Abstract
Background
Infrainguinal bypass (IB) surgery is an effective means of improving arterial circulation to the lower extremity for patients with critical limb ischemia (CLI). However, wound complications (WC) of the surgical incision following IB can impart significant morbidity.
Methods
A retrospective analysis of WC from the 1404 patients enrolled in a multicenter clinical trial of vein bypass grafting for CLI was performed. Univariate and multivariable regression models were used to determine WC predictors and associated outcomes, including graft patency, limb salvage, quality of life (QoL), resource utilization (RU), and mortality.
Results
A total of 543 (39%) patients developed a reported WC within 30 days of surgery, with infections (284, 52%) and hematoma/hemorrhage (121, 22%) being the most common type. Postoperative anticoagulation (odds ratio [OR], 1.554; 95% confidence interval [CI] 1.202 to 2.009; P = .0008) and female gender (OR, 1.376; 95% CI, 1.076 to 1.757; P = .0108) were independent factors associated with WC. Primary, primary-assisted, and secondary graft patency rates were not influenced by the presence of WC; though, patients with WC were at increased risk for limb loss (hazard ratio [HR], 1.511; 95% CI 1.096 to 2.079; P = .0116) and higher mortality (HR, 1.449; 95% CI 1.098 to 1.912; P = .0089). WC was not significantly associated with lower QoL at 3 months (4.67 vs 4.79, P = .1947) and 12 months (5.02 vs 5.13, P = .2806). However, the subset of patients with serious WC (SWC) demonstrated significantly lower QoL at 3 months compared with patients without WC, (4.43 vs 4.79, respectively, P = .0166), though this difference was not seen at 12 months (4.94 vs 5.13, P = .2411). Patients with WC had higher RU than patients who did not have WC. Mean index length of hospital stay (LOS) was 2.3 days longer, mean cumulative 1-year LOS was 8.1 days longer, and mean number of hospitalizations was 0.5 occurrences greater for patients with WC compared with patients without WC (all P < .0001).
Conclusions
WC is a frequent complication of IB for CLI, associated with increased risk for major amputation, mortality, and greater RU. Further detailed investigation into the link between female gender and oral anticoagulation use with WC may help identify causes of WC and perhaps prevent or lessen their occurrence.
Infrainguinal bypass (IB) surgery has proven to be an effective means of improving arterial circulation to the lower extremity for both disabling claudication and critical limb ischemia (CLI). Wound complications (WC) of the surgical incision following IB, however, continue to be a source of significant morbidity, occurring with an incidence between 17% to 44%.1,2 WC can include infection, hematoma, seroma or lymphatic leak, necrosis, dehiscence, and erythema.3–;6 These complications can potentially jeopardize the underlying graft, placing it at risk of infection, hemorrhage, or thrombosis and ultimately have a negative impact on the operative limb.1,3,5 Less devastating are the economic consequences of WC, which are incurred by both the patient and health care system. These expenses include further hospitalization, additional procedures, and the use of outpatient resources such as rehabilitation and visiting nursing services.3,4
Previous studies have identified a diverse set of risk factors implicated in the abnormal healing of infrainguinal surgical incisions. Significant patient predictors identified have included female gender,4,7–9 older age,3,9 obesity,3,9,10 and diabetes.11,12 Significant surgical predictors include aspects of perioperative management,2,9,11 surgical technique,1,13 and graft conduit used.13,14
Although WC has been implicated in deleterious outcomes, few studies have correlated these complications with clinical endpoints such as graft patency, limb salvage, and survival rates. Nam et al, using life table analysis, showed that there were no differences in primary patency, secondary patency rates, limb salvage, nor survival rates between patients with and without WC.4 Limb loss rates across several studies ranged between 0% to 3% among patients with WC.1,2,4,13,14 Resource utilization (RU) related to WC has also been infrequently assessed, with the most common measure used was postoperative length of stay. Three studies reported significantly longer hospital stays for patients with WC.1,9,13 Kent et al, however, reported no difference in postoperative length of stay between the two groups; additionally, they reported that estimated per patient costs related to WC after IB was $688, specific to their institution.3
Our study is a post-hoc analysis of the WC from the PRoject of Ex-Vivo vein graft ENgineering via Transfection III (PREVENT III) database.15 PREVENT III was a randomized, double-blinded, multicenter, phase III trial of a pharmacologic agent (edifoligide) to prevent vein graft failure in patients who underwent IB for CLI. The study population consists of 1404 patients selected from both community and university hospitals across the United States and Canada. The large and diverse study base of PREVENT III provides a wider scope with which to identify the predictors of wound complications post IB in current practice.
The purpose of our study is fivefold: (1) to evaluate the incidence of WC in our cohort and compare these values with previous studies; (2) to identify significant predictors of WC; (3) to assess if WC affect traditional IB endpoints including primary, primary-assisted, and secondary patency, and limb salvage and survival rates; (4) to estimate the wound related economic burden imposed on the health care system through examining RU; and (5) to determine how quality of life is affected by WC.
METHODS
Prevent III database
PREVENT III was a double-blinded, randomized, multicenter, placebo-controlled clinical trial testing the efficacy of edifoligide (E2F decoy)16 in preventing vein graft neointimal hyperplasia in patients who underwent IB for CLI.15 E2F is a transcription factor involved in cell-cycle regulation and inhibition of E2F blocks cellular proliferation. Edifoliglide is a short double-stranded oligodeoxynucleotide that contains a binding site for E2F, and, thus, acts as a competitive inhibitor. Edifoliglide has been demonstrated to inhibit smooth muscle cell proliferation and reduce intimal hyperplasia in animal models of vascular injury. The study population included 1404 patients with CLI at 83 inpatient sites across the United States and Canada with clinical follow-up to 1 year. Enrollment criteria included patients aged 18 and older with a diagnosis of CLI, (defined as arterial insufficiency with gangrene, a non-healing ischemic ulcer, or rest pain.) Failure of edifoligide to decrease vein graft failure in Prevent III, and in a companion study of coronary bypass grafts (Prevent IV), has been previously reported.17,15 To date, it is the largest randomized clinical trial in patients with CLI.
Adverse event data
PREVENT III mandated documentation of any adverse event (AE) within the first 30 days following surgery, including events related to the surgical incision. Information about the date of onset and resolution of the event and the type of WC were recorded. An AE could be further defined as “serious”, by a study investigator, based on standard Food and Drug Administration clinical trials reporting standards (21 CFR Part 312). These criteria included prolongation of hospital stay or the need for additional procedures. Serious adverse events (SAE) required completion of the adverse event case-report form as well as a descriptive narrative detailing the complication as well as diagnostic and treatment modalities used as a part of its management. The additional detailed data provided in SAE was used in a subset analysis of patients with serious WC (SWC).
Study design
Adverse events in PREVENT III were categorized using the organ system and preferred terms from the Medical Dictionary for Regulatory Affairs (MedDRA) (MedDRA Maintenance and Support Services Organization, Reston, Va). We defined patients with WC as those having infection, necrosis, hematoma/hemorrhage, or seroma/ lymphocele at the surgical incision or harvest site within 30 days of the bypass surgery. Inclusion terms for the infection category included infection, cellulitis, and abscess; while inclusion terms for the necrosis category included necrosis, dehiscence, gangrene, delayed healing, and eschar. Ischemic ulcers or foot gangrene present prior to IB were not counted as WC, but were tracked for their relation to the development of WC. A subset analysis was also performed for patients with SWC utilizing the more detailed descriptions of treatment and outcomes available in their SAE reports.
Patient variables selected to test as possible predictors of WC, included age, gender, race, baseline weight, smoking status, randomization to study drug, dialysis-dependence, hypertension, diabetes, anemia (indicated by hematocrit<30%), poor nutritional status (indicated by total lymphocyte count <1500 cells/mm3),4 baseline medications, previous infrainguinal reconstruction, and presence of tissue loss (defined as gangrene or ulcer). Surgical variables were also selected to test as possible predictors of WC, including duration of surgical procedure, conduit length, site of distal anastomosis (tibial or pedal arteries versus above or below-knee popliteal artery), conduit diameter (<3 mm vs ≥3 mm), use of a composite conduit, whether the conduit was from a source other than the great saphenous vein, and whether a concomitant procedure was performed (including debridement and/or amputation). Quality of life (QoL) was assessed by the VascuQol questionnaire, a validated instrument assessing pain, symptoms, activities, social life and emotional state in patients with vascular disease. 18,19 Indicators used to assess RU included index length of hospital stay (iLOS), cumulative length of hospital stay (cLOS) over 1 year, and number of rehospitalizations (NOR) over 1 year.20
Statistical methods
Univariate analysis was performed to test for associations between patient and surgical variables and developing either any WC or SWC. Categorical variables were tested using a Fisher exact test, and continuous variables were tested using a t-test for independent samples. Multivariable analysis, using logistic regression modeling with backwards selection, was performed to identify independent patient and surgical predictors of any WC and SWC. The statistical significance of individual variables was evaluated using a Wald test, and a P value ≤ .20 was our inclusion criteria for the models.
Comparisons of mean values of iLOS, cLOS, and NOR were made between patients with any WC and SWC to patients without WC. A natural logarithm-transformed linear regression model was used to evaluate iLOS and cLOS, and a Poisson regression model was used to evaluate NOR to better fit the non-normal distribution of the data.
Mean global QoL scores were evaluated both within and between subgroups at baseline, 3 months and 12 months. A paired t-test was used to assess for statistically significant differences in mean QoL scores from baseline to 3 months, and from baseline to 12 months in the full cohort, in patients with any WC, SWC, and without WC. A mixed effects regression model was used to evaluate for differences in the overall change in QOL score over 1 year, between patients with any or SWC and patients without WC.
Differences in rates of primary, primary-assisted, and secondary patency, amputation, and survival were compared between patients with any WC and SWC and without WC over 1 year. This was evaluated using both a log-rank test for univariate analysis and Cox proportional hazards models for multivariable analysis.
All tests were considered statistically significant at an alpha level of .05 (P = .05). All analyses were performed using SAS version 9.1.3 (Cary, NC).
RESULTS
PREVENT III cohort
The details of the PREVENT III cohort as well as study outcomes have been previously reported15 and are summarized here briefly to provide context for this study. There were 1404 patients who had lower extremity vein bypass as part of the PREVENT III trial (897 males, 507 females, mean age 69 ± 12 years). The primary indication for surgery was ischemic rest pain in 25%, non-healing ulceration in 39% and ischemic gangrene in 36%. Sixty four percent had diabetes, 73% were smokers, 12% were on dialysis, and 28% had undergone a previous infrainguinal bypass. Overall, 222 patients (15.8%) died during the study, 18 were lost to follow-up, and 26 withdrew from the study.
A single segment of great saphenous vein (reversed or non-reversed) was used in 81% of cases. The proximal anastomosis was to the common femoral, superficial femoral, or profunda femoris artery in 78% of cases; while the distal anastomosis was to the popliteal artery in 30%, a tibial artery in 55%, and to the dorsal pedal or a plantar artery in 13% of the cases. Perioperative (30-day) death rate was 2.7%. Major morbidity occurred in 17.6%. The 30-day graft occlusion rate was 5.2% and the 30-day major amputation rate 1.8%. Assisted primary patency at 1 year was 77%. Limb salvage at 1 year was 88% and survival at 1 year 84%.
Wound complications
Of the 1404 patients in the PREVENT III cohort, 543 (39%) developed a reported WC within 30 days of surgery. Among patients with WC, the two most common types of complications were infections (n = 284, 52%) and hematoma/hemorrhage (n = 121, 22%) (Table I). Among the 543 patients with WC, 155 patients (28.5% of WC and 11% of the total population) were classified as SWC. Infections (n = 107; 69%) and necrosis (n = 43; 28%) were most common among patients with SWC. SWC occurred most frequently in the groin (n = 36; 23%), followed by the thigh (n = 32; 21%), the calf (n = 26; 17%), and the foot (n = 7; 5%). SWC were managed non-operatively using dressings and systemic antibiotics in 136 (88%) of patients while 23 (15%) of patients required surgical intervention.
Table I.
Types of wound complications
| Variables | Wound complication (N = 543) n (%) |
|---|---|
| Type of complication | |
| Infection | 284 (52%) |
| Necrosis | 94 (17%) |
| Hematoma/hemorrhage | 121 (22%) |
| Seroma/lymphocele | 59 (11%) |
| Other | 84 (15%) |
Wound categories not mutually exclusive.
Among infection-related SWC (n = 107; 69%), the most common organisms isolated from the wound were Gram positive (n = 25; 23%), including methicillin-resistant staphylococcus aureus in eight (7%) patients, though 71% of patients with infection-related SWC had no recorded wound culture. These infection-related SWC patients received broad-spectrum antibiotic coverage with multiple agents, with the most common being vancomycin (n = 52; 49%), and flouroquinolones (n = 50; 47%).
Predictors of wound complications
In univariate analysis, female gender (odds ratio [OR] = 1.380) (95% confidence interval [CI], 1.10 to 1.72; P = .0052) and Hct < 30% (OR, 1.4720; 95% CI, 1.0884 to 1.9909; P = .0147) were significantly associated with developing WC (Table II, online only). Among surgical variables, having a vein bypass conduit diameter <3 mm (OR, 1.7166; 95% CI, 1.1053 to 2.6661; P = .0162), the site of the distal anastomosis being a tibial or pedal vessel (OR 1.2690; 95% CI, 1.0047 to 1.6027; P = .0456), and the use of postoperative oral anticoagulation (OR, 1.4931; 95% CI, 1.1751 to 1.8972; P = .0013) were significantly associated with developing WC. In multivariable analysis, only postoperative oral anticoagulation (OR, 1.554; 95% CI 1.202 to 2.009; P = .0008) and female gender (OR, 1.376; 95% CI, 1.076 to 1.757; P = .0108) remained as independent factors associated with WC. Nineteen percent of patients had both antiplatelet and anticoagulation medications upon discharge. Anticoagulation was independently associated with WC while antiplatelet therapy was not. No statistically significant synergistic effect between antiplatelet and anticoagulation medications were seen with respect to WC in this study.
Female gender (OR = 1.7899; 95% CI 1.2666 to 2.5291; P = .0013) and use of postoperative oral anticoagulation (OR = 1.8217; 95% CI 1.2666 to 2.6201; P = .0018) were also significantly associated with developing SWC in univariate analysis (Table II, online only) and multivariable analysis, (OR = 1.616; 95% CI 1.117 to 2.336; P = 0.0108) (OR = 2.108; 95% CI 1.421 to 3.128; P = .0002), respectively.
Relationship of wound complications to patient outcomes
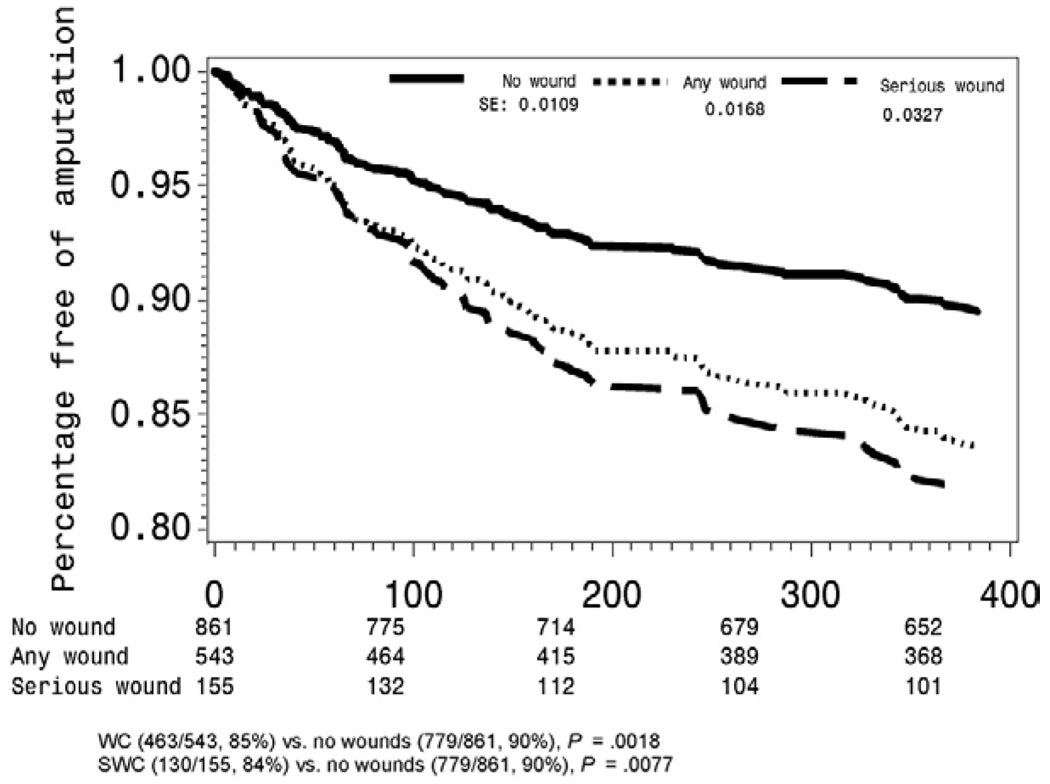
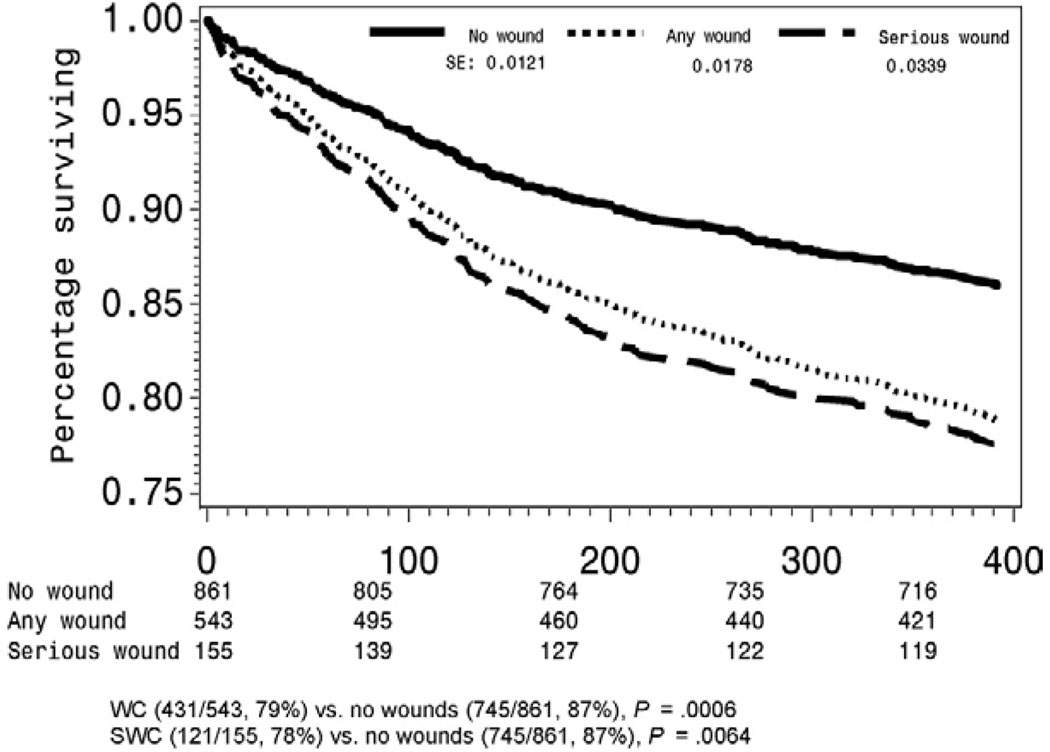
Primary (hazard ratio [HR], 1.059; 95% CI, 0.893 to 1.257; P = .5084), primary-assisted (HR, 1.041; 95% CI, 0.833 to 1.301; P = .7255), and secondary graft patency (HR, 1.057; 95% CI, 0.831 to 0.344; P = .6530) rates were not influenced by the presence of WC. However, patients with WC were at increased risk for limb loss (HR, 1.511; 95% CI 1.096 to 2.079; P = .0116) (Fig 1) and higher mortality (HR, 1.449; 95% CI 1.098 to 1.912; P = .0089)(Fig 2).
Fig 1.
Limb salvage among wound complication groups.
Fig 2.
Mortality among wound complication groups.
Similarly, primary (HR, 1.208; 95% CI 0.926 to 1.575; P = .1629), primary-assisted (HR, 0.954; 95% CI, 0.657 to 1.386; P = .8064), and secondary graft patency (HR, 0.992; 95% CI, 0.665 to 1.479; P = .9674) rates were not influenced by the presence of SWC. However, SWC patients were also at increased risk for major limb amputation (HR, 1.957; 95% CI, 1.218 to 3.145 P = .0055)(Fig 1) and mortality (HR, 1.621; 95% CI, 1.057 to 2.488; P = .0267) (Fig 2), and these risks were greater for SWC patients than WC patients (relative risk [RR], 1.295 for major limb amputation and 1.119 for mortality).
Influence of wound complications on quality of life and resource utilization
Overall, mean global QoL scores improved from baseline, at 3 months, and 12 months, respectively (2.82 ± 1.14; 4.75 ± 1.39; 5.09 ± 1.38)(Table III).19 WC was not significantly associated with lower QoL at 3 months (4.67 vs 4.79, P = .1947) and 12 months (5.02 vs 5.13, P = .2806). Mixed regression model analysis of the overall change in mean global QoL score over one year was also found not to be statistically significant between patients with and without WC (coefficient, −0.1118; 95% CI, −0.2741 to −0.0504; P = .1764). Patients with WC had higher RU than patients who did not have WC. Mean iLOS was 2.3 days longer, mean cLOS was 8.1 days longer, and mean NOR was 0.5 occurrences greater for patients with WC compared with patients without WC (all P < .0001)(Table IV).
Table III.
Impact of wound complications on quality of life
| Variables | All patients mean |
No wound complication mean |
Any wound complication mean |
Serious wound complication mean |
Any WC vs no WC P value |
Serious WC vs no WC P value |
|---|---|---|---|---|---|---|
| Baseline score | 2.82 ± 1.14 | 2.82 ± 1.15 | 2.81 ± 1.14 | 2.71 ± 0.98 | .8769 | .2077 |
| 3 months score | 4.75 ± 1.39 | 4.79 ± 1.38 | 4.67 ± 1.40 | 4.43 ± 1.29 | .1947 | .0166 |
| 12 months score | 5.09 ± 1.38 | 5.13 ± 1.36 | 5.02 ± 1.41 | 4.94 ± 1.40 | .2806 | .2411 |
WC, Wound complications.
Table IV.
Impact of wound complications on resource utilization
| Variables | All patients mean |
No wound complication mean |
Any wound complication mean |
Serious wound complication mean |
Any WC vs no WC P value |
Serious WC vs no WC P value |
|---|---|---|---|---|---|---|
| Index LOS (d) | 8.9 ± 9.3 | 8.0 ± 7.7 | 10.3 ± 11.3 | 10.7 ± 10.8 | <.0001 | <.0001 |
| Cumulative LOS (d) | 24.1 ± 27.4 | 20.9 ± 26.2 | 29.1 ± 28.6 | 34.5 ± 32.4 | <.0001 | <.0001 |
| # of rehospitalizations | 1.5 ± 1.7 | 1.3 ± 1.6 | 1.8 ± 1.8 | 2.2 ± 1.7 | <.0001 | <.0001 |
WC, Wound complications; LOS, length of hospital stay.
Patients with SWC demonstrated significantly lower QoL at 3 months compared with patients without WC, (global score 4.43 vs 4.79, respectively, P = .0166), though this difference disappeared by 12 months (4.94 vs 5.13, P = .2411)(Table III). Mixed regression model analysis of the overall change in mean, global QoL score over 1 year was also found not to be statistically significant between SWC and patients without WC (coefficient, −0.1744; 95% CI −0.4291 to −0.0803; P = .1792). Similar to WC, patients with SWC had greater iLOS, cLOS, and NOR. Mean iLOS was 2.7 days longer, mean cLOS was 13.5 longer, and NOR was 0.9 occurrences greater in patients with SWC compared with patients without WC (all P < 0.0001)(Table IV).
DISCUSSION
The 1404 patient in the PREVENT III study provide a large, multicentered study cohort to examine incidence, risk factors and outcomes associated with WC. To our knowledge, this is the largest published prospective cohort of patients undergoing peripheral bypass for CLI. Our study showed that WC and SWC occurred with an incidence of 39% and 11%, respectively, and was associated with female gender and usage of postoperative oral anticoagulation. Graft patency, (including primary, primary-assisted, and secondary,) was not affected by the presence of WC. Limb salvage and survival, however, were negatively associated with WC. Patients with WC had higher adjusted rates of amputation and lower adjusted rates of survival. Patients with WC had lower, but not statistically significant, QoL than patients without WC. Patients with SWC, however, did have significantly lower QoL scores at 3 months; a finding that was not continued at 12 months. WC patients also incurred greater RU, as measured by short-term and year-long criteria.
Our overall 30-day WC incidence of 39% falls within the higher range of previously reported incidences (17% to 44%),1,2 though differences in study design make direct comparisons difficult. One explanation for our higher incidence may be the more comprehensive inclusion criteria for WC in this study, which is nested in a prospective clinical trial. Similarly, the detailed reporting of serious postoperative complications in the PREVENT III study afforded us a comprehensive analysis of the nature and management of SWC. Previous studies have used a narrow definition for SWC including mostly graft infection and exposure1–3,13,14, occurring with an incidence of between 1% to 43%,1,3 and managed with operative treatment (debridement, skin grafting, muscle flaps, graft revision or excision, and amputation), 1–3,14 In our study, SWC occurred in 11% of the cohort and was not limited to graft involvement alone, but included all WC type categories mentioned above. Despite having 11% SWC, the occurrence of graft-related complications was very low (1 graft infection; 0 graft exposures).
Female gender and the use of postoperative oral anticoagulation were factors consistently associated with WC (and SWC). Several potential explanations for female gender as a risk factor for WC exist, including the amount of fat relative to total mass, the distribution of fat in the lower extremities, differences in native skin flora, and hormonal differences. Weight was not a significant factor associated with WC, though better measures of body fat (BMI, anthropometric measures) were not available for analysis.
The use of oral coagulation postoperatively (and associated preoperative use) was also related to hemorrhagic and other types of WC; but the use of other anticoagulants, such as heparin, was not associated with WC. Twenty percent of patients were on oral anticoagulation before surgery, a number which increased to 27% upon discharge. Of note, PREVENT III study protocol discouraged the use of anticoagulation for the maintenance of graft patency, but allowed its use for non-graft related issues, such as arrhythmia. One possible explanation for our findings is warafin’s reduction of vitamin K-dependent coagulation proteins, which are also responsible for vascular repair, cell-cell adhesion, cell cycle regulation and cell signal transduction,21 all of which may affect wound healing. Prior studies that randomized coumadin use in bypass patients did not describe greater WC rates, though WC may not have been a measured study endpoint.22,23 Furthermore, patients randomized to postoperative anticoagulation for graft protection in these studies did not have the same preoperative warfarin exposure as the patients in this study.
The association of oral anticoagulation with WC found in this study does not undermine the importance of anticoagulation for patients who require it for cardiovascular conditions such as arrhythmia, prosthetic valves, or DVT. However, the role of anticoagulation for graft patency remains debatable.24 Our findings add further insight into the management of CLI patients who have undergone IB, and may provide clinicians with an additional factor that influences the use of postoperative anticoagulation for graft patency.
WC and SWC was associated with major limb amputation, but not associated with worsened graft patency. This suggests that wounds were an additional risk factor in amputation. WC was also associated with lower 1-year survival. Detailed analysis of available reporting data showed no cases where WC leads directly to death by way of sepsis or hemorrhage. Patients who develop WC may represent a subgroup with low metabolic reserve that are also susceptible to other morbidity and mortality risks. WC may also negatively affect the decision of a surgeon to salvage failing or failed grafts. The association of WC (and SWC) with major amputation was seen independent of patency outcomes. This supports the concept that WC and graft patency are just a subset of factors leading to amputation.
SWC was associated with worse QoL at 3 months. The trend of lower (but not statistically significant) QoL among WC patients for other comparisons suggests an impact too small to measure within this study. Furthermore, the impact of SWC appears to lessen at 12 months, though we have no data on when the SWC was clinically resolved.
The limitations of this study stem mainly from its nested design. PREVENT III was not designed primarily to examine the incidence and consequences of WC. And although detailed information about WC was collected as a part of adverse reporting in PREVENT III, we cannot be certain about the consistency of the diagnosis of WC as they were applied by the various site physicians. There may also be inclusion bias for reporting minor or questionable WC because of participation in the study; similarly, there may also be exclusion bias for reporting WC in patients with other, more significant medical issues. Secondly, while we have detailed information about the onset and nature of the WC, we have no information on its resolution. Thus, more detailed conclusions about treatment efficacy and its impact on QoL and RU cannot be made. In addition, WC is a complex process with many different possible contributors and outcomes. The associations of WC to death and amputation seen in our study may reflect that WC is also a proxy for other health conditions not controlled in our analysis. Finally, as with most QoL and RU analyses, death and censorship can have a significant unknown impact on results. In the PREVENT III cohort, previous work as also shown an association of amputation with QoL survey non-response. 19 Accordingly, missing data (from death, study drop out, and survey non-response) was controlled by previously published mixed methods regression techniques in the QoL analysis19 and modified survival regression techniques in the RU analysis.20 These limitations with-standing, our study provides a detailed analysis of WC in a large number of patients who underwent IB for CLI at multiple centers.
CONCLUSIONS
WC is a frequent complication of IB for CLI, associated with increased risk for major amputation, mortality, and greater RU. Further detailed investigation into the link between female gender and oral anticoagulation use with WC may help identify causes of WC and perhaps prevent or lessen their occurrence.
Supplementary Material
Footnotes
Competition of interest: Drs Bandyk, Clowes, Moneta, Belkin, and Conte have each served as a paid consultant to Corgentech, Inc. Dr Conte has served as a paid consultant to Bristol-Myers Squibb. Dr Moneta owns stock in Bristol-Myers that predated the PREVENT III trial.
Presented at the Sixty-First Annual Meeting of the Society for Vascular Surgery, Baltimore, Md, Jun 7–10, 2007.
AUTHOR CONTRIBUTIONS
Conception and design: LN, SB, MC
Analysis and interpretation: LN, SB, DB, MB, AC, GM, MC
Data collection: LN, SB
Writing the article: LN, SB, MC
Critical revision of the article: LN, SB, MC
Final approval of the article: LN, SB, DB, MB, AC, GM, MC
Statistical analysis: LN, SB
Obtained funding: MB, AC, GM, MC
Overall responsibility: LN
REFERENCES
- 1.Wengrovitz M, Atnip RG, Gifford RR, Neumyer MM, Heitjan DF, Thiele BL. Wound complications of autogenous subcutaneous infrainguinal arterial bypass surgery: predisposing factors and management. J Vasc Surg. 1990;11:156–161. doi: 10.1067/mva.1990.16918. discussion 161–3. [DOI] [PubMed] [Google Scholar]
- 2.Reifsnyder T, Bandyk D, Seabrook G, Kinney E, Towne JB. Wound complications of the in situ saphenous vein bypass technique. J Vasc Surg. 1992;15:843–848. doi: 10.1067/mva.1992.36658. discussion 848–50. [DOI] [PubMed] [Google Scholar]
- 3.Kent KC, Bartek S, Kuntz KM, Anninos E, Skillman JJ. Prospective study of wound complications in continuous infrainguinal incisions after lower limb arterial reconstruction: incidence, risk factors, and cost. Surgery. 1996;119:378–383. doi: 10.1016/s0039-6060(96)80135-8. [DOI] [PubMed] [Google Scholar]
- 4.Nam JH, Gahtan V, Roberts AB, Kerstein MD. Influence of incisional complications on infrainguinal vein bypass graft outcome. Ann Vasc Surg. 1999;13:77–83. doi: 10.1007/s100169900224. [DOI] [PubMed] [Google Scholar]
- 5.Whittemore AD, Belkin M. Vascular surgery: infrainguinal bypass. 4th ed. Vol 1. Philadelphia (PA): WB Saunders; 2000. [Google Scholar]
- 6.Wolterbeek JH, van Leeuwen AA, Breslau PJ. Skin closure after infrainguinal bypass surgery: a prospective randomized study. Eur J Vasc Endovasc Surg. 2002;23:321–324. doi: 10.1053/ejvs.2001.1593. [DOI] [PubMed] [Google Scholar]
- 7.Belkin M, Conte MS, Donaldson MC, Mannick JA, Whittemore AD. The impact of gender on the results of arterial bypass with in situ greater saphenous vein. Am J Surg. 1995;170:97–102. doi: 10.1016/s0002-9610(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 8.Roddy SP, Darling RC, 3rd, Maharaj D, Chang BB, Paty PS, Kreienberg PB, et al. Gender-related differences in outcome: an analysis of 5880 infrainguinal arterial reconstructions. J Vasc Surg. 2003;37:399–402. doi: 10.1067/mva.2003.99. [DOI] [PubMed] [Google Scholar]
- 9.Olsen MA, Sundt TM, Lawton JS, Damiano RJ, Jr, Hopkins-Broyles D, Lock-Buckley P, et al. Risk factors for leg harvest surgical site infections after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2003;126:992–999. doi: 10.1016/s0022-5223(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee ES, Santilli SM, Olson MM, Kuskowski MA, Lee JT. Wound infection after infrainguinal bypass operations: multivariate analysis of putative risk factors. Surg Infect (Larchmt) 2000;1:257–263. doi: 10.1089/109629600750067183. [DOI] [PubMed] [Google Scholar]
- 11.Richet HM, Chidiac C, Prat A, Pol A, David M, Maccario M, et al. Analysis of risk factors for surgical wound infections following vascular surgery. Am J Med. 1991;91:170S–172S. doi: 10.1016/0002-9343(91)90364-4. [DOI] [PubMed] [Google Scholar]
- 12.Goshima KR, Mills JL, Sr, Hughes JD. A new look at outcomes after infrainguinal bypass surgery: traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. J Vasc Surg. 2004;39:330–335. doi: 10.1016/j.jvs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz ME, Harrington EB, Schanzer H. Wound complications after in situ bypass. J Vasc Surg. 1988;7:802–807. doi: 10.1067/mva.1988.avs0070802. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Cogbill TH, Strutt PJ, Gundersen AL. Wound complications after infrainguinal bypass. Classification, predisposing factors, and management. Arch Surg. 1988;123:859–862. doi: 10.1001/archsurg.1988.01400310073012. [DOI] [PubMed] [Google Scholar]
- 15.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 16.Ehsan A, Mann MJ, Dell’Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121:714–722. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 17.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Namini H, Seely L. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg. 2005;42:456–464. doi: 10.1016/j.jvs.2005.05.001. discussion 464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–687. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–983. doi: 10.1016/j.jvs.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen LL, Lipsitz SR, Bandyk DF, Clowes AW, Moneta GL, Belkin M, et al. Resource utilization in the treatment of critical limb ischemia: the effect of tissue loss, comorbidities, and graft-related events. J Vasc Surg. 2006;44:971–975. doi: 10.1016/j.jvs.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer C, Shearer MJ, Zittermann A, Bolton-Smith C, Szulc P, Hodges S, et al. Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr. 2004;43:325–335. doi: 10.1007/s00394-004-0480-4. [DOI] [PubMed] [Google Scholar]
- 22.Johnson WC, Williford WO. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg. 2002;35:413–421. doi: 10.1067/mva.2002.121847. [DOI] [PubMed] [Google Scholar]
- 23.Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998;28:446–457. doi: 10.1016/s0741-5214(98)70130-2. [DOI] [PubMed] [Google Scholar]
- 24.Dorffler-Melly J, Buller HR, Koopman MM, Prins MH. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD000536. CD000536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.