Abstract
Background. Redox active substances (e.g., Thioredoxin-1, Macrophage Migration Inhibitory Factor) seem to be central hubs in the septic inflammatory process. Materials and Methods. Blood samples from patients with severe sepsis or septic shock (n = 15) were collected at the time of sepsis diagnosis (t0), and 24 (t24) and 48 (t48) hours later; samples from healthy volunteers (n = 18) were collected once; samples from postoperative patients (n = 28) were taken one time immediately after surgery. In all patients, we measured plasma levels of IL-6, TRX1 and MIF. Results. The plasma levels of MIF and TRX1 were significantly elevated in patients with severe sepsis or septic shock. Furthermore, TRX1 and MIF plasma levels showed a strong correlation (t0: r sp = 0.720, ρ = 0.698/t24: r sp = 0.771, ρ = 0.949). Conclusions. Proinflammatory/~oxidative and anti-inflammatory/~oxidative agents show a high correlation in order to maintain a redox homeostasis and to avoid the harmful effects of an excessive inflammatory/oxidative response.
1. Introduction
Severe sepsis, septic shock, and the resulting multiple organ failure/dysfunction syndrome represent an ongoing challenge in intensive care units [1–5]. With mortality ranging from 40% to 70%, septic shock is the most common cause of death in intensive care medicine [2, 6]. Despite intensive basic research and clinical studies, the pathophysiology of sepsis is still poorly understood. Inflammation leads to oxidative stress because of the production of reactive oxygen species (ROS). Oxidative stress is a major contributing factor to the high mortality rates associated with several diseases such as endotoxic shock. Immune cells therefore need adequate levels of antioxidant defenses in order to avoid harmful effects of an excessive ROS production and to keep a well-balanced redox homeostasis. In this context, two substances have recently become of great interest: (1) Macrophage Migration Inhibitory Factor (MIF), a proinflammatory protein, which is released by immune cells and shows elevated levels in sepsis syndrome, as well as (2) Human Thioredoxin-1 (TRX1), a potent antioxidant that modulates inflammation, cell growth, and apoptosis, which seems to counteract the proinflammatory and pro-oxidant effects of MIF [7–9]. Therefore, these two biomarkers appear to be central hubs in the inflammatory setting and are therefore of great interest. However, no sufficient knowledge exists about the role of these key mediators in severe sepsis or septic shock. The aims of this study were therefore twofold: (1) to assess the plasma levels of each parameter in different inflammatory settings (healthy volunteers, postoperative, and septic patients) and (2) to establish whether plasma levels of TRX1 and MIF correlate with each other.
2. Materials and Methods
The observational clinical study was approved by the local ethics committee and was conducted in the surgical and medical intensive care units of the University Hospitals of Heidelberg and Mannheim, Germany. All study and control patients or their legal designees signed written informed consent. In total, 61 individuals in three groups were enrolled in the study (Table 1). The three groups included 15 patients with severe sepsis or septic shock (referred to as the septic group), 28 patients after major abdominal surgery (the postoperative group), and 18 healthy volunteers (the volunteer group). The 15 patients were classified as having severe sepsis or septic shock based on the criteria of the International Sepsis Definitions Conference [10]. Patients were eligible for enrollment with an onset of sepsis syndrome ≤24 hours. The initial blood draw was also performed within this period. In contrast, patients with an onset of sepsis syndrome >24 hours were excluded from the study. The management of patients with severe sepsis or septic shock in the intensive care unit included early goal-directed therapy (according to Rivers and colleagues [11]), elimination of the septic focus, and broad-spectrum antibiotics [12, 13]. The second group included 28 patients undergoing major abdominal surgery, with negative parameters for systemic inflammatory response syndrome (Table 1). As a control group, we chose 18 healthy young volunteers without any signs of infection (Table 1). Blood samples from patients with severe sepsis were collected within 24 hours after the diagnosis of sepsis, and also 24 and 48 hours later. In the septic group, severity of illness was estimated using the Acute Physiology and Chronic Health Evaluation (APACHE) II score as well as the Sequential Organ Failure Assessment (SOFA) score and the Simplified Acute Physiology Score (SAPS) II. Patients with sepsis were re-evaluated for survival 90 days after enrollment in the study. This evaluation was performed using available hospital records. In case of the patient's discharge from the hospital, the family doctor was contacted. If necessary, we furthermore got in contact with the patient himself. Blood samples from the postoperative group were collected once immediately after surgery, and the samples from the volunteer group were taken one time. After blood collection, plasma of all study participants was immediately obtained by centrifugation, transferred into cryotubes, and stored at −80°C until further processing. Human Interleukin-6 (IL-6) was measured in order to determine the ongoing inflammatory response. Furthermore, the activation of the redox system was evaluated. Therefore, we measured plasma levels of Human Thioredoxin-1 (TRX1) and Macrophage Migration Inhibitory Factor (MIF). We used ELISA kits to determine plasma concentrations of Interleukin-6 (IL-6, R&D Systems, Minneapolis, MN, USA), Human Thioredoxin-1 (Human TRX1, LabFrontier Co., Ltd, Seoul, Korea), and Macrophage Migration Inhibitory Factor (Human MIF, RayBiotech, Inc., Norcross, GA, USA) according to the manufacturers' instructions.
Table 1.
Baseline data of 15 patients in the septic group, 28 patients in the postoperative group, and 18 individuals in the volunteer group.
| Septic group | |
|---|---|
| Demographic data | |
| Age, y | 61.0 ± 13.7 |
| Male sex | 9 (60.0%) |
| Primary site of infection / septic focus | |
| Lung | 3 (20.0%) |
| Gastrointestinal tract | 6 (40.0%) |
| Genitourinary tract | 1 (6.7%) |
| Surgical site | 3 (20.0%) |
| Other | 2 (13.3%) |
| Outcome | |
| Survivor | 8 (53.3%) |
|
| |
| Postoperative group | |
|
| |
| Demographic data | |
| Age, y | 62.3 ± 14.2 |
| Male sex | 15 (53.6%) |
| Primary site of surgery | |
| Pancreas | 13 (46.4%) |
| Colon | 5 (17.9%) |
| Liver | 2 (7.1%) |
| Genitourinary | 3 (10.7%) |
| Other abdominal | 5 (17.9%) |
|
| |
| Volunteer group | |
|
| |
| Demographic data | |
| Age | 34.5 ± 8.6 |
| Male sex | 10 (55.6%) |
Data are presented by number (%), except for age (mean ± standard deviation).
All assays were performed in duplicate. The resulting study data were entered into an electronic database (Microsoft Excel 2002, Microsoft Corporation, Redmond, WA, USA) and evaluated using SPSS software (Statistical Product and Services Solutions, Version 16.0, SPSS Inc, Chicago, IL, USA). Categorical data were summarized by means of absolute and relative frequencies (counts and percentages). Quantitative data were summarized using the number of observations, mean and standard deviation, minimum, median with quartiles, or differences of the quartiles and maximum. Wherever appropriate, data were visualized using box-and-whisker plots. The Kolmogorov-Smirnov test was applied to check for normal distribution. Due to abnormally distributed data, nonparametric methods for evaluation were used (chi-square test for categorical data, Mann-Whitney U-test as well as Wilcoxon test for continuous data). Correlation analysis was performed using two-sided Spearman's rank correlation test as well as Pearson's product-moment correlation test. A P value < .05 was considered statistically significant. Concerning symbolism and higher orders of significance: P < .05:*, P < .01:**, P < .001:***.
3. Results
Age and sex of patients in the septic (61 years; 9 male sex) and postoperative (62 years; 15 male sex) groups were comparable (Table 1). In the septic group, patients who survived or died showed no significant differences in their demographic data (data not shown). In contrast, healthy volunteers (35 years; 10 male sex) were significantly younger compared with the septic and postoperative groups (Table 1). In the septic group, 8 of 15 patients (53.3%) survived (Table 1). No one in the postoperative or volunteer groups died during the study. The primary site of infection in the septic group was the gastrointestinal tract (6 patients, 40.0%). Furthermore, the septic focus was found to be in the respiratory tract (3 patients, 20%) or dedicated as a surgical complication (3 patients, 20%) (Table 1). A positive culture from the site of infection was obtained in 67% of all septic patients. In these patients, cultures were found to be gram-negative in 70% and gram-positive in 30%. Patients in the postoperative group primarily underwent surgery of the pancreas, whereas surgeries of the colon, liver, and the genitourinary tract were less frequent (Table 1).
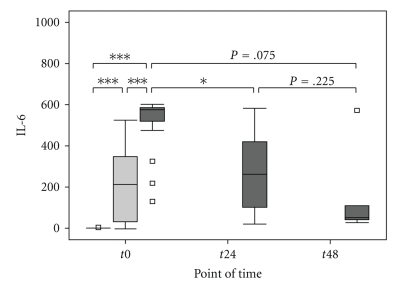
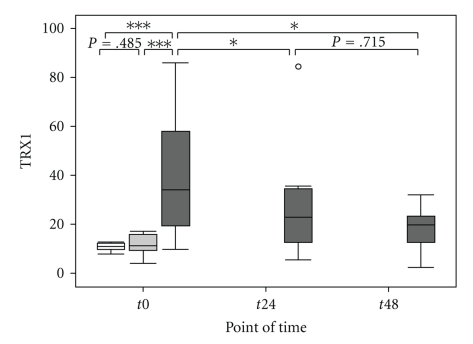
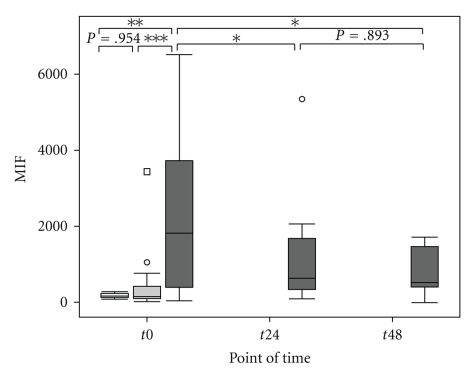
Septic patients were considered to be severely injured during the entire study period, as assessed by the APACHE II, SOFA, and SAPS II score, but showed no significant differences between the surviving and nonsurviving subgroups of septic patients (Table 2). Plasma levels of IL-6 were significantly elevated at the onset of sepsis compared with the postoperative and the volunteer groups (Figure 1 and Table 3). Furthermore, plasma levels of IL-6 were significantly elevated in the postoperative group compared with healthy volunteers (Figure 1 and Table 3). In the septic group, the level of IL-6 decreased significantly within 24 hours after sepsis onset (t0 → t24: P = .021*, t24 → t48: P = .225, t0 → t48: P = .075), but still remained significantly higher than the volunteer group (t24: P < .001***, t48: P < .001***) (Figure 1). Il-6 levels did not differ between the surviving and nonsurviving subgroup of septic patients at any time (Table 2). The plasma levels of TRX1 were significantly elevated at the time of diagnosis of sepsis, compared with levels in the postoperative and volunteer groups (Figure 2 and Table 3). TRX1 plasma levels decreased significantly within 48 hours after sepsis onset (t0 → t24 : P = .046*, t24 → t48 : P = .715, t0 → t48 : P = .028*), but still remained significantly elevated than the volunteer group (t24 : P = .114, t48 : P = .042*). In comparison to the postoperative group, TRX1 plasma levels of septic patients failed scarcely to show a significant difference at t24, as well as t48 (t24 : P = .061, t48 : P = .069) (Figure 2). TRX1 plasma levels did not differ between the postoperative and volunteer groups (TRX1: P = .458) (Figure 2 and Table 3). Furthermore, between the surviving and nonsurviving subgroups of septic patients, TRX1 plasma levels did not show any significant difference (Table 2). The plasma levels of MIF were significantly elevated at the time of diagnosis of sepsis, compared with levels in the postoperative and volunteer groups (Figure 3 and Table 3). MIF plasma levels decreased significantly within 48 hours after sepsis onset (t0 → t24: P = .050*, t24 → t48: P = .893, t0 → t48: P = .028*), but still remained significantly elevated than the volunteer group (t24: P = .030*, t48: P = .048*) and the postoperative group (t24: P = .023*, t48: P = .069) (Figure 3). MIF plasma levels did not differ between the postoperative and volunteer groups (MIF: P = .954) (Figure 3 and Table 3). Furthermore, between the surviving and nonsurviving subgroups of septic patients, MIF plasma levels did not show any significant difference (Table 2).
Table 2.
Comparison of IL-6, TRX1, and MIF plasma levels, as well as APACHE II, SAPS II, and SOFA scores in survivors and nonsurvivors in the septic group at baseline and at 24 and 48 hours.
| Survivor (n = 8) | Nonsurvivor (n = 7) | P-value | ||
|---|---|---|---|---|
| IL-6 (pg/ml) | t0 | 578.7; 451.4–579.9 | 576.1; 521.9–581.6 | >.05 |
| t24 | 262.2; 103.4–300.3 | 371.2; 153.6–553.7 | >.05 | |
| t48 | 53.0; 41.0–79.6 | 51.7; 47.4–312.0 | >.05 | |
| P-value | t0-t24-t48 | >.05 | >.05 | |
|
| ||||
| TRX1 (ng/ml) | t0 | 26.8; 14.2–44.7 | 57.5; 22.0–82.2 | >.05 |
| t24 | 25.8; 13.4–47.8 | 22.9; 16.3–28.0 | >.05 | |
| t48 | 12.9; 9.5–16.1 | 23.8; 21.1–27.9 | >.05 | |
| P-value | t0-t24-t48 | >.05 | >.05 | |
|
| ||||
| MIF (pg/ml) | t0 | 1645.8; 345.1–4078.7 | 1821.1; 971.9–3147.6 | >.05 |
| t24 | 624.7; 396.3–1899.5 | 889.0; 432.4–1467.3 | >.05 | |
| t48 | 421.8; 315.3–692.7 | 951.1; 425.8–1469.0 | >.05 | |
| P-value | t0-t24-t48 | >.05 | .05* | |
|
| ||||
| APACHE II (points) | t0 | 43.0; 35.0–45.0 | 33.0; 32.5–37.0 | >.05 |
| t24 | 35.0; 29.5–40.8 | 43.0; 38.8–45.0 | >.05 | |
| t48 | 34.0; 23.5–38.5 | 35.0; 35.0–36.0 | >.05 | |
| P-value | t0-t24-t48 | >.05 | >.05 | |
|
| ||||
| SOFA (points) | t0 | 13.0; 11.0–15.0 | 16.5; 15.0–18.0 | >.05 |
| t24 | 13.0: 11.5–15.5 | 16.5; 14.3–18.0 | >.05 | |
| t48 | 14.0: 12.0–15.0 | 18.0; 9.0–18.0 | >.05 | |
| P-value | t0-t24-t48 | >.05 | >.05 | |
|
| ||||
| SAPS II (points) | t0 | 60.5; 54.8–66.3 | 81.0; 72.3–89.3 | >.05 |
| t24 | 73.0; 60.0–77.0 | 76.5; 66.0–84.5 | >.05 | |
| t48 | 63.0; 57.5–75.5 | 76.0; 71.0–90.0 | >.05 | |
| P-value | t0-t24-t48 | >.05 | >.05 | |
Data are presented by median and interquartile range (Q1–Q3).
Figure 1.
Comparison of Interleukin-6 (IL-6) in the volunteer, postoperative, and septic groups at baseline and at 24 and 48 hours in the septic group. Concentrations of Interleukin-6 (IL-6; (pg/ml)) were measured from the sera of healthy volunteers (“Healthy”, n = 18, white box), postoperative patients after major abdominal surgery (“Post-OP”, n = 28, light grey box), and patients with sepsis (“Sepsis”, n = 15, dark grey box), at t0 (measured once for the volunteer group, immediately after surgery for the postoperative group, and at the time of diagnosis of sepsis for the sepsis group). In addition, for the septic group, the 2 other times of data collection are represented, t24 and t48 for 24 and 48 hours, respectively, after the diagnosis of sepsis. Data in box plots are given as median, 25th percentile, 75th percentile, and the 1.5 interquartile range. Outliers are shown in form of circles (1.5–3 interquartile ranges above 75th percentile or below 25th percentile) or rectangles (>3 interquartile ranges above 75th percentile or below 25th percentile). Abbreviations: IL-6, Interleukin-6.
Table 3.
Comparison of IL-6, TRX1, and MIF plasma levels in the volunteer, postoperative, and septic group at baseline.
| Healthy (n = 18) | Post-OP (n = 28) | Sepsis (n = 15) | |
|---|---|---|---|
| IL-6 (pg/ml) | 0.0; 0.0–0.8 | 216.7; 48.8–360.5 | 577.7; 521.9–583.2 |
| P-value | Healthy versus Post-OP: P < .001*** | ||
| Healthy versus Sepsis: P < .001*** | |||
| Post-OP versus Sepsis: P <.001*** | |||
|
| |||
| TRX1 (ng/ml) | 11.0; 10.0–12.1 | 11.3; 9.6–15.6 | 34.0; 19.7–57.7 |
| P-value | Healthy versus Post-OP: P = .458 | ||
| Healthy versus Sepsis: P < .001*** | |||
| Post-OP versus Sepsis: P <.001*** | |||
|
| |||
| MIF (pg/ml) | 161.6; 148.5–214.0 | 156.4; 115.1–398.7 | 1821.1; 412.2–3708 |
| P-value | Healthy versus Post-OP: P = .954 | ||
| Healthy versus Sepsis: P = .005** | |||
| Post-OP versus Sepsis: P < .001*** | |||
Data are presented by median and interquartile range (Q1–Q3).
Figure 2.
Comparison of Thioredoxin-1 (TRX1) measurements in the volunteer, postoperative, and septic groups at baseline and at 24 and 48 hours in the septic group. Concentrations of Thioredoxin-1 (TRX1; (ng/ml)) were measured from the sera of healthy volunteers (“Healthy,” n = 18, white box), postoperative patients after major abdominal surgery (“Post-OP,” n = 28, light grey box), and patients with sepsis (“Sepsis,” n = 15, dark grey box), at t0 (measured once for the volunteer group, immediately after surgery for the postoperative group, and at the time of diagnosis of sepsis for the sepsis group). In addition, for the septic group, the two other times of data collection are represented, t24 and t48 for 24 and 48 hours, respectively, after the diagnosis of sepsis. Data in box plots is given as median, 25th percentile, 75th percentile, and the 1.5 interquartile range. Outliers are shown in form of circles (1.5-3 interquartile ranges above 75th percentile or below 25th percentile) or rectangles (>3 interquartile ranges above 75th percentile or below 25th percentile). Abbreviations: TRX1, Thioredoxin-1.
Figure 3.
Comparison of Macrophage Migration Inhibitory Factor (MIF) measurements in the volunteer, postoperative, and septic groups at baseline and at 24 and 48 hours in the septic group. Concentrations of Macrophage Migration Inhibitory Factor (MIF; (pg/ml)) were measured from the sera of healthy volunteers (“Healthy,” n = 18, white box), postoperative patients after major abdominal surgery (“Post-OP,” n = 28, light grey box), and patients with sepsis (“Sepsis,” n = 15, dark grey box), at t0 (measured once for the volunteer group, immediately after surgery for the postoperative group, and at the time of diagnosis of sepsis for the sepsis group). In addition, for the septic group, the two other times of data collection are represented, t24 and t48 for 24 and 48 hours, respectively, after the diagnosis of sepsis. Data in box plots is given as median, 25th percentile, 75th percentile, and the 1.5 interquartile range. Outliers are shown in form of circles (1.5–3 interquartile ranges above 75th percentile or below 25th percentile) or rectangles (>3 interquartile ranges above 75th percentile or below 25th percentile). Abbreviations: MIF, Macrophage Migration Inhibitory Factor.
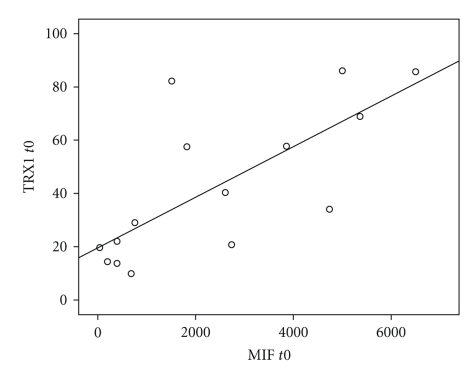
A correlation analysis using two-sided Spearman's rank correlation test, as well as Pearson's product-moment correlation test, indicated a strong correlation between TRX1 and MIF plasma levels in patients with severe sepsis or septic shock especially at the onset of sepsis syndrome (t0: r sp = 0.720, ρ = 0.698) and 24 hours later (t24: r sp = 0.771, ρ = 0.949) (Figure 4). In contrast, between TRX1 and IL-6 plasma levels as well as between MIF and IL-6 plasma levels in septic patients, no significant correlations could be observed at the onset of sepsis (TRX/IL-6, t0: r sp = 0.143, ρ = 0.106; MIF/IL-6, t0: r sp = 0.029, ρ = 0.127) and 24 hours later (TRX/IL-6, t24: r sp = 0.107, ρ = 0.087; MIF/IL-6, t24: r sp = 0.310, ρ = 0.232).
Figure 4.
Strong correlation between TRX1 and MIF plasma levels in patients with severe sepsis or septic shock at the onset of sepsis syndrome. The scatter plot visualizes concentrations of Macrophage Migration Inhibitory Factor (MIF; (pg/ml)) as well as accompanying concentrations of Thioredoxin-1 (TRX1; (ng/ml)) from patients with severe sepsis or septic shock at the onset of sepsis syndrome (t0) (“Sepsis,” n = 15, black circles). Abbreviations: MIF, Macrophage Migration Inhibitory Factor; TRX1, Thioredoxin-1.
4. Discussion
The present study demonstrates that proinflammatory/~oxidative (Macrophage Migration Inhibitory Factor, MIF) as well as anti-inflammatory/~oxidative (Human Thioredoxin-1, TRX1) agents are significantly raised in patients with severe sepsis and septic shock. Positive correlations between these two mediators may suggest a linked role in the pathophysiology of sepsis.
Severe sepsis as well as septic shock and related multiple organ dysfunction syndrome is still the most common cause of death in intensive care medicine [1–6]. Many of the pathophysiological changes during sepsis are related to inflammation [14]. Not surprisingly, different markers of systemic inflammation (e.g., IL-6) and mediators involved in the redox homeostasis (e.g., TRX1, MIF) are significantly elevated during ongoing sepsis [15], whereas only IL-6 differed between patients after major abdominal surgery and healthy volunteers. This reflects generalized infection during sepsis, while patients after major abdominal surgery experience only a mild activation of their inflammatory system [16]. IL-6 is released into the bloodstream after any kind of tissue insult [17]. Therefore, IL-6 is the most commonly described cytokine after surgery. Concentrations of IL-6 peak 24 hours after the surgical procedure and return to the baseline value in a few days in noninfected patients [18]. In accordance to our investigation, IL-6 peak concentrations are reported to be generally in the range of 200–300 pg/ml [19]. The highest IL-6 serum/plasma concentrations are reached during sepsis, therefore it appears to be a good marker of the severity of infection [20, 21]. (Human) Thioredoxin-1 (TRX1) is an anti-inflammatory agent, whose anti-inflammatory effects are not yet completely understood. TRX1 is a redox-sensitive molecule that has pleiotropic cellular effects, functioning as a protective cellular antioxidant and regulator of transcription factor activity [22–27]. Together with the TRX-reductase and NADPH, TRX1 represents a major intracellular reducing agent containing a redox-active disulfide/dithiol within the conserved active site sequence Cys-Gly-Pro-Cys, and therefore protecting cells from oxidative stress [22–27]. Beside these antioxidative effects, TRX1 plays an important role in the modulation of the immune system by regulating DNA binding of several transcription factors (e.g., p53, NFkappaB, activator protein-1) [28–30]. Several investigations have shown that extracellular levels of TRX1 are increased in conditions of oxidative stress and inflammation [7, 30–39]. In accordance to these investigations, the present study was able to demonstrate that plasma TRX1 levels were significantly higher in patients with sepsis compared to healthy volunteers and postoperative patients. The elevated plasma levels of TRX1 then most likely reflect the increased oxidative stress in septic patients. In the literature, it was demonstrated that increased TRX1 plasma levels induced resistance to harmful conditions in animal models (e.g., LPS-induced acute hepatitis, cecal ligation, and puncture) [40–47] due to the ability of TRX1 to relieve local oxidative stress and to modulate neutrophiles extravasation into sites of inflammation [48]. Furthermore, TRX1 seems to be able to counteract the proinflammatory and pro-oxidant effects of Macrophage Migration Inhibitory Factor (MIF) [49].
MIF is another member of the TRX superfamily, but functions as a classical proinflammatory cytokine [9]. Macrophage Migration Inhibitory Factor (MIF) activates T-cells and macrophages, amplifies the production of other proinflammatory cytokines, and upregulates the expression of Toll-Like Receptor-4 (TLR-4) by phagocytes [9]. Because of the prevention of p53-dependent apoptosis of activated macrophages, high concentrations of MIF result in sustained inflammatory responses. Therefore, MIF seems to counteract the antioxidative effects of TRX. Accordingly, we were also able to demonstrate significantly higher MIF-levels in patients of the septic group in comparison to patients of the postoperative or volunteer groups. MIF is secreted by leukocytes, and its synthesis is induced by bacterial endo- and exotoxins and several proinflammatory mediators (Tumor Necrosis Factor-α/TNF-α, Interferon-γ/IFN-γ, Complement Factor 5a/C5a). For the acute phase of sepsis, it has been demonstrated that high plasma levels are harmful and correlate with sepsis severity [8]. The neutralization of MIF results in an attenuation of the inflammatory response and improved survival in experimental sepsis [8, 50]. In the light of these observations, the relationship between TRX1 and MIF in sepsis is of great interest. To our knowledge, only little work has been done so far in simultaneously determining plasma levels of TRX1 and MIF in human sepsis or septic animal models [49]. In accordance to the results of Leaver and colleagues [51], we were able to show raised plasma levels of TRX1 and MIF in patients with SIRS/sepsis, whereby plasma levels of TRX1 and MIF showed a unique correlation. Unfortunately, neither TRX1 nor MIF showed significant differences between the surviving and nonsurviving subgroups of the septic patients and therefore could not be used as an early predictor for survival in patients with severe sepsis or septic shock. This may be due to the small cohort of septic patients, so a re-evaluation in a larger cohort of septic patients needs to be performed. Furthermore, the detailed mechanisms of interaction between TRX1 and MIF are not yet completely understood. Son et al. recently suggested that cell surface TRX1 serves as one of the MIF binding molecules or MIF-receptor components and inhibits MIF-mediated inflammatory signals [52]. As a possible limitation of this investigation, the significant lower age of healthy volunteers in comparison to the postoperative group as well as the septic group has to be stated. At last, we are not able to estimate exactly the influence of patients' age on the redox marker measurements in the different study groups. Unfortunately, many previously published investigations dealing with these parameters are afflicted with the same problem [47, 51]. Nevertheless, it remains unlikely that the factor age might have considerably influenced the presented study results, since TRX1 and MIF plasma levels showed significant differences in patients of the same age (postoperative cohort versus septic cohort) and were comparable in patients of significant different ages (healthy volunteers versus postoperative cohort). Due to the positive correlations of TRX1 and MIF, our investigation supports a linked role of these two mediators in the pathophysiology of sepsis. This has important implications for further research on the pathogenesis of severe sepsis or septic shock.
5. Conclusions
In summary, our results suggest that substances involved in the redox homeostasis (e.g., TRX1, MIF) represent central hubs in the septic inflammatory response, as assessed by significantly elevated plasma levels of these mediators in patients with severe sepsis or septic shock. Proinflammatory/~oxidative and anti-inflammatory/~oxidative agents show a high correlation in order to maintain a redox homeostasis and to avoid harmful effects of an excessive inflammatory/oxidative response. For this reason, the detailed mechanisms of the TRX1 and MIF interaction, as well as their use as possible targets for therapeutic manipulation, represent areas for further research.
Acknowledgments
The authors thank U. Krauser and C. Liebetrau for their excellent technical assistance.
References
- 1.Alberti C, Brun-Buisson C, Goodman SV, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically III infected patients. American Journal of Respiratory and Critical Care Medicine. 2003;168(1):77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Réa network. American Journal of Respiratory and Critical Care Medicine. 2003;168(2):165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. Journal of the American Medical Association. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 4.Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Medicine. 2004;30(4):580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Burke-Gaffney A, Callister MEJ, Nakamura H. Thioredoxin: friend or foe in human disease? Trends in Pharmacological Sciences. 2005;26(8):398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Calandra T, Echtenacher B, Le Roy D, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nature Medicine. 2000;6(2):164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 9.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nature Reviews Immunology. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 11.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England Journal of Medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 12.Russell JA. Management of sepsis. The New England Journal of Medicine. 2006;355(16):1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 13.Weigand MA, Bardenheuer HJ, Böttiger BW. Clinical management of patients with sepsis. Anaesthesist. 2003;52(1):3–22. doi: 10.1007/s00101-002-0436-0. [DOI] [PubMed] [Google Scholar]
- 14.Schouten M, Wiersinga WJ, Levi M, Van Der Poll T. Inflammation, endothelium, and coagulation in sepsis. Journal of Leukocyte Biology. 2008;83(3):536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 15.Weigand MA, Schmidt H, Pourmahmoud M, Zhao Q, Martin E, Bardenheuer HJ. Circulating intercellular adhesion molecule-1 as an early predictor of hepatic failure in patients with septic shock. Critical Care Medicine. 1999;27(12):2656–2661. doi: 10.1097/00003246-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA, Xu D, Kaiser VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Frontiers in Bioscience. 2006;11(1):520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 17.Van Snick J. Interleukin-6: an overview. Annual Review of Immunology. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 18.Fassbender K, Pargger H, Muller W, Zimmerli W. Interleukin-6 and acute-phase protein concentrations in surgical intensive care unit patients: diagnostic signs in nosocomial infection. Critical Care Medicine. 1993;21(8):1175–1180. doi: 10.1097/00003246-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Butler J, Chong GL, Baigrie RJ, Pillai R, Westaby S, Rocker GM. Cytokine responses to cardiopulmonary bypass with membrane and bubble oxygenation. Annals of Thoracic Surgery. 1992;53(5):833–838. doi: 10.1016/0003-4975(92)91446-g. [DOI] [PubMed] [Google Scholar]
- 20.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Annals of Surgery. 1992;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hack CE, De Groot ER, Felt-Bersma RJF, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74(5):1704–1710. [PubMed] [Google Scholar]
- 22.Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. European Journal of Biochemistry. 2000;267(20):6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxidants and Redox Signaling. 2000;2(4):811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 24.Mitsui A, Hirakawa T, Yodoi J. Reactive oxygen-reducing and protein-refolding activities of adult T cell leukemia-derived factor/human thioredoxin. Biochemical and Biophysical Research Communications. 1992;186(3):1220–1226. doi: 10.1016/s0006-291x(05)81536-0. [DOI] [PubMed] [Google Scholar]
- 25.Mustacich D, Powis G. Thioredoxin reductase. Biochemical Journal. 2000;346(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Tagaya Y, Maeda Y, Mitsui A, et al. ATL-derived factor (ADF), an IL-2 receptor/TAC inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO Journal. 1989;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagaya Y, Wakasugi H, Masutani H, et al. Role of ATL-derived factor (ADF) in the normal and abnormal cellular activation: involvement of dithiol related reduction. Molecular Immunology. 1990;27(12):1279–1289. doi: 10.1016/0161-5890(90)90032-u. [DOI] [PubMed] [Google Scholar]
- 28.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirota K, Murata M, Sachi Y, et al. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-κB. Journal of Biological Chemistry. 1999;274(39):27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 30.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annual Review of Pharmacology and Toxicology. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- 31.Abdiu A, Nakamura H, Sahaf B, Yodoi J, Holmgren A, Rosén A. Thioredoxin blood level increases after severe burn injury. Antioxidants and Redox Signaling. 2000;2(4):707–716. doi: 10.1089/ars.2000.2.4-707. [DOI] [PubMed] [Google Scholar]
- 32.Callister ME, Burke-Gaffney A, Quinlan GJ, et al. Extracellular thioredoxin levels are increased in patients with acute lung injury. Thorax. 2006;61(6):521–527. doi: 10.1136/thx.2005.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jekell A, Hossain A, Alehagen U, Dahlström U, Rosén A. Elevated circulating levels of thioredoxin and stress in chronic heart failure. European Journal of Heart Failure. 2004;6(7):883–890. doi: 10.1016/j.ejheart.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Jikimoto T, Nishikubo Y, Koshiba M, et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Molecular Immunology. 2002;38(10):765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 35.Kato A, Odamaki M, Nakamura H, Yodoi J, Hishida A. Elevation of blood thioredoxin in hemodialysis patients with hepatitis C virus infection. Kidney International. 2003;63(6):2262–2268. doi: 10.1046/j.1523-1755.2003.t01-3-00002.x. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki K, Noda N, Okada S, et al. Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy. 1998;11(4):277–288. doi: 10.1023/a:1008032703468. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, De Rosa S, Roederer M, et al. Elevation of plasma thioredoxin levels in HIV-infected individuals. International Immunology. 1996;8(4):603–611. doi: 10.1093/intimm/8.4.603. [DOI] [PubMed] [Google Scholar]
- 38.Gromer S, Urig S, Becker K. The thioredoxin system—from science to clinic. Medicinal Research Reviews. 2004;24(1):40–89. doi: 10.1002/med.10051. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura H. Thioredoxin as a key molecule in redox signaling. Antioxidants and Redox Signaling. 2004;6(1):15–17. doi: 10.1089/152308604771978309. [DOI] [PubMed] [Google Scholar]
- 40.Hattori I, Takagi Y, Nakamura H, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxidants and Redox Signaling. 2004;6(1):81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino T, Nakamura H, Okamoto M, et al. Redox-active protein thioredoxin prevents proinflammatory cytokine- or bleomycin-induced lung injury. American Journal of Respiratory and Critical Care Medicine. 2003;168(9):1075–1083. doi: 10.1164/rccm.200209-982OC. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Nakamura H, Shioji K, et al. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110(10):1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi S, Nishio A, Nakamura H, et al. Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. American Journal of Physiology. 2006;290(4):G772–G781. doi: 10.1152/ajpgi.00425.2005. [DOI] [PubMed] [Google Scholar]
- 44.Okubo K, Kosaka S, Isowa N, et al. Amelioration of ischemia-reperfusion injury by human thioredoxin in rabbit lung. Journal of Thoracic and Cardiovascular Surgery. 1997;113(1):1–9. doi: 10.1016/S0022-5223(97)70393-3. [DOI] [PubMed] [Google Scholar]
- 45.Okuyama H, Nakamura H, Shimahara Y, et al. Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology. 2003;37(5):1015–1025. doi: 10.1053/jhep.2003.50203. [DOI] [PubMed] [Google Scholar]
- 46.Shioji K, Kishimoto C, Nakamura H, et al. Overexpression of thioredoxin-1 in transgenic mice attenuates Adriamycin-induced cardiotoxicity. Circulation. 2002;106(11):1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 47.Hofer S, Rosenhagen C, Nakamura H, et al. Thioredoxin in human and experimental sepsis. Critical Care Medicine. 2009;37(7):2155–2159. doi: 10.1097/CCM.0b013e31819fff67. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura H, Herzenberg LA, Bai J, et al. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15143–15148. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamaki H, Nakamura H, Nishio A, et al. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131(4):1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Al-Abed Y, Dabideen D, Aljabari B, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. Journal of Biological Chemistry. 2005;280(44):36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 51.Leaver SK, MacCallum NS, Pingle V, et al. Increased plasma thioredoxin levels in patients with sepsis: positive association with macrophage migration inhibitory factor. Intensive Care Medicine. 2009;36(2):336–341. doi: 10.1007/s00134-009-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Son A, Kato N, Horibe T, et al. Direct association of thioredoxin-1 (TRX) with macrophage migration inhibitory factor (MIF): regulatory role of TRX on MIF internalization and signaling. Antioxidants & Redox Signaling. 2009;11:2595–2605. doi: 10.1089/ars.2009.2522. [DOI] [PubMed] [Google Scholar]