Abstract
In this work, we report an electrochemical surface plasmon resonance/waveguide (EC-SPR/waveguide) glucose biosensor, which could detect enzymatic reactions in a conducting polymer/glucose oxidase (GOx) multilayer thin film. In order to achieve a controlled enzyme electrode and waveguide mode, GOx (negatively charged) was immobilized with a water-soluble conducting N-alkylaminated polypyrrole (positively charged) using the layer-by-layer (LbL) electrostatic self-assembly technique. The electrochemical and optical signals were simultaneously obtained from the composite LbL enzyme electrode upon addition of glucose as mediated by the electroactivity and electrochromic property of the polypyrrole layers. The signal enhancement in the EC-SPR detection is obtained by monitoring the doping-dedoping events on the polypyrrole. The real time optical signal could be distinguished between the change in the dielectric constant of the enzyme layer and other non-enzymatic reaction events such as adsorption of glucose and change of refractive index of solution. This was possible by a correlation of both the SPR mode, m=0, and m=1 mode of the waveguide in an SPR/waveguide spectroscopy experiment.
Keywords: surface plasmon, glucose, biosensor, conducting polymer, enzyme, waveguide
Introduction
Electrically conducting and π-conjugated polymers have been shown to be highly sensitive materials for monitoring and mediating enzymatic interactions. This is attributed to the high conductivity and specific electrochemical redox properties of conducting polymers, which can be paired with redox events present in enzymatic reactions.1,2,3 For example, the redox property of conducting polymers changes upon reduction of the enzyme glucose oxidase (GOx). This change can then be detected either as an electrochemical signal or an optical signal. Upon introduction of glucose, glucose oxidase is reduced and glucose converted to gluconic acid (gluconolactone). In conventional glucose sensors without conducting polymer mediators, the reduced glucose oxidase drives the conversion of O2 to H2O2, in the presence of O2,. The H2O2 can then undergo oxidation to give H2O. On the other hand, in the case of conducting polymer mediators, the oxidation of a reduced chemical specie/polymer material can be directly measured by an electrode surface (see supporting information, SI Figure 1). Thus, electroactive conducting polymers can be considered as “wiring” materials for the electrochemical activation of redox enzymes.4 Redox enzymes usually have poor direct electrical conductivity contact and communication with metallic electrodes. An electroactive conducting polymer provides the matrix for electron transfer from the enzyme to the electrode, correlated with changes in the electrochemical (I-V) signal.
The surface plasmon resonance (SPR) optical technique is a powerful tool for characterizing dielectric properties of surfaces, interfaces, and thin films. In bio-sensor applications, this technique allows for investigation of biomolecular adsorption/desorption events at surfaces and provides in-situ time dependent surface coverage measurements without the need for labeling. We have previously demonstrated the electrochemical-SPR (EC-SPR) technique for the characterization of conducting polymer thin films including polyaniline, PEDOT, polyvinylcarbazole, and polythiophenes.5–9 In EC-SPR measurements, the gold substrate that carries the optical surface mode is simultaneously used as the working electrode in a standard three-electrode electrochemical experiment. One of the advantages in using the EC-SPR technique is that the electrochemical and optical properties are simultaneously obtained on surfaces and ultrathin films at the nanometer scale. This involves the in-situ monitoring of the film thickness and electrochromic properties during the anion doping/de-doping process of deposited conducting polymers. More recently, we have demonstrated the in-situ electrochemical - surface plasmon spectroscopy - atomic force microscopy (EC-SPS-AFM) technique for “real time” dynamic and simultaneous acquisition of the dielectric (optical), surface morphological, and electrochemical data of a depositing conducting polymer thin film at the electrode/electrolyte interface.11 This enabled differentiation of cyclic voltammetry (CV) and potentiostatically (chronoamperometrically) deposited films between their dielectric, electrochemical, and morphological properties in real time.
However, there are not too many examples of laser-excited evanescent wave SPR techniques for glucose sensing,11 as compared to the quartz crystal microbalance (QCM) or electrochemical –QCM techniques.12 One of the reasons for this is that for SPR, it is not easy to distinguish between changes in thickness, dielectric constant of the layer, adsorption of glucose, and refractive index change of buffer, i.e. the real-time event is sensitive to both enzymatic and non-enzymatic reactions.13 Another is the poor stability of the composite conducting polymer/GOx enzyme film that is prepared in-situ.17 This has been commonly done by direct in-situ anodic pyrrole electropolymerization with GOx at the electrode surface.16 In this work, we have endeavored to apply a combined EC-SPR and waveguide approach (Figure 1) to enable better differentiation of these factors. First, a water-soluble, positively charged N-alkylaminated polypyrrole was synthesized for pairing with the negatively charged GOx. The composite enzyme electrode was fabricated by the assembly of the polypyrrole and the GOx via the layer-by-layer (LbL) electrostatic self-assembly technique.14,20 Compared to the as deposited LbL film, it was observed that an enhancement in sensitivity for an SPR/amperometric sensor can be achieved by taking advantage of the doping-dedoping properties of these films in a neutral buffer solution condition. Since the LbL film can be homogeneously assembled, dips from both SPR and waveguide were obtained when thicker films were fabricated. By doing the measurement using the m=0 and m=1 modes in an SPR/waveguide spectroscopy experiment, we were able to separate the signal specific only to the GOx enzymatic activity via changes in the dielectric constant. Compared to QCM measurements, this set-up allows one to distinguish the contribution of the GOx enzymatic activity from non-specific adsorption and/or changes in the subphase dielectric constant.12 Thus we have harnessed the EC-SPR and waveguide techniques for demonstrating a glucose-biosensor with possible applications for other enzymatic biological opto-electronic differentiation.
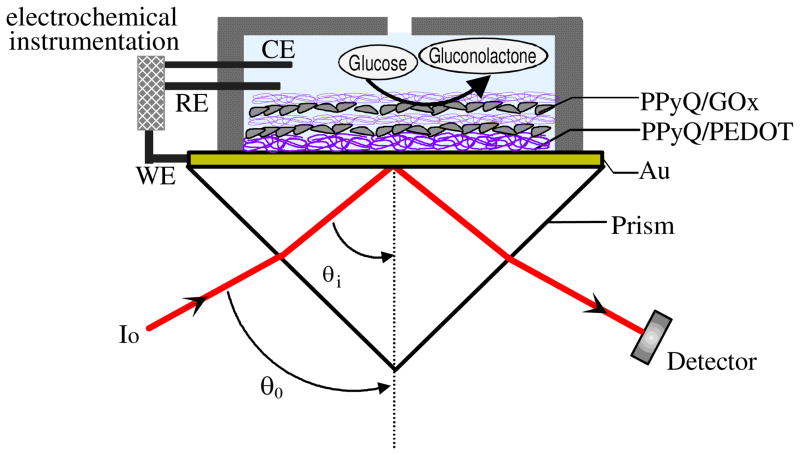
Figure 1.
An EC-SPR glucose biosensor schematic diagram in a Kretschmann configuration with a three-electrode cell set-up
Experimental Details
Materials
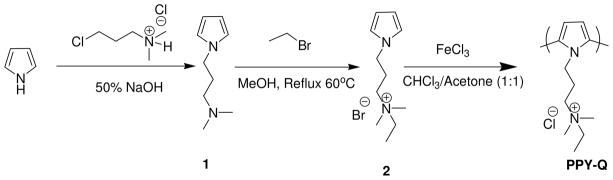
Glucose oxidase (GOx: from Aspergillus niger) was obtained from Aldrich and used immediately. Scheme 1 illustrates the synthesis route for the water-soluble polypyrrole (PPY-Q) or poly(N,N-dimethyl ethyl-3-(1H-pyrrol-1-yl)propan-1-ammonium chloride). The compound 2, which is a precursor for the synthesis of PPY-Q was also found to be water soluble. The synthetic details and characterization data are mentioned in the supporting information and the procedure previously reported elsewhere.15 The polymer PPY-Q was also found to be soluble in organic solvents such as acetone, THF, DMSO, and DMF. The PEDOT: PSS aqueous solution was made by mixing 50ml Milli-Q water and 5ml PEDOT: PSS colloid suspension (available from BAYTRON P, Bayer Corporation) after filtration using a syringe driven filter “Millex” with diameter 0.70 μm (Millipore). Poly(dially dimethyl ammonium chloride) or PDADMAC (from Aldrich) solution was prepared using Milli-Q water with a concentration of 2 mg/ml. 3-mercapto-1-propanesulfonic sodium was also obtained from Aldrich and used as received. Adsorption and sensing was done on Phosphate Buffered Saline (PBS) at pH~ 7, a water-based salt solution containing sodium chloride, sodium phosphate, and/or potassium chloride and potassium phosphate.
Scheme 1.
Synthesis scheme for the water soluble polypyrrole, PPY-Q.
Layer-by-Layer Adsorption
The LbL adsorption of the polyanion and the polycation was performed following the popular multilayer electrostatic deposition approach.16,17 The gold surface of the flat solid substrate was functionalized by immersing the slide for 90 min. in an ethanol solution of 3-mercapto-1-propanesulfonic sodium salt (0.2 mg/ml) (followed by rinsing), creating a uniformly charged (negative) substrate surface. The gold film with a thickness of ca. 50 nm, was deposited by vacuum evaporation onto an LaSFN9 glass slide. The Au/glass or glass substrates with the functionalized surfaces were alternately immersed for 15 minutes in PBS at pH~ 7, buffer solutions of the polycation and the polyanion (or glucose oxidase) until the desired number of layers was achieved. The glucose oxidase was stored in refrigerator at 4°C and used immediately for the LbL fabrications for EC-SPR experiment. For EC-SPR/waveguide experiments, glucose oxidase was used at 25°C, and the immersion time was increased up to several hours. Rinsing with PBS (pH 7.4) was done between depositions.
Electrochemical Measurement
Electrochemical experiments were performed in a conventional 3-electrode cell with the Au/glass substrate as the working electrode, a platinum wire as the counter electrode, and an Ag/AgCl (3 M NaCl) reference electrode. A potentiostat (Princeton Applied Research, PARSTAT 2263A) was used for the cyclic voltammetry or amperometric biosensor experiments.
Electrochemical-SPR
Figure 1 shows the attenuated total reflection (ATR) setup used for the excitation of surface plasmons in the Kretschmann configuration combined with an electrochemical cell. A triangular LaSFN9 prism was used.17 The Au/glass substrates were clamped against the Teflon cell with an O-ring providing a liquid-tight seal. The Teflon cell was then mounted to the 2-axis goniometer for investigations by SPR. Surface plasmons are excited at the metal-dielectric interface, upon total internal reflection of a p-polarized laser light beam. A He-Ne laser (λ=632.8 nm) was used in all measurements. The optical/electrochemical processes on the gold were detected by monitoring the reflectivity either as a function of the incident angle θ0.or at a fixed incident angle. WINSPALL program was used for modeling (thickness and dielectric constant determination)
Results and Discussion
Fabrication of LbL film
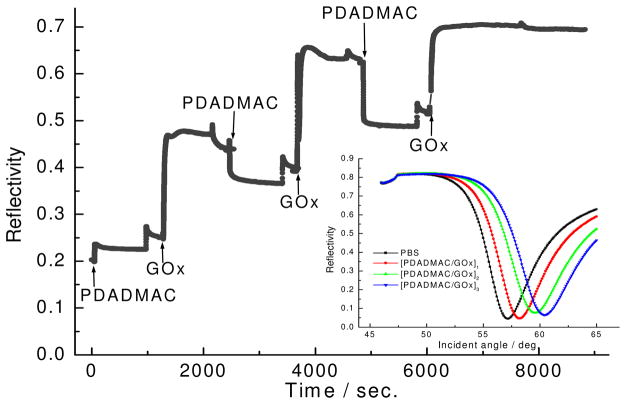
In order to fabricate a controlled enzyme electrode and to investigate the sensing properties without conducting polymers, GOx (−) was first immobilized with the non-electrically conducting PDADMAC (+) polyelectrolyte using the LbL technique. Figure 2 shows the reflectivity change measured in situ by SPR during the multilayer fabrication process. The inset shows angular scans taken after the deposition of each bilayer. As shown in this figure, a monotonous reflectivity increase with increasing number of layers indicates a regular and step deposition of PDADMAC/GOx layers. One can see that for each bilayer, the reflectivity change caused by GOx is much larger than the PDADMAC, indicating a larger amount of deposition of GOx. The decrease of the reflectivity during the deposition process of PDADMAC indicates some amount of desorption of GOx during this process. The thickness obtained by Fresnel modeling is 1 nm for PDADMAC and 9 nm for GOx.
Figure 2.
SPR kinetic curve and angular scans during LBL deposition. Inset is the reflectivity-angular scan for each deposited layer.
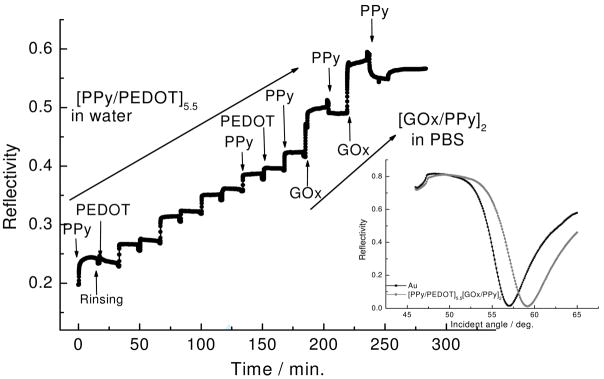
The conducting polymer/enzyme electrode was then fabricated for optimum electro-activity (see also SI Figure 2). In this case, it was necessary to first deposit PPy-Q (+) and PEDOT:PSS (−) layers on the Au electrode in order to enhance the electron transfer between the glucose and Au electrode. Figure 3 shows the deposition of 5.5 bilayers of PPy-Q and PEDOT:PSS followed by the deposition of 2 bilayers of PPy-Q and GOx in PBS. The inset shows the angular scan taken before and after the entire deposition. A linear increase of the reflectivity was observed, which indicates homogeneous deposition for the PPy-Q/PEDOT:PSS bilayer system (2.3 nm thickness/bilayer). Although some amount of desorption of GOx was again observed during the deposition of PPy-Q, a more linear increase of the PPy-Q/GOx was also observed. One can see that the reflectivity is stable after each deposition of PPy-Q/GOx (~6 nm thickness) pairs, indicating that stable immobilization of GOx was essentially obtained with the conducting polymers using the LbL deposition technique.
Figure 3.
SPR kinetic curve and angular scan during LBL deposition of the composite layers. Inset is the reflectivity-angular scan for each deposited layer.
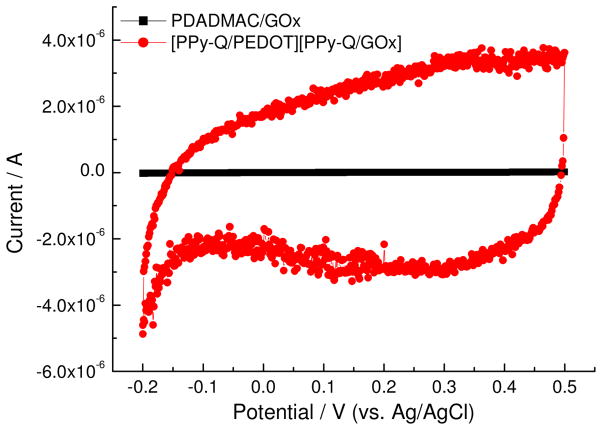
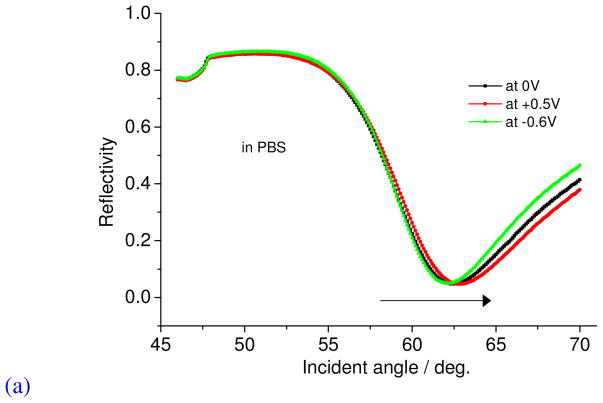
The electroactivity between the two films was then compared in electrolyte buffer solution and the doping-dedoping events monitored. In principle, electroactivity should involve current increase or decrease with CV scanning indicating the transport of ions and electron-transfer (see Figure SI 3). Figure 4 shows the cyclic voltammograms of the PDADMAC/GOx functionalized electrode and PPy-Q/PEDOT:PPSS-PPy-Q/GOx functionalized electrode in PBS (pH 7.4) between −0.2 and 0.5 V. As shown in this figure, the PPy-Q/PEDOT:PSS and PPy-Q/GOx functionalized electrode showed a good current cycle (electroactivity) in PBS while PDADMAC/GOx showed little current change in this region. This indicates that the conducting polymer/glucose oxidase electrode can be used at a low potential in neutral solution. It is very important to operate the glucose sensing at potentials lower than 0.3 V in order to exclude the possibility of the non-enzymatic oxidation of glucose and the oxidation of other analytes such as acetaminophen, ascorbic acid, and uric acid.18 These other analytes can be present in real samples, e.g. blood or urine, for laboratory analysis. It should be noted that the glucose sensing at 0.3 V is also not affected by hydrogen peroxide, which depends on the residual oxygen in the cell.4 The SPR reflectivity curves of a PEDOT film prepared at constant potentials, i.e. 0 V, −0.6 V, and +0.5 V are shown in Figure 5a. These shifts in reflectivity are important because it highlights the electrochromic properties of the deposited PPy-Q/GOx films. The angular measurements at each potential were performed 2.5 minutes after changing the potential in neutral solution. The resonance angle of the SPR curves repeatedly changed in going from the doped state to the de-doped state of the polymer film, and from the dedoped state to doped state stability and reproducibility.
Figure 4.
Cyclic voltammetry of functionalized Au electrodes with the two different LbL film compositions.
Figure 5.
(a) SPR angular- reflectivity curves of PPy-Q/PEDOT:PPSS-PPy-Q/GOx film at different applied potentials (b) SPR Glucose Sensing as a function of concentration (time) at different doping states.
EC-SPR experiments for the detection of glucose
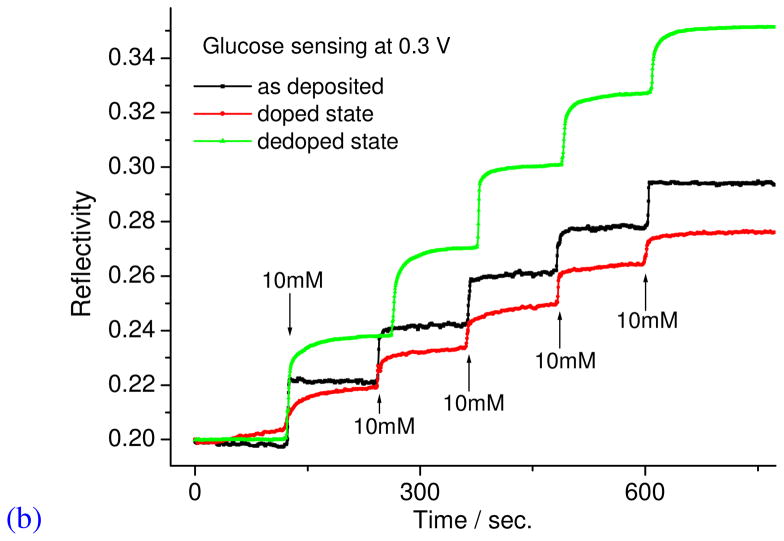
Since the electrochromic property was obtained in neutral solution, it should be possible to carry out glucose sensing after doping, dedoping, and at the as-deposited states as shown in Figure 5b. The sensing was carried out in neutral PBS buffer upon the addition of 10 mM of glucose every 5 minutes. As shown in this Figure, the degree of optical signal change is dependent on the doped/dedoped state film before sensing. From these results it indicates that the doped/dedoped state did not move back even when the potential was set at 0.3 V during the sensing experiment. This is probably because the ions are more difficult to diffuse out the layer-by-layer films in PBS solution. Furthermore, this result indicates that the dedoped state has the highest reflectivity change or is more sensitive for the optical signal. This is not counterintuitive since the polypyrrole film is oxidized in the glucose sensing (reduction) event and thus the dedoped state shows the highest change, i.e. from a dedoped state to a more doped state.11,12 The results obtained also highlights the fact that the change of reflectivity can also be controlled by the doping state of the conducting polymer films. It should be noted that the magnitude of this shift is also a function of the electrochromic behavior of the films and the wavelength of the light source for the ATR experiment.19
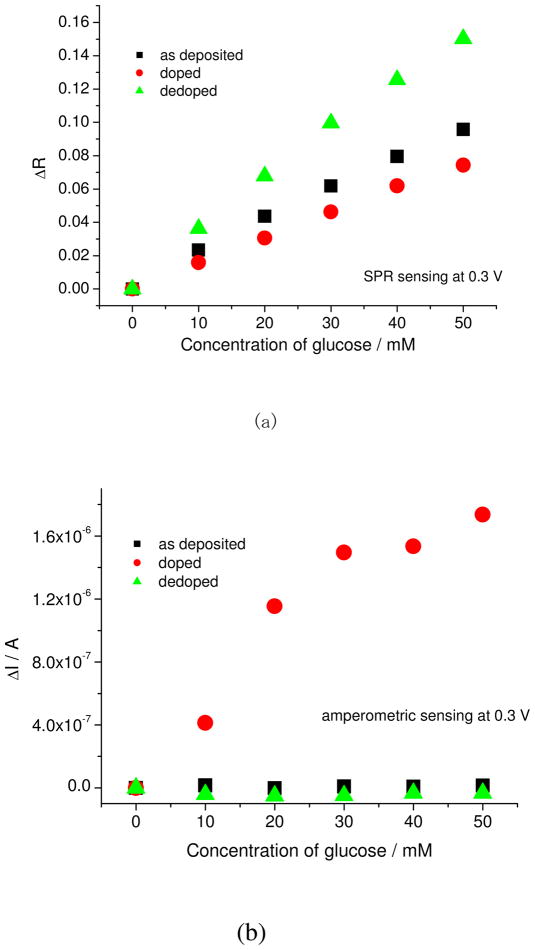
Figure 6 shows the plots of the simultaneous SPR/amperometric glucose sensing. As explained, the dedoped state showed the highest reflectivity change. In the case of amperometric sensing, the dedoped state and as-deposited state showed a smaller change in current, while the doped state showed a very large change in current, i.e. micron ampere. This is because the LbL film at the doped state facilitates better electron transfer from the glucose to the electrode. The sensitivity of doped state was 0.3 μA/10 mM, while the as-deposited state was 2.8 nA/10 mM. This means that the doping state can have a big advantage on the chosen transduction method for sensing, and that amperometric and SPR sensing methods are complementary to each other.
Figure 6.
Plot of amperometric vs SPR sensing with increasing concentration of glucose.
EC-SPR/Waveguide experiments for the detection of glucose
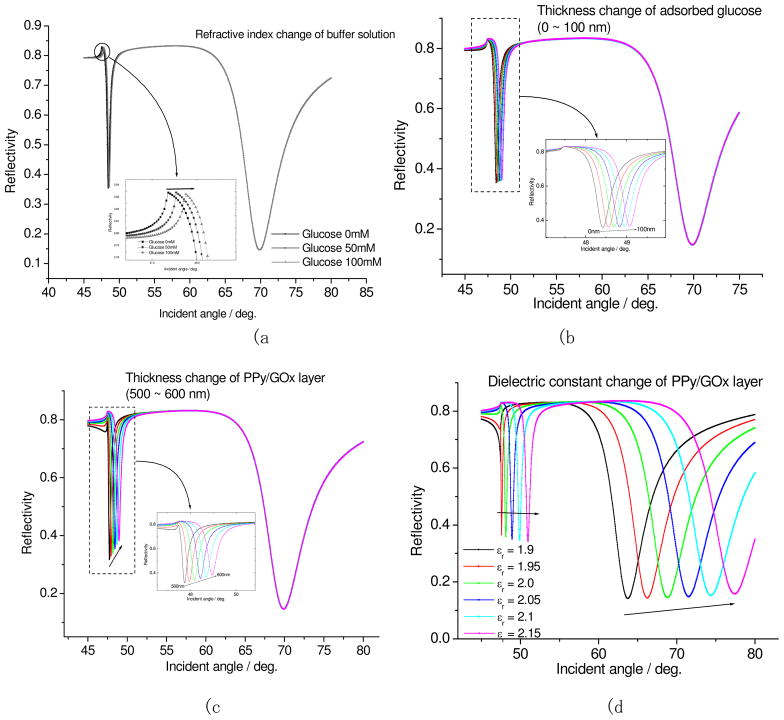
Although much enhancement in the current was obtained by doping, the SPR signal still includes the component of the refractive index change of the solution with addition of glucose. We were able to overcome this problem by utilizing an SPR/waveguide spectroscopy set-up. 19 Figure 7 shows the simulations of the surface plasmon resonance/waveguide mode on assumptions of a refractive index change of the buffer (a), adsorption of glucose (b), thickness change of PPy/GOx layer (c), and dielectric constant change of PPy/GOx layer (d). In this simulation, the thickness of PPy/GOx layer was assumed to be 500 nm (waveguiding). As shown in this Figure, the minimum angle of waveguide mode (m=1 mode) shifts to higher angle in all the cases. On the other hand, in the case of surface plasmon mode (m=0 mode), the minimum angle shifts only to higher angle in the case of a direct dielectric constant change of the PPy/GOx layer. This is because the evanescent field of surface plasmon (~150 nm) is inside the PPy/GOx layer, such that the curve does not depend on the PPy/GOx surface adsorption of glucose, thickness change of PPy/GOx layer, and the refractive index of the buffer. Since the enzymatic reaction is generated upon the addition of glucose, the GOx and PPy are reduced due to the electron transfer event. Hence the signal can be detected as the change of the dielectric constant of PPy/GOx layer. If the waveguide mode produces changes, then this correspondingly includes changes in the thickness of the film. This simulation shows that the SPR/waveguide mode can exclude the possibility of the signal change due to non-enzymatic reactions or simple analyte adsorption (non-specific) if needed. The Reflectivity vs. parameter plots on SI-Figure 4 (supporting information), further highlights the fact that changes in the SPR mode can only be due to the dielectric changes caused by enzymatic activity. This is very important for real glucose sensing because blood plasma can contain not only glucose but also ascorbic acid, uric acid, acetaminophen and other potential interfering analytes. Also, blood plasma can have substantially different refractive indexes depending on a person’s physiological or disease condition.
Figure 7.
Simulation of surface plasmon resonance/waveguide mode with glucose addition in various parameters. (a) refractive index change of buffer solution, (b) thickness change of absorbed glucose, (c) thickness change of PPy/GOx layer, (d) dielectric constant change of PPy/GOx layer.
To demonstrate the simulated waveguiding principles, actual thick films > 500 nm of the composite sensing elements were fabricated. Figure 8 shows the reflectivity angular curves for a 25 bilayer of PPyPEDOT-PPyGOx LbL film in PBS solution at several constant potentials. As shown in this Figure, both SPR (m=0) mode and waveguide (m=1) mode were clearly observed. Since the waveguide can be excited only with a uniform and smooth surface coverage, this indicated that the PPy/LbL film is smooth and formed an ordered structure suitable as a mediator for EC-SPR/waveguide glucose sensing. The SPR dip was sensitively moved toward lower angles when the potential was lower (more negative), while the waveguide mode was almost constant. Based on the model calculations shown in Figure 7, this indicates that the dielectric constant of the LbL film is lowered with decreasing potential (more negative), while the thickness of the film remained constant at each potential. The lowering of the dielectric potential is a result of the dedoping of the conducting polymer layer as no glucose has yet been introduced. This indicates that the information of the dielectric constant and thickness can indeed be separated using this protocol.
Figure 8.
Reflectivity angular scan of PPyPEDOT-PPyGOx on an SPR/Waveguide combined modes at various potentials.
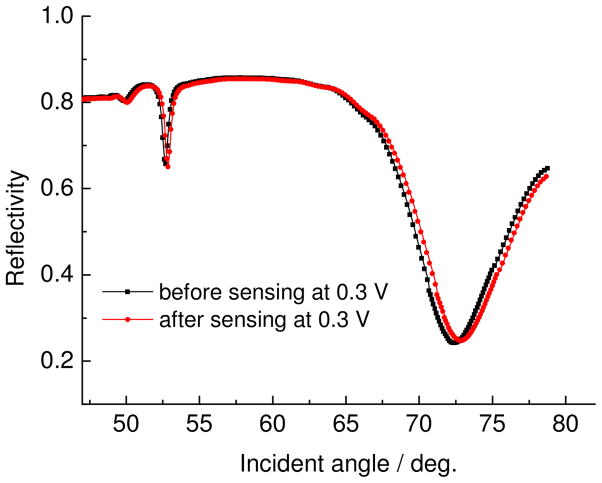
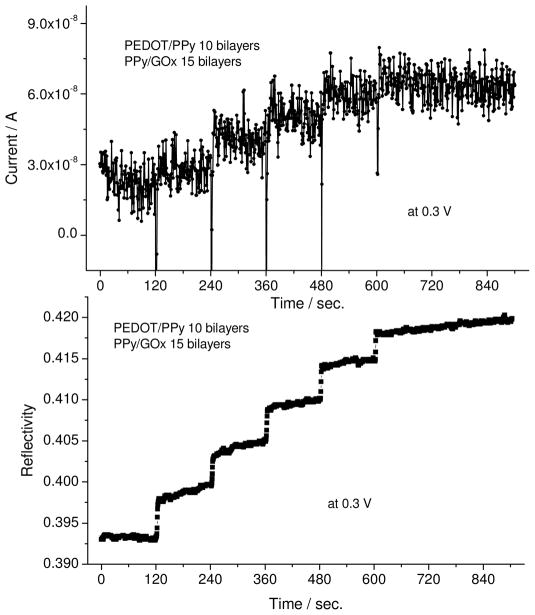
Figure 9 shows the reflectivity-angular scan for 25 bilayers of PEDOT/PPy-PPyGOx LbL films before and after sensing with 10 mM glucose. As shown in this Figure, both the SPR mode and waveguide mode were clearly observed and were shifted. The SPR minimum angle, m=0, and the waveguide mode, m=1, shifted to a slightly higher angle after addition of glucose at 0.3 V. First of all, this indicated that the enzyme showed high activity with the addition of glucose within the PPY matrix. The shift in evanescent optical properties (dielectric constant) of the SPR mode is a consequence of change in electrochemical activity of the PPY present with GOx/glucose enzymatic activity. The dielectric change followed the digestion of the glucose substrate by the GOx enzyme. This can be explained by an increase in the dielectric constant as a function of increased oxidation state of the conducting polymer with the formation of gluconate species. The thickness increase can be attributed to the incorporation of glucose in to the film. This means that the accompanying activity of the enzyme resulted in a non-negligible thickness change (as in the case if it were just non-specific adsorption on the surface) because of incorporation of glucose inside the film. Based on modeling with WINSPALL, this thickness change is about 5 nm. To verify this digestion by the enzyme, direct SPR/amperometric measurements were followed with incremental addition of glucose. Figure 10 shows the simultaneous observation of SPR reflectivity change and current change upon successive addition of 10 mM glucose. Both the reflectivity and the current increased when increments of 10 mM glucose was added into buffer solution. This stepwise increase in current and reflectivity indicate that glucose is continually “sensed” without blockage or non-specific adsorption on the surface, although no saturation experiments were performed. This may well contain some pairing of the buffer ions (chloride and phosphonate) on the conducting polymer,7,8 as the enzyme activity leads to a greater oxidized state of the conducting polymers (doping). In general, these films were very robust during the whole duration of these experiments. Further long-term stability studies may be necessary for evaluating their practical device applications.
Figure 9.
Angular SPR curves Before and After sensing of glucose at 0.3V and 10 concentration of glucose.
Figure 10.
Simultaneous observation of current (above) and SPR reflectivity (below) incremental addition of glucose.
Conclusion
In this work, we have demonstrated the feasibility and practical aspect of utilizing the EC-SPR and waveguide mode spectroscopy for glucose sensing using a water soluble N-alkylaminated polypyrrole as mediating layers in a composite GOx enzyme electrode. The fact that LbL electrostatic assembly technique can be utilized indicates the versatility of the process and the ease of future preparation for other enzymes and conducting polymers. The electrochemical and optical signals were obtained from the composite LbL enzyme electrode upon addition of glucose because the electroactivity and electrochromic property of polypyrrole film (absent with the PDADMAC layer) enhanced signaling. This was essentially due to the doping-dedoping activity of the polypyrrole mediator. Furthermore, the use of an electrochemical surface plasmon resonance/waveguide (EC-SPR/waveguide) set-up was found to be key in distinguishing enzymatic activity from changes in the film thickness and the subphase dielectric constant. This enabled real time optical signal change to be separated from the change of dielectric constant of the enzyme layer and the changes from non-enzymatic reactions such as adsorption of glucose and change of refractive index of the solution. This was done by analyzing both the SPR mode, m=0 and m=1 mode of waveguide in SPR/waveguide spectroscopy at various parameters and comparing their modeled behavior. In the future, this protocol should be useful for other types of enzymatic based sensors.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the partial funding from the National Science Foundation ARRA-CBET-0854979 and Robert A. Welch Foundation, E-1551. A.B. acknowledges the NIH Keck Center Nanobiology Fellowship (NIH Grant No.3 R90 DK071504-03). R.C.A. acknowledges previous support for visits at the Austrian Institute of Technology (AIT) with Prof. Knoll.
Footnotes
Supporting Information Available: Schematic diagrams, Spectroelectrochecxmistry, EC-SPR of multilayers, modeling, synthesis procedure details and 1H NMR. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wang D, Gong X, Heeger PS, Rininsland F, Bazan GC, Heeger AJ. PNAS. 2002;99:49. doi: 10.1073/pnas.012581399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter K, Nilsson R, Inganäs O. Nature Mater. 2003;2:419. doi: 10.1038/nmat899. [DOI] [PubMed] [Google Scholar]
- 3.Wallace GG, Kane-Maguire LAP. Adv Mater. 2002;14:953. [Google Scholar]
- 4.Kros A, Hövell S, Sommerdijk N, Nolte R. Adv Mater. 2002;13:1555. [Google Scholar]
- 5.Baba A, Advincula RC, Knoll W. Novel Methods to Study Interfacial Layers. In: Möbius D, Miller R, editors. Studies in Interface Science. Vol. 11. Elsevier Science; 2001. p. 55. [Google Scholar]
- 6.Raitman O, Katz E, Willner I, Chegel V, Popova G. Angew Chem Int Ed. 2001;40:3649. doi: 10.1002/1521-3773(20011001)40:19<3649::aid-anie3649>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Baba A, Advincula RC, Knoll W. J Phys Chem B. 2002;106:1581. [Google Scholar]
- 8.Baba A, Park MK, Advincula RC, Knoll W. Langmuir. 2002;18:4648. [Google Scholar]
- 9.Chegel V, Raitman O, Katz E, Gabai R, Willner I. Chem Commun. 2001;883 [Google Scholar]
- 10.Kang X, Jin Y, Chen G, Dong S. Langmuir. 2002;18:1713. [Google Scholar]
- 11.Baba A, Knoll W, Advincula R. Rev Sci Instr. 2006;77:064101. [Google Scholar]
- 12.Calvo EJ, Forzani E, Tero M. J Electroanal Chem. 2002;538–539:231. [Google Scholar]
- 13.(a) Raitman O, Katz E, Bückmann AF, Willner I. J Am Chem Soc. 2002;124:6478. [Google Scholar]; (b) Hsieh H, Pfeiffer Z, Amiss T, Sherman D, Pitner B. Biosensors and Bioelectronics. 2004;19:653. doi: 10.1016/s0956-5663(03)00271-9. [DOI] [PubMed] [Google Scholar]; (c) Iwasaki Y, Horiuchi T, Niwa O. Anal Chem. 2001;73:1595. doi: 10.1021/ac0012851. [DOI] [PubMed] [Google Scholar]; (d) Lam W, Chua L, Wong C, Zhang Y. Sensors and Actuators B: Chemical. 2005;105:138. [Google Scholar]
- 14.(a) Hodak J, Etchenique R, Calvo EJ, Singhal K, Bartlett PN. Langmuir. 1997;13:2708. [Google Scholar]; (b) Constantine CA, Gattás-Asfura KM, Mello SV, Crespo G, Rastogi V, Cheng TC, DeFrank JJ, Leblanc RM. Langmuir. 2003;19:9863. [Google Scholar]; (c) Ferreira M, Fiorito PA, Oliveira ON, Torresi SIC. Biosensors and Bioelectronics. 2004;19:1611. doi: 10.1016/j.bios.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 15.(a) Yasuzawa M, Kunugi A. Electrochemistry Communications. 1999:459–462. [Google Scholar]; (b) Werner H, Raden D, Murty D. J Org Chem. 1956:896–898. [Google Scholar]
- 16.(a) Decher G, Hong JD. Ber Bunsen-Ges Phys Chem. 1991;95:1430. [Google Scholar]; (b) Decher G, Hong JD. Makromol Chem Makromol Symp. 1991;46:321. [Google Scholar]
- 17.Advincula R. IEICE Transactions on Electronics. 2000;7:1104. [Google Scholar]
- 18.(a) Umana M, Waller J. Anal Chem. 1986;58:2979–2983. [Google Scholar]; (b) Bélanger D, Nadreau J, Fortier G. J of Electroanalytical Chem. 1989;274:143. [Google Scholar]; (c) Foulds N, Lowe C. J Chem Soc, Faraday Trans 1. 1986;82:1259. [Google Scholar]; (d) Bélanger D, Fortier G. Biotechnology and Bioengineering. 2004;37:854. doi: 10.1002/bit.260370909. [DOI] [PubMed] [Google Scholar]; (e) Yasuzawa M, Nieda T. Sensors and Actuators B: Chemical. 2000;66:77. [Google Scholar]
- 19.Aulasevich A, Roskamp R, Jonas U, Menges B, Dostalek K, Knoll W. Macromol Rapid Commun. 2009;30:872. doi: 10.1002/marc.200990017. [DOI] [PubMed] [Google Scholar]
- 20.Ram M, Adami M, Paddeu S, Nicolini C. Nanotechnology. 2000;11:112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.