use of SPSS permitted more ease and flexibility for these analyses, particularly by allowing to enter a laterality factor and to test for correlations with clinical variables. Significant BOLD signal changes were submitted to mixed ANOVAs with diagnosis and sex as between-subjects factors, and amygdala laterality as within-subjects factor. Post-hoc analyses included simple effects and Tukey’s honestly significant difference. The clinical variables used in correlation analyses included disease severity indexed by the presence of salt-wasting; glucocorticoid dose of current replacement therapy; and performance scores of aversiveness ratings and reaction times.
Finally, a voxel-wise whole brain analysis provided data on the whole brain. Because of the exploratory nature of these analyses, we set the threshold for significance at P<0.001-uncorrected and cluster > 20 voxels.
RESULTS
Sample
The groups did not differ on gender, age, or IQ (7 female/7 male in each group; age: t(26)= 0.27, p=0.8; IQ: t(26)= 1.25, p=0.2) (Table 1). None of the subjects met criteria for a DSM-IV axis-I diagnosis.
Performance Scores
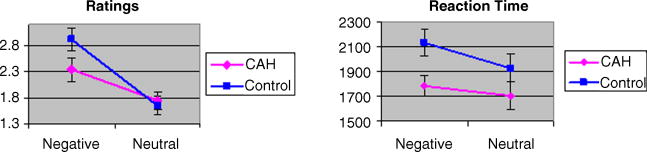
Means and standard deviations of rating scores and reaction times are presented in Table 2 and depicted in Fig. 1.
Table 2.
Means and standard deviations of performance scores (ratings of and reaction times to negative emotions and neutral emotions) by group and sex
| CAH | HEALTHY | |||
|---|---|---|---|---|
| Male (n=7) | Female (n=7) | Male (n=7) | Female (n=7) | |
| Negative Ratings | 2.2 (0.9) | 2.4 (0.8) | 3.1(0.4) | 2.7 (1.1) |
| Neutral Ratings | 1.6 (0.5) | 1.9 (0.7) | 1.8 (0.4) | 1.5 (0.9) |
| Negative RT | 1734.5 (392.1) | 1830.6 (270.2) | 2314.1 (306.5) | 1946.3 (427.8) |
| Neutral RT | 1728.0 (541.9) | 1686.0 (294.7) | 2110.1(329.0) | 1746.4 (444.8) |
Fig. 1.
Means (standard error) of ratings (left) and reaction times (right) for negative (fearful and angry) and neutral facial expressions.
Two 3-way repeated-measures analyses of variance examined the effects of Diagnosis, Sex, and Emotion on aversiveness ratings and on reaction time.
With respect to ratings, there was a significant interaction of Diagnosis-by-Emotion, with a large effect size (F(1,24)=5.6, p = 0.03, d′=0.93). This interaction reflected lower ratings of negative emotions in the CAH group than in the controls (F(1,24)=3.2, p=0.09, d′= 0.69), in contrast to the absence of group differences in the ratings of neutral emotion (F(1,24)=0.12, p = 0.73, d′=0.13). In addition, there was a main effect of Emotion, reflecting, as expected, that ratings of negative emotion (fearful & angry) were significantly higher than ratings of neutral emotion (F(1,24)=43.2, p<0.001). Finally, there was no main effect of group on face ratings (F(1,24)=0.95, p = 0.34), and there was no significant influence of Sex as a main effect (F(1,24)= 0.01, p=0.92) or in interaction with Diagnosis (F(1,24)=1.35, p=0.26) or Emotion (F=(1,24)=0.16, p=0.69).
With respect to reaction time, there was no interaction of Diagnosis-by-Emotion (F(1,24)=2.52, p = 0.13, d′=0.61). However, similarly to the face ratings, there was a main effect of emotion (F(1,24)=12.2, p = 0.002), reflecting longer reaction time for negative than for neutral expressions. The main effect of diagnosis fell short of significance (F(1,24)=4.11, p = 0.054, d′=0.61), indicating a trend for longer reaction times in controls than CAH patients. Finally, Sex had no influence on reaction time as a main effect (F(1,24)=1.46, p=0.24) or in interaction with Diagnosis (F(1,24)=1.96, p=0.17) or Emotion (F(1,24)=0.71, p=0.41). Healthy controls showed a strong effect of emotion (F(1,13)=17.90, p=0.001), but not the CAH group (F(1,13)=1.46, p=0.25) (see Fig. 1).
Amygdala ROI Analysis
Diagnosis and Diagnosis-by-Sex
Diagnosis showed no significant effect on right or left amygdala BOLD signal change. However, the interaction of diagnosis-by-sex was significant in the left amygdala (T(24)=2.93; Pcorrected=0.031) and marginally significant in the right amygdala (T(24)=2.29; Pcorrected=0.079), suggesting that the effect of CAH on amygdala function was modulated by sex (Table 3, Fig. 2).
Table 3.
Peak Activations in regions of interest, at Pcorrected <0.05 and cluster > 30 voxels, for the contrast Girls (CAH - Control) vs. Boys (CAH – Control), which is the equivalent of the interaction of Diagnosis by Sex on the BOLD signal change variable (change from condition of neutral faces to condition of negative faces) (Cluster K represents the number of voxels activated at Pcorrected <0.05; and x,y,z are MNI coordinates of activated peak voxels, in mm)
| Diagnosis × Sex | Cluster (K) | Pcorrected | T | x | y | z |
|---|---|---|---|---|---|---|
| Left Amygdala | 122 | 0.031 | 2.93 | −18 | −8 | −6 |
| Right Amygdala | 63 | 0.079 | 2.39 | 32 | −2 | −12 |
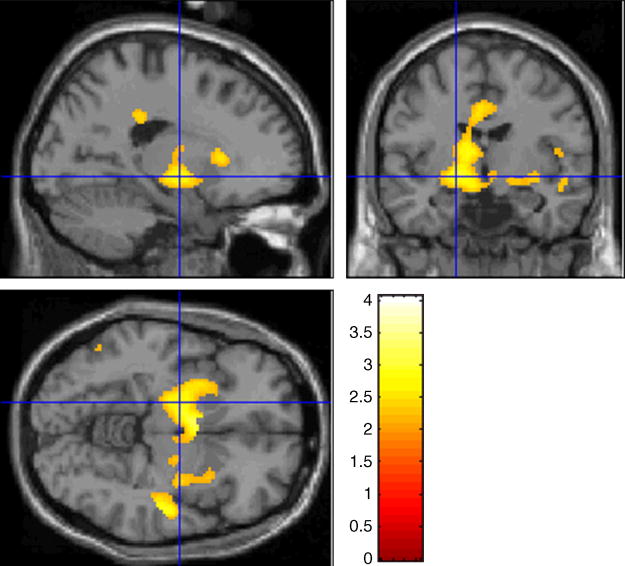
Fig. 2.
Bilateral amygdala activation in the contrast of diagnosis-by-gender interaction rendered on a single subject T1 image provided by SPM99 (left amygdala peak activation at the intersection of vertical and horizontal axis: MNI -18 -8 -6 mm).
BOLD signal change values were extracted for each individual at the peak activation voxel of the left amygdala (MNI x=−18mm, y=−8mm, z=−6mm) and at its mirror voxel in the right amygdala (MNI x=18mm, y=−8mm, z=−6mm). The overall ANOVA with diagnosis and sex as between-subjects factors and laterality as within-subjects factor revealed a significant three-way interaction between diagnosis, sex and laterality (F= 6.45, df= 1,24, P=0.018), and as expected a significant diagnosis-by-sex interaction (F(1,24)=7.33; P=0.012) (Fig. 3). Laterality had no significant effects on amygdala activation as a main effect or in interaction with diagnosis or sex.
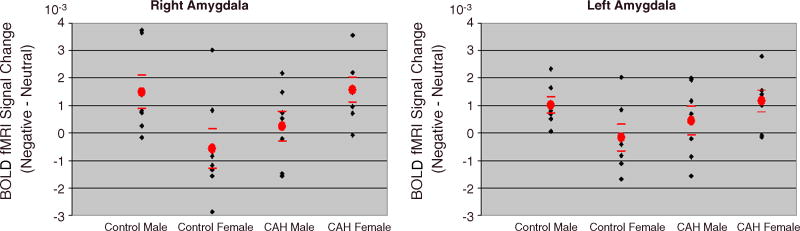
Fig. 3.
Scatter plots of individual fMRI BOLD signal changes in the right (MNI 18, -8, -6 mm) and left amygdala (MNI -18, -8, -6 mm) by Gender and Diagnosis. Means of BOLD signal differences are depicted by plain circles, and standard errors of the means by horizontal lines on each side of the means.
Further analysis of the diagnosis-by-sex interaction using ANOVA with diagnosis as the between-subjects factor was performed for each sex group separately. In females, both right and left amygdalae were significantly more strongly activated in the CAH group than the control group (overall amygdala: F(1,12)= 5.97, p=0.031; right amygdala: F(1,12)=4.4, P=0.059, d′=1.01; left amygdala: F(1,12)=6.4, P=0.027, d′=0.99). In males, there was no effect of diagnosis on amygdala activation (p’s > 0.1; d′=0.51for right amygdala, and d′=0.83 for left amygdala) (Fig. 3).
Finally, because of the potentially virilizing effect of CAH on the amygdala function in females, amygdala activation was compared between females with CAH and control males. No significant differences were found between females with CAH and control males (F(1,12)=0.02, p=0.9), suggesting the presence of a potentially virilizing effect of the amygdala in CAH females (see Fig. 3).
Correlations between clinical variables and regional activations
Finally, no associations were found between amygdala activation and performance scores (r’s<0.20, p’s>0.30), age at treatment onset, dose of corticosteroid replacement, 24-hour urinary free cortisol, adrenal hormone levels, or severity of disease (salt waster vs. non-salt-waster).
Whole brain exploratory analysis
The peak activations of the whole brain analyses, thresholded at P < 0.001 uncorrected, and cluster > 20 voxels are presented in Table 4.
Table 4.
Whole Brain Voxel-Wise Activations at P<0.001, and cluster > 20 voxels (x,y,z are MNI coordinates in mm; BA = Brodmann Areas)
| Cluster (K) | T | x | y | z | Side | Regions | BA |
|---|---|---|---|---|---|---|---|
| CAH vs. Healthy | |||||||
| 294 | 5.12 | 18 | −46 | −18 | right | Fusiform Gyrus | 37 |
| 172 | 4.47 | −16 | −38 | −20 | left | Fusiform Gyrus | 20 |
| 37 | 4.33 | 10 | −80 | −16 | right | Fusiform Gyrus | 18 |
| 47 | 3.85 | −4 | −76 | 44 | left | Precuneus | 19 |
| 45 | 3.71 | 16 | −76 | 40 | right | Precuneus | 19 |
| 23 | 3.81 | 46 | −58 | −2 | right | Middle Temporal Gyrus | 37 |
| 22 | 3.77 | 46 | 0 | −16 | right | Middle Temporal Gyrus | 21 |
| 24 | 3.86 | −18 | 16 | 14 | left | Caudate | |
| Diagnosis (CAH vs. Control) × Gender (Male vs. Female) | |||||||
| 391 | 4.05 | −56 | −28 | 48 | left | Inferior Parietal Lobe | 40 |
| 754 | 3.81 | 48 | −28 | 52 | right | Inferior Parietal Lobe | 40 |
Diagnosis
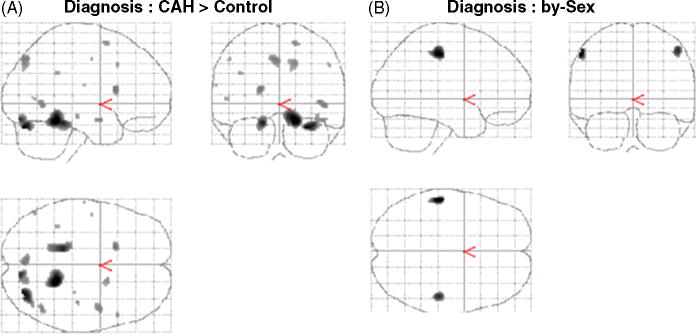
Adolescents with CAH showed greater activation than healthy adolescents during the presentation of negative vs. neutral faces, most significantly bilaterally in the fusiform gyrus and occipital cortex (Fig. 4-A, Table 4).
Figure 4.
Voxel-wise whole brain analyses (SPM 99, Puncorrected<0.001): A. Significant main effect of Diagnosis. B. Significant interactions of Diagnosis-by-Sex
No regions were more activated in healthy adolescents than in adolescents with CAH.
Diagnosis-by-Sex
The Diagnosis-by-Sex interaction was significant bilaterally in the inferior parietal lobule (LPI) (Fig. 4-B, Table 4).
DISCUSSION
We found altered amygdala function in females with CAH. However, no difference in amygdala function was observed in males with CAH compared to control males. Amygdala activation in females with CAH was bilaterally higher than amygdala activation in control females, and was similar to amygdala activation in control males, suggesting a virilizing effect. The left amygdala seemed to be more substantially affected, but this laterality effect was not statistically significant.
These findings are partially consistent with previous work in our laboratory that showed reduced amygdala volume in 27 children with CAH (11 females; age 4–16 years) compared to 47 sex- and age-matched healthy controls (Merke et al., 2003). Although our previous structural study showed a volumetric reduction in the size of the amygdala in patients with CAH independent of sex, it is of interest to note that the effect size (ES) (Cohen 1998) in girls (ES d′ = 1.56) was almost twice as large as the effect size in boys (ES d′ = 0.91). Our current functional study shows altered amygdala activation in females only.
In the present work, the amygdala function was probed through the now standard way of examining fMRI BOLD signal changes in the amygdala, i.e., during exposure to displays of faces expressing anger or fear vs. neutral expressions (see review, Adolphs 2002). Behaviorally, the CAH group rated the aversiveness of negative vs. neutral emotions less differently than did the control group, and this effect was mostly related to lower ratings of negative emotions by the CAH group compared to the control group. Moreover, reaction time to negative vs. neutral emotions tended to be shorter in the CAH group compared to the control group, echoing aversiveness ratings. These group differences in performance did not influence the fMRI findings. However, they suggested that patients with CAH may not differentiate aversive from neutral emotions as well as controls. In addition, the diagnosis effect on performance scores was not modulated by sex, suggesting that these performance differences might reflect an alternative (non-androgen) disease-specific effect on neural processes. CAH-related alterations in the HPA axis may play a role, but further studies are needed to explore this possibility.
Previous psychological and behavioral studies of patients with CAH have focused on possible androgen effects on the brain. Compared with their unaffected sisters or control girls, female with CAH have more male-typical childhood play and behavior (Berenbaum1999; Berenbaum & Snyder 1995; Dittmann, Kappes, Kappes, Borger, Meyer-Bahlburg, Stegner, Willig, & Wallis 1990; Hines 2003; Meyer-Bahlburg, Dolezal, Baker, Carlson, Obeid, & New 2004; Servin, Nordenstrom, Larsson, & Bohlin 2003), are more likely to report physical aggression (Berenbaum et al., 1997) and have better spatial abilities (Hampson, Rovet, & Altmann 1998; Hines, Fane, Pasterski, Mathews, Conway, & Brook 2003). The children in our study were not evaluated for male-typical behavior. However, the females with CAH had amygdala activation similar to control males, possibly reflecting an early organizational effect of excess androgen on these brain structures or associated circuits.
The organizational effects of androgen on the development of the brain provide a likely explanation to our findings. The amygdala contains many androgen receptors (DonCarlos, Garcia-Ovejero, Sarkey, Garcia-Segura, & Azcoitia 2003; Wood 1997), which have been shown to modulate amygdala size in animals and humans (Goldstein, Seidman, Horton, Makris, Kennedy, Caviness, Jr., Faraone, & Tsuang 2001). Androgens have also been proposed to influence emotional learning (Vazquez-Pereyra et al., 1995) through their action on the limbic system (Vazquez-Pereyra et al., 1995; Naghdi and Asadollahi, 2004) (Naghdi et al., 2004; Vazquez-Pereyra et al., 1995).
Whereas our main interpretation relies on the neurodevelopmental consequences of elevated androgens, the distinct or interactive contribution of glucocorticoid imbalance cannot be ruled out, particularly based on the role of glucocorticoids on amygdala function. The corticotrophin releasing hormone (CRH), whose expression in the amygdala is stimulated by circulating glucocorticoid (Makino, Gold, & Schulkin 1994), has been found to play a critical role in the processing of negative emotions, such as aggression, and behavioral responses to stress, particularly fear-related behaviors (LeDoux 2000). The mechanism underlying glucocorticoid potential influence in our findings is complex. Both early lack of circulating glucocorticoid and later excess of iatrogenic glucocorticoid (Merke, Bornstein, Avila, & Chrousos 2002) could alter amygdala function. However, glucocorticoid imbalance would not explain the female selectivity of the perturbation observed in the present study because alterations in the HPA axis due to CAH are comparable in both sexes (Merke et al., 2005). Further functional neuroimaging studies that target processes known to be highly influenced by corticosteroid function, such as memory (Wolf, Kuhlmann, Buss, Hellhammer, & Kirschbaum 2004) or stress response (de Kloet et al., 2005), could help to disambiguate the respective role of sex steroids and corticosteroids on amygdala function and associated behavior.
Finally, we present findings of a complementary whole brain analysis. Two findings merit some comments. First, the interaction of Diagnosis-by-Sex contrast showed that the most robust effect laid in the inferior parietal lobule (LPI). The LPI is highly sexually dimorphic (Frederikse, Lu, Aylward, Barta, & Pearlson 1999), and has been associated with sex differences in visual-spatial abilities (Gron, Wunderlich, Spitzer, Tomczak, & Riepe 2000). Furthermore, the examination of this interaction using post-hoc analyses indicated that the pattern of activation in girls with CAH was more similar to that of control boys than of control girls, supporting the hypothesis of an early organizational effect of testosterone on brain function in girls with CAH. The second notable finding is the main effect of Diagnosis that is predominant on the fusiform gyrus. This structure processes complex visual stimuli, particularly of social nature (e.g., faces, body movements) (Kanwisher, McDermott, & Chun 1997), and is tightly connected with the amygdala (Morris, Friston, Buchel, Frith, Young, Calder, & Dolan 1998; Pessoa, McKenna, Gutierrez, & Ungerleider 2002; Sabatinelli, Bradley, Fitzsimmons, & Lang 2005; Taylor, Liberzon, & Koeppe 2000). This finding is consistent with the presence of disease specific abnormalities across sex, which might affect the coding of social stimuli, as suggested by performance perturbation in the CAH group relative to the control group found in the present work. These deficits might reflect impaired corticosteroid function rather than sex steroid since they affect equally boys and girls.
A number of caveats should be mentioned. First, our sample size was relatively small, and may have limited the exploration of correlations of brain activation with behavioral performance and treatment. However, the detection of a significant interaction between sex and diagnosis suggested that this effect was strong. Second, it was impossible to separate out the complicated effects of cortisol replacement medication and multiple hormones at the time of testing. However, our CAH patients were on approximately physiological dose replacement, overall disease control was good, and our hormonal measurements did not appear to influence the results. Third, the task was relatively complex, which may have led to some dispersion of effects because of increased noise. However, this task has been used in our laboratory for several years (McClure et al., 2004; Monk et al., 2003; Nelson et al., 2003; Pine, Cohen, Gurley, Brook, & Ma 1998), with a large number of healthy adolescents, and pediatric patients with anxiety and mood disorders. It has been shown to reliably activate the amygdala, to be sensitive to development (McClure et al., 2004) function (Monk et al., 2003) and to psychopathology (Pine et al., 1998; McClure et al., in preparation). Fourth, there are virtually no data available on sex differences in amygdala response to evocative faces in healthy adolescents. Therefore, the sex differences found in the present study are important to validate in future work. Finally, our control sample consisted of healthy adolescents matched on all demographic characteristics, but without the stress of having a chronic disease. However, the use of a comparison group with a chronic disease also presents drawbacks, since the nature of the chronic disease can itself affect brain function in unique ways.
Despite these limitations, this first study of cerebral function in adolescents with CAH suggests altered amygdala function in this population, possibly reflecting an organizational effect of sex steroids. Future cognitive and neuroimaging studies in patients with CAH and other endocrine imbalances will provide further insight into the influence of hormones on the developing brain.
Acknowledgments
We thank the staff of the NIH MR Center for making this study possible and Harvey Iwamoto for his assistance in programming the task. FSM was supported by a postdoctoral fellowship from the Fonds de la recherche en santé du Québec (FRSQ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Horm Behav. 1999;35:102–110. doi: 10.1006/hbeh.1998.1503. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Duck SC, Bryk K. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:727–733. doi: 10.1210/jcem.85.2.6397. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22:505–515. doi: 10.1016/s0306-4530(97)00049-8. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Snyder E. Early Hormonal influences on childhood sex-typed activity and playmate preferences: implications for the development of sexual orientation. Dev Psychol. 1995;31:31–42. [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de BC, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. NJ: Lawrence Erlbaum Associates, Inc; 1998. [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Borger D, Meyer-Bahlburg HF, Stegner H, Willig RH, Wallis H. Congenital adrenal hyperplasia. II: Gender-related behavior and attitudes in female salt-wasting and simple-virilizing patients. Psychoneuroendocrinology. 1990;15:421–434. doi: 10.1016/0306-4530(90)90066-i. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Frederikse ME, Lu A, Aylward E, Barta P, Pearlson G. Sex differences in the inferior parietal lobule. Cereb Cortex. 1999;9:896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3:404–408. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- Hall CM, Jones JA, Meyer-Bahlburg HF, Dolezal C, Coleman M, Foster P, Price DA, Clayton PE. Behavioral and physical masculinization are related to genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:419–424. doi: 10.1210/jc.2003-030696. [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet JF, Altmann D. Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Dev Neuropsychol. 1998;14:299–320. [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex steroids and human behavior: prenatal androgen exposure and sex-typical play behavior in children. Ann NY Acad Sci. 2003;1007:272–282. doi: 10.1196/annals.1286.026. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM. Impaired cognitive function in women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91:1376–81. doi: 10.1210/jc.2005-1959. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. 155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Intern Med. 2002;136:320–334. doi: 10.7326/0003-4819-136-4-200202190-00012. [DOI] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF. Gender and sexuality in classic congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:155–71. viii. doi: 10.1016/s0889-8529(08)70024-0. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Arch Sex Behav. 2004;33:97–104. doi: 10.1023/b:aseb.0000014324.25718.51. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testosterone on long-term memory in male adult rats. Behav Brain Res. 2004;153:1–6. doi: 10.1016/j.bbr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Nass R, Baker S. Androgen effects on cognition: congenital adrenal hyperplasia. Psychoneuroendocrinology. 1991;16:189–201. doi: 10.1016/0306-4530(91)90078-8. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Celotti F, Motta M. Sexual differentiation of the brain: role of testosterone and its active metabolites. J Endocrinol Invest. 2004;27:120–127. [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;20(99):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Worthman CM, Costello EJ, Angold A. Testosterone, antisocial behavior, and social dominance in boys: pubertal development and biosocial interaction. Biol Psychiatry. 2004;55:546–552. doi: 10.1016/j.biopsych.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Servin A, Nordenstrom A, Larsson A, Bohlin G. Prenatal androgens and gender-typed behavior: a study of girls with mild and severe forms of congenital adrenal hyperplasia. Dev Psychol. 2003;39:440–450. doi: 10.1037/0012-1649.39.3.440. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, Bilder RM. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Vazquez-Pereyra F, Rivas-Arancibia S, Loaeza-Del Castillo A, Schneider-Rivas S. Modulation of short term and long term memory by steroid sexual hormones. Life Sci. 1995;56:L255–L260. doi: 10.1016/0024-3205(95)00067-g. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kuhlmann S, Buss C, Hellhammer DH, Kirschbaum C. Cortisol and memory retrieval in humans: influence of emotional valence. Ann N Y Acad Sci. 2004;1032:195–7. 195–197. doi: 10.1196/annals.1314.019. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Worsley KM, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. Testosterone levels in healthy men and the relation to behavioural and physical characteristics: facts and constructs. Eur J Endocrinol. 2001;144:183–197. doi: 10.1530/eje.0.1440183. [DOI] [PubMed] [Google Scholar]