Abstract
Accumulating evidence indicates a key role of inflammation in hypertension and cardiovascular disorders. However, the role of inflammatory processes in neurogenic hypertension remains to be determined. Thus, our objective in the present study was to test the hypothesis that activation of microglial cells and generation of proinflammatory cytokines in the PVN contributes to neurogenic hypertension. Intracerebroventricular infusion of minocycline, an anti-inflammatory antibiotic, caused a significant attenuation of mean arterial pressure, cardiac hypertrophy and plasma norepinephrine induced by chronic Ang II infusion. This was associated with decreases in the numbers of activated microglia and mRNAs for interleukin-1β interleukin-6, and tumor necrosis factor α , and an increase in the mRNA for interleukin-10 in the PVN. Over expression of interleukin-10 induced by recombinant adeno-associated virus-mediated gene transfer in the PVN mimicked the antihypertensive effects of minocycline. Furthermore acute application of a pro-inflammatory cytokine, interleukin-1β, into the left ventricle or the PVN in normal rats resulted in a significant increase in MAP. Collectively this indicates that angiotensin II-induced hypertension involves activation of microglia, and increases in proinflammatory cytokines in the PVN. These data have significant implications on the development of innovative therapeutic strategies for the control of neurogenic hypertension.
Keywords: angiotensin II, hypertension, minocycline, interleukin 10, microglia, paraventricular nucleus, cytokine
Introduction
Inflammation has been implicated in hypertension and cardiovascular diseases in both animal models and human diseases.1, 2 Increases in levels of plasma proinflammatory cytokines (PIC) and other markers of inflammation are associated with the progression of hypertension while immune suppression produces beneficial outcomes.3, 4 Despite evidence for the participation of peripheral cytokines and inflammation in cardiovascular disease, little is known about their involvement in neurogenic hypertension. Studies from Francis and collaborators have indicated that angiotensin (Ang) II-induced hypertension involves activation of tumor necrosis factor (TNF) α and nuclear factor kappa B (NFκB) and production of reactive oxygen species (ROS) in the brain.5, 6 These observations have led us to propose that Ang II-induced neurogenic hypertension involves activation of microglial cells and production of PIC within the brain. Our objective in the present study was to test this hypothesis.
We focused on the paraventricular nucleus (PVN) and a chronic Ang II infusion rat model of hypertension for this study, based on the following rationales: (i) The PVN integrates signals/inputs from circumventricular organs (CVOs), and other cardiovascular-relevant brain areas and transmits them to the rostroventrolateral medulla and other downstream areas to influence sympathetic nerve activity7; and (ii) chronic Ang II infusion is an established animal model of hypertension with strong neurogenic components.8 Our studies demonstrate that Ang II-induced hypertension involves activation of microglia and increases in PIC within the PVN.
Materials and Methods
Animals
Adult male Sprague-Dawley (SD) rats (Charles River Laboratories) aged 5 weeks (n=118) were individually housed in a temperature-controlled room (22-23°C) with 14:10 hr light-dark cycle. Tap water and laboratory chow were available ad libitum. All experimental procedures were approved by the University of Florida Institute Animal Care and Use Committee.
Surgical preparation
Implantation of telemetry transducers
Six-week-old male SD rats were anesthetized with a mixture of O2 (1 L/min) and isoflurane (3-4%). Rat telemetry transducers (TA11PA-C40, DSI, St, Paul, MN, USA) were implanted into the abdominal aorta on day -10 (for minocycline experiments) or day -28 (for viral experiments), as described previously9. A bolus injection of Buprenophine (0.05 mg/kg, s.c.) was administered after each surgery.
ICV minocycline infusion
Ten days after implantation of telemetry transducers one group of rats were implanted with ICV cannulae for infusion of minocycline on day 0 (Figure 1a) as described previously9, 10. In brief, rats were anesthetized with a 4% isoflurane/O2 mixture and the head was positioned in a Kopf stereotaxic apparatus. An infusion cannula (Alzet, Durect Corp, CA) was implanted into the left cerebroventricle (1.3 mm caudal to bregma, 1.5 mm lateral to the midline, 3.5 mm ventral to the dura). A four-week osmotic minipump (implanted s.c.) was connected to the infusion cannula via the catheter tube to deliver minocycline (Sigma, 5 μg/hr).
Figure 1. ICV minocycline attenuates Ang II-induced cardiovascular effects.
(a) Outline of experimental protocol. (b) Minocycline abolishes Ang II-induced hypertension. * P<0.05, † P<0.01 vs. the other three groups at the same time points. (c) Minocycline infusion attenuates cardiac hypertrophy induced by Ang II. Bar graphs are mean ± SEM of HW/BW ratio following each treatment (n=6 in each group). * P<0.05 vs. other three groups. Mino: minocycline. (d) Minocycline inhibits the increase in plasma NE induced by Ang II infusion. * P<0.05 vs. control; † P<0.05 vs. Ang II by one-way ANOVA followed by Bonferoni. (Control n=5; n=6 in the other three groups)
Implantation of s.c. osmotic mini pump
On day 0, each group of rats were further randomly assigned to two subgroups to receive either Ang II (200 ng/kg/min) or 0.9% saline delivered via an osmotic minipump (#2004, Alzet, CA) implanted s.c. between the scapulae.
Injection of AAV vector into the PVN
To viral experiments, 14 days recovery post the transducer implantation surgery rats were anesthetized with 4% isoflurane mixed with O2, and randomly assigned to three groups to receive either AAV5-CBA-IL10 (1.2 × 10e12 genome copy [gc]), AAV5-CBA-eGFP (1.2 × 10e12 gc) or 0.1 M PBS (vehicle control) injected into the PVN. A single injection of vector or PBS was made using a pneumatic picopump (WPI, USA) through a glass micropipette (tip o.d. = 30-50 μm) bilaterally into the PVN (1.6-1.8 mm caudal to the Bregma, 0.2-0.3 mm lateral to the midline, 7.6-7.8 mm ventral to the dura) by pressure microinjection (200 nl, 30 s).
AAV5-CBA-IL10 vector
Rat interleukin (IL)-10 cDNA was amplified by PCR and cloned into a TA cloning vector, pCR-XL-TOPO (Invitrogen, Carlsbad, CA), generating construct pCR-XL-TOPO-IL10; DNA sequencing was performed to confirm the nucleotide sequences of IL-10 cDNA on the TA cloning vector were correct. Then the rat IL-10 cDNA fragment was digested from pCR-XL-TPOP-IL10 plasmid, separated using 0.8% argrose gel, purified with QIAquick Gel extraction kit (Qiagen, USA), and subsequently cloned into an AAV5-ITR-containing vector, PTR2-CB, under the control of hybrid cytomegalovirus enhancer/chicken β actin (CBA) promoter, generating a new construct, AAV5-CBA-IL10.
Recombinant AAV virus production
The AAV virus production and titer determination were performed by the vector core of the University of Florida, Gene Therapy Center, as previously described11. Briefly, rAAV production was performed by co-transfection of the rAAV5-ITR vector construct and a combined Ad/AAV helper construct. Transfections were performed by calcium phosphate co-precipitation in an Ad-E1a- and E1b-expressing permissive human cell line (HEK293). rAAV was purified from cells by iodixanol density gradient centrifugation.
RNA isolation, reverse transcription, and Real-Time PCR
For analysis of cytokine mRNAs, the hypothalamic tissue including PVN was dissected as previous described12. In brief, rat brains were isolated and cut into a coronal segment (−0.92 to −2.13 mm posterior to bregma, according to Paxinos and Watson13). From the coronal section we excised a block of the hypothalamus containing the PVN (~2 mm wide and 1.5 mm high). Total RNA was isolated using RNeasy kits (Qiagen, Valencia, Ca, USA) according to the manufacturer’s instructions and 200 ng purified RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Latoratories, CA, USA). The IL-1β, IL-6, TNFα and IL-10 mRNA levels were analyzed by quantitative real-time PCR using specific primers and probes in a PRISM 7000 sequence detection system (Applied Biosystems, CA).14 Data were normalized to 18s ribosomal RNA.
Cardiovascular measurements
Measurements of mean arterial blood pressure (MAP) and heart rate (HR) were made via the telemetry transducers. The start of Ang II infusion via the osmotic pump was set as day 0. Raw data were analyzed using Dataquest IV software (DSI). MAP and HR were sampled between 9 am to 5pm every 3-4 days. The data from 10am to 12pm were used for the analysis of MAP and HR.
Plasma norepinephrine levels were measured using HPLC as described previously15.
Immunocytochemistry
Forebrains were cut into 35 μm coronal sections. For IL-10 immunostaining sections were incubated with a cocktail of polyclonal rabbit anti-IL10 antibody (1:200, Abbiotec Inc., CA) and mouse monoclonal anti-NeuN antibody (1:100, Chemicon International, CA) for 24 h at 4°C. Secondary antibodies were Alex Fluor 594 anti-rabbit (1:500) for IL-10 and Alex Fluor 488 anti-mouse (1:1000) for NeuN (all from Molecular Probes, OR). For microglial immunostaining, primary antibody was a mouse monoclonal antibody OX42 (BD Bioscience Inc, 1:250), which is a specific marker for microglia16, and the secondary antibody was a goat anti-mouse IgG (1:2000) conjugated with 3,3′-Diaminobenzidine Tetrahydrochloride (DAB). PVN sections were examined using an Olympus BX41 fluorescence microscope.
Microglia quantification
OX42 antibody is a specific microglial marker and stains all microglia. When microglia are activated they undergo morphological changes. This is associated with retracted processes giving a thick and stubby appearance17, 18 . In addition activated microglia have enlarged perikarya and concentrated OX-42 staining. We defined activated microglia as cells which exhibit strong OX-42 immunoreactivity, an enlarged soma and fewer and shorter (less than the cell soma diameter, 4~4.5 μm) processes. The dimensions of 30 activated microglia from 3 consecutive sections in each animal were measured, and average group (n=4) data was used to determine activated microglia in brain regions. The area of the PVN that was chosen for microglial measurements was the same anterior level (−1.8 to 1.9 mm from bregma) for all three groups. The number of microglia in a 0.2×0.2 mm2 area was counted from at least three different adjusted sections from each animal. Morphological analysis and quantification of microglia was performed within the PVN using a light microscope at 400× magnification. Tissue sections from the cortex and lateral hypothalamus were used as control regions. There were no differences in the average length of microglial processes in these regions in both vehicle- and Ang II-treated animals, indicating a lack of microglial activation by Ang II. The analysis of activated microglia was performed in a double-blind fashion.
Cardiac pathology
At the end of the experiment, rats were euthanized and hearts were removed for cardiac morphology and histological analyses as described previously9. In brief, hearts were removed and weighed, the cross sections of ventricles were fixed in 10% neutral buffered formalin for 24 hr, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (HE) for the myocyte diameter assay, or stained with Picro-Sirius Red to determine interstitial fibrosis. Twenty images from different (non-overlapping) regions of the left ventricle wall were examined using the Image J program from the National Institutes of Health.
Effect of acute IL-1β on blood pressure
Blood pressure was recorded via the indwelling transducer implanted two weeks ahead as described earlier. To determine the effect of ICV IL-1β on blood pressure, IL-1β (10 ng/ml, 500 nl) was pressure injected to the left ventricle (A/P:-1.3 mm vs. bregma, M/L: 1.2 mm, D/V: 3.4 mm) via a pneumatic picopump. In a different rat, the IL-1 receptor antagonist (IL-1RA) (100 ng/ml, 500 nl) was microinjected into the left ventricle, followed 20 min later by ICV delivery of IL-1β. To further determine the site-specific effect of IL-1β on blood pressure, this PIC was microinjected (10 ng/ml, 100 nL) into the left PVN (A/P:−1.8 mm vs. bregma, M/L: 0.3 mm, D/V: 7.8 mm).
Statistics
Data were expressed as mean ± SE. Statistical significance was evaluated with the use of a one-way or two-way ANOVA followed by a layered Bonferroni post hoc test. Statistical tests were performed with Prism software (v4.0, GraphPad, Inc., San Diego, CA, USA).
Results
ICV minocycline attenuates Ang II-induced hypertension
Chronic s.c. infusion of Ang II in SD rats caused an increase in MAP which was sustained for four weeks (Figure 1b). Simultaneous ICV infusion of minocycline (5 μg/hr) resulted in attenuation of the Ang II-induced increase in MAP (Ang II: 156±7.2 mmHg [n=9]; Ang II+minocycline: 104±4.2 mmHg [n=8]; P<0.05). A modest attenuation was observed as early as 8 days, an effect which became significant by day 12 and persisted thereafter throughout the experiment (Figure 1b). Minocycline treatment had no effects on HR. Furthermore minocycline alone had no effect on MAP (control 101±2.7 mmHg [n=7] vs. minocycline 106±1.3 mmHg [n=4]).
Minocycline attenuates Ang II-induced hypertrophy
To determine the beneficial effects of minocycline on Ang II-induced cardiac hypertrophy, heart weight vs. body weight (HW/BW) ratio and cardiac interstitial fibrosis was determined. As shown in Figure 1c, Ang II infusion leads to a significant increase in HW/BW ratio. However, Ang II infusion in the presence of minocycline normalized the ratio to control values. Consistently, the fibrotic area was greater in Ang II treated rats vs. minocycline plus Ang II treated rats (data not shown).
Minocycline decreases Ang II-induced plasma norepinephrine
Chronic Ang II infusion resulted in a 2.7 fold increases in plasma NE (Figure 1d), one index of increased sympathetic nerve activation. ICV infusion of minocycline at a dose that inhibits Ang II-induced hypertension abolished the increase in plasma NE produced by Ang II infusion (Figure 1d).
Effects of minocycline on PVN microglia and cytokine mRNA expression
Staining with OX-42 antibody showed a significant increased in the activated microglial cells in the PVN of Ang II treated rats (Figure 2a). Chronic Ang II infusion resulted in a 23-fold increase in activated microglial cells (Figure 2b) and minocycline reduced these numbers by 80%. In addition Ang II reduced the length of microglial cell processes, an effect that was attenuated by minocycline (Figure 2b). Ang II infusion increased the total number of activated microglia and minocycline was able to attenuate this effect (Figure 2c). Figure 3 shows that Ang II infusion caused respective 4.3, 2.8 and 3-fold increases in IL-1β, IL-6 and TNFα mRNA levels while it decreased IL-10 mRNA by 38%. These effects were reversed in the PVN of Ang II-infused rats that were treated simultaneously with minocycline.
Figure 2. Effect of ICV minocycline on activation of microglia in the PVN.
Microglia within the PVN under each treatment condition were identified via OX-42 immunostaining, and their activation was assessed as detailed in the Methods.
(a) Representative micrographs showing the activation of PVN microglia under each treatment condition, 4 weeks after the start of Ang II infusions. The scale bar equals 10 μm. (b) Quantification of the length of processes of microglia in the PVN under each treatment condition. * P<0.001 vs. other groups by Two-way ANOVA, n=4 in each group. (c) Quantification of the numbers of activated microglial based on the morphological analysis. Mino: minocycline. * P<0.05 v.s. control by one-way ANOVA followed by Bonferoni.
Figure 3. Effect of ICV minocycline on PVN cytokine mRNA expression.
Following euthanization of the rats used in the experiment in Figure 1, brains were removed and mRNA levels of cytokines in the PVN were assessed by Real-Time RT-PCR. * P<0.05 vs. corresponding control; † P<0.05 vs. Ang II by One-way ANOVA (n=6 in each group). Mino: minocycline.
IL-10 over expression in the PVN attenuates Ang II-induced hypertension
In view of our data that Ang II induced hypertension involves microglial activation, increases in PIC and decreases in IL-10 levels in the PVN, we hypothesized that over expression of IL-10 would overcome and attenuate this hypertensive effect. AAV5-IL-10 or AAV-GFP was injected bilaterally into the PVN fourteen days prior to chronic Ang II infusion (Figure 4a). In the AAV5-IL-10 injected rats there was a significant decrease in the Ang II-induced hypertension compared with rats injected with AAV-GFP or PBS at the same site (Figure 4b). This inhibition became statistically significant at 17 days after the start of Ang II infusion (Ang II+AAV-GFP, 149±6.0 mmHg [n=6] vs. Ang II+AAV-IL-10, 109±5 mmHg [n=6], p< 0.001). IL-10 over expression had no effect on basal MAP in control SD rats, and none of the treatments produced significant changes in HR (data not shown). Over expression of IL-10 in the PVN also attenuated the cardiac hypertrophy produced by Ang II infusion (Figure 4c) similar to the effect of minocycline (Figure 1c). Heart weight/body weight (HR/BW) ratio was reduced by 22% in the Ang II+AAV-IL-10 group compared with the Ang II+AAV-eGFP group (3.0±0.12 g/kg vs. 3.8±0.14 g/kg, P<0.05). Similarly, enhanced IL-10 expression attenuated the increase in myocyte diameter and interstitial fibrosis produced by Ang II infusion (Figure 4c).
Figure 4. Increased expression of IL-10 in the PVN blunts Ang II-induced hypertension.
SD rats were microinjected with either AAV5-IL-10, AAV5-GFP or PBS into the PVN as detailed in the Methods. (a) Outline of experimental protocol. (b) Time dependent changes in MAP in each treatment group. Dashed lines indicated the treatment time points. * P<0.05, ** P<0.01, *** P<0.001 vs. GFP or PBS groups at the same time points. (c) Effect of IL-10 transduction on Ang II-induced cardiac hypertrophy. * P<0.05 vs. IL-10 + saline; † P<0.05 IL-10 + Ang II; ‡ P<0.05 vs. GFP or PBS plus Ang II infusion (n=4 in each group).
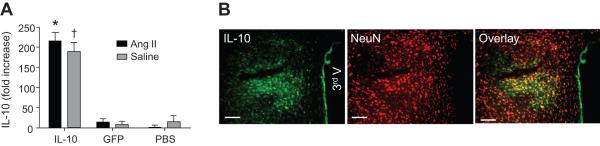
The locations of the AAV microinjections and IL-10 over expression were confirmed by real time RT-PCR and immunocytochemistry (Figure 5). The data indicate that microinjection of AAV5-IL-10 bilaterally into the PVN produced increases of IL-10 mRNA levels in rats that had received either Ang II or 0.9% saline infusion (Figure 5a). Further, AAV5-mediated transduction was highly efficient, robust, and restricted to the PVN region. To further determine the cellular distribution, NeuN, a neuronal marker, was co-stained with IL-10 in the same coronal section. The majority of IL-10 immunoreactivity was co-localized with NeuN (Figure 5b).
Figure 5. AAV5-IL-10 induced neuronal expression of IL-10 in the PVN.
(a) IL-10 mRNA levels in the PVN at 28 days following microinjections of AAV5-IL-10, AAV5-GFP or PBS. * P<0.05 vs. GFP or PBS group. † P<0.05 vs. GFP or PBS groups. (b) Representative fluorescence micrographs showing IL10- and NeuN- immunoreactivity in the PVN following AAV5-CBA-IL10 microinjection. The right panel shows the merged images of IL10 and NeuN. Bar = 50 um.
If over expression of IL-10, an anti-inflammatory cytokine, blocks Ang II-induced hypertension, then one would anticipate that microinjection of PIC (such as IL-1β) in the PVN, would increase MAP. Figure 6a is a representative experiment demonstrating that microinjection of IL-1β (10 ng/ml, 500 nl) into the left ventricle resulted in a 25 mmHg increase in MAP in normal SD rats. This effect was attenuated by IL-1 receptor antagonist (100 ng/ml, 500 nl). Furthermore, microinjection of IL-1β (100 ng/ml, 100 nl) unilaterally into left PVN induced an 8 mmHg increase in MAP in normal SD rats as shown in Figure 6b. This is consistent with observations from the literature.19
Figure 6. IL-1β increases MAP in anesthetized rats.
(a) Representative tracings showing the effects of ICV injection of IL-1β (10 ng/ml, 500 nl) on MAP in the absence or presence of the IL-1 receptor antagonist (IL-1RA: 100 ng/ml, 500 nl). (b) Representative tracing showing effect of PVN injection of IL-1β (10 ng/ml, 100 nl) on MAP.
Discussion
The present study provides evidence that microglial cells in the PVN and cytokines produced within the brain play a central role in neurogenic hypertension. Inhibition of microglial activation or over expression of IL-10 in the PVN attenuates Ang II-induced hypertension and its associated cardiac pathophysiology. The evidence for these conclusions are: (i) ICV infusion of minocycline, an anti-inflammatory antibiotic with demonstrated neuroprotective effects20, attenuates Ang II-induced hypertension and raised plasma NE; (ii) minocycline also inhibits Ang II-stimulated microglial activation in the PVN, consistent with its demonstrated actions in the brain,21 and also the Ang II-induced increases in PIC levels at this nucleus; (iii) IL-10 over expression in the PVN and not in the RVLM (data not shown) attenuates hypertension while IL-1β increases MAP.
Evidence has emerged from the last several years that implicate the involvement of cytokines in cardiovascular diseases such as hypertension and heart failure.2, 5 For example, levels of PVN PIC are increased in animal models of hypertension and hypothalamic infusion of IL-1β increases blood pressure and sympathetic outflow.22 In addition, central gene transfer of IL-10 reduces hypothalamic inflammation in rats with heart failure following myocardial infarction.23 Our present observations complement these findings. Furthermore, they provide evidence that the production of cytokines within the brain during hypertension involves microglial cells. This includes the observations that intracerebroventricular (ICV) infusion of minocycline and over expression of IL-10 attenuate Ang II-induced hypertension. Thus, it is tempting to propose a novel mechanism of dysregulation in neurogenic hypertension. We propose that circulating Ang II activates cardiovascular-responsive areas in the CVOs resulting in the generation and transmission of hypertensive signals to the PVN. Microglia in the PVN are activated and release PIC which directly or indirectly increase the activity of PVN sympathetic neurons. These and other pathways converge into the RVLM leading to increases in sympathetic outflow7. So what is (are) the hypertensive signal(s) from the CVOs that activates PVN microglial? Available evidence suggests that Ang II is a likely candidate: (i) Levels of PVN Ang II and AT1 receptors are increased in many animal models of hypertension;24 (ii) Our preliminary data shows that microglial cells express AT1 receptors and that chronic silencing of the PVN AT1 receptor by AAV-mediated expression of an AT1 receptor specific shRNA causes a significant decreases in high blood pressure in the spontaneously hypertensive rat (SHR, data not shown). However, the role of other signaling molecules downstream of the AT1 receptor, such as ROS25, 26, cannot be ruled out at the present time. In addition, the role of other cell types (i.e. astroglial cells, neurons) in the PVN in cytokine actions remains to be explored2. Finally, this study raises important questions: (i) Are anti-hypertensive effects of minocycline truly central or does the ICV infused antibiotic cross the blood-brain-barrier and exert a peripheral effect. The latter seems to be an unlikely possibility since the dose of minocycline that normalizes Ang II induced increases in hypertension following ICV administration is only ‘~30% as effective when administered subcutaneously (data not shown). Since minocycline is able to freely pass through the BBB would it be possible to administer it in a high enough dose peripherally that could influence the PVN and thus be beneficial for the control of neurogenic hypertension?27 Experiments are under way to support/refute this view. (ii) The mechanism of minocycline actions in the PVN needs to be elucidated. Minocycline is a broad spectrum antibiotic of the tetracycline family, has been implicated to be neuroprotective in several diseases e.g. sclerosis, neurodegenerative disorder, stroke and Parkinson disease28. It has been shown to inhibit protein kinase C (PKC) α phosphorylation and nuclear translocation of PKCα/βII and interferon γ regulatory factor, which further inhibit class II activator, another transcription factor, to promote the gene expression for microglial activation29. In addition to anti-inflammation, minocycline and other tetracycline derivatives are proposed to attenuate apoptosis and inhibit production of ROS via an action on mitochondria28. Thus, the anti-hypertensive effect of minocycline may involve one or more of these signaling pathways leading to inhibition of microglial activation.
Perspectives
The present study described that central application of minocycline, an anti-inflammatory agent and inhibitor of microglial activation, increased expression of the anti-inflammatory cytokine IL-10 in the PVN, elicit an anti-hypertensive effect, and alleviate cardiac damage in an Ang II-infused rat model. Thus, microglia may serve as a source of inflammatory cytokines in the CNS cardiovascular control centers and have a key role in the development of neurogenic hypertension. Thus, these results may indicate novel targets for the treatment of hypertension.
Acknowledgements
We thank Fan Lin for her excellent technical support.
Source of Funding This work was supported by NIH grants: HL33610 (M.K.R) & HL 076803 (C.S.); and American Heart Association 09POST2230164 (P.S.).
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 2.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37:e52–e57. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schillaci G, Pirro M, Gemelli F, Pasqualini L, Vaudo G, Marchesi S, Siepi D, Bagaglia F, Mannarino E. Increased C-reactive protein concentrations in never-treated hypertension: The role of systolic and pulse pressures. J Hypertens. 2003;21:1841–1846. doi: 10.1097/00004872-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Stumpf C, John S, Jukic J, Yilmaz A, Raaz D, Schmieder RE, Daniel WG, Garlichs CD. Enhanced levels of platelet P-selectin and circulating cytokines in young patients with mild arterial hypertension. J Hypertens. 2005;23:995–1000. doi: 10.1097/01.hjh.0000166840.63312.12. [DOI] [PubMed] [Google Scholar]
- 5.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 8.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–933. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, Scheuer DA, Shi P, Sumners C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J. 2008;22:3175–3185. doi: 10.1096/fj.08-108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mecca AP, O’Connor TE, Katovich MJ, Sumners C. Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp Physiol. 2009;94:937–946. doi: 10.1113/expphysiol.2009.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- 12.Jiang N, Shi P, Li H, Lu S, Braseth L, Cuadra AE, Raizada MK, Sumners C. Phosphate-activated glutaminase-containing neurons in the rat paraventricular nucleus express angiotensin type 1 receptors. Hypertension. 2009;54:845–851. doi: 10.1161/HYPERTENSIONAHA.109.134684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 4th ed Academic Press; San Diego: 1998. [Google Scholar]
- 14.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 16.Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- 17.Marco P, Sola RG, Ramon y Cajal S, DeFelipe J. Loss of inhibitory synapses on the soma and axon initial segment of pyramidal cells in human epileptic peritumoural neocortex: Implications for epilepsy. Brain Res Bull. 1997;44:47–66. doi: 10.1016/s0361-9230(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K, Kohsaka S. Microglia: Activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Chen J, Yin X, Zhao H. Angiotensin II receptor 1 involved in the central pressor response induced by interleukin-1 beta in the paraventricular nucleus. Neurol Res. 2008;31:420–424. doi: 10.1179/174313208X353677. [DOI] [PubMed] [Google Scholar]
- 20.Graber JJ, Dhib-Jalbut S. Protective autoimmunity in the nervous system. Pharmacol Ther. 2009;121:147–159. doi: 10.1016/j.pharmthera.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Miyaoka T. Clinical potential of minocycline for schizophrenia. CNS Neurol Disord Drug Targets. 2008;7:376–381. doi: 10.2174/187152708786441858. [DOI] [PubMed] [Google Scholar]
- 22.Kannan H, Tanaka Y, Kunitake T, Ueta Y, Hayashida Y, Yamashita H. Activation of sympathetic outflow by recombinant human interleukin-1 beta in conscious rats. Am J Physiol. 1996;270:R479–R485. doi: 10.1152/ajpregu.1996.270.2.R479. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101:304–312. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 24.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itani H, Liu X, Sarsour EH, Goswami PC, Born E, Keen HL, Sigmund CD. Regulation of renin gene expression by oxidative stress. Hypertension. 2009;53:1070–1076. doi: 10.1161/HYPERTENSIONAHA.109.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 27.Brogden RN, Speight TM, Avery GS. Minocycline: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs. 1975;9:251–291. doi: 10.2165/00003495-197509040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Orsucci D, Calsolaro V, Mancuso M, Siciliano G. Neuroprotective effects of tetracyclines: Molecular targets, animal models and human disease. CNS Neurol Disord Drug Targets. 2009;8:222–231. doi: 10.2174/187152709788680689. [DOI] [PubMed] [Google Scholar]
- 29.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]