Abstract
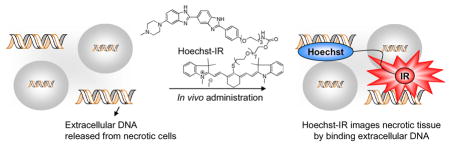
Cell necrosis is central to the progression of numerous diseases and imaging agents that can detect necrotic tissue have great clinical potential. We demonstrate here that a small molecule, termed Hoechst-IR, composed of the DNA binding dye Hoechst and the near infrared dye, IR-786, can image necrotic tissue in vivo via fluorescence imaging. Hoechst-IR detects necrosis by binding extracellular DNA released from necrotic cells and was able to image necrosis generated from a myocardial infarction and lipopolysaccharide/D-galactosamine (LPS-GalN) induced sepsis.
Necrotic tissue is a central characteristic of numerous diseases including sepsis, cancer, cardiac dysfunction, atherosclerosis and several others. 1 The imaging of necrotic tissue therefore has the potential to significantly improve the diagnosis of a wide variety of human diseases. However, imaging necrosis in vivo has been challenging because of a lack of imaging contrast agents.
A molecular target with considerable potential for imaging tissue necrosis is extracellular DNA (E-DNA). 2 Cell necrosis results in the disruption of the cell membrane, which causes the release of the cellular contents, including genomic DNA, into the extracellular space. The intracellular DNA concentration is in the millimolar range, and therefore high concentrations of E-DNA are potentially generated in the extracellular space of tissue with high levels of necrosis. Under certain pathological conditions, E-DNA can be detected in the blood, however due to the large blood volume and the presence of serum nucleases, the E-DNA concentration in the blood should be significantly lower than in necrotic tissue. E-DNA is also not generated during apoptosis, the primary mechanism of cell death in healthy tissue, and therefore the E-DNA concentration in healthy tissue should be very low. Finally, E-DNA can potentially be imaged with small molecule based imaging agents, because numerous small molecules have been developed that can bind DNA with nanomolar affinity and high specificity. 3 However, despite its potential, the development of an imaging agent that can image E-DNA in vivo still remains a major challenge.3c In this report, we demonstrate that a small molecule, termed Hoechst-IR, can image necrotic tissue in vivo via fluorescence imaging. Hoechst-IR detects necrosis by binding E-DNA released from necrotic cells and was able to image necrosis generated from a myocardial infarction and lipopolysaccharide/D-galactosamine (LPS-GalN) induced sepsis.
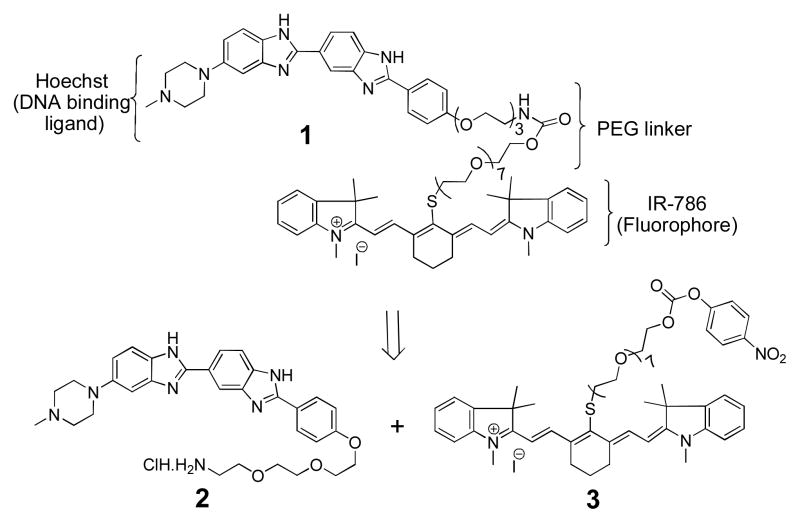
The molecular design of Hoechst-IR (1) is shown in Scheme 1 and is composed of two moieties namely Hoechst, a DNA binding agent, and the near infrared (NIR) dye IR-786. Hoechst was chosen as the E-DNA binding component of Hoechst-IR because of its nanomolar affinity for DNA, well documented biocompatibility in humans,4 and also because it can be modified at its terminal phenol without losing DNA binding activity.5 An 11-unit PEG linker was incorporated into Hoechst-IR to increase its hydrophilicity and prevent it from crossing the cell membrane and binding intracellular DNA. Lastly, Hoechst-IR contains the NIR fluorophore, IR-786, which allows for imaging of E-DNA, using near IR wavelengths.6
Scheme 1.
Structure and retrosynthesis of Hoechst-IR (1)
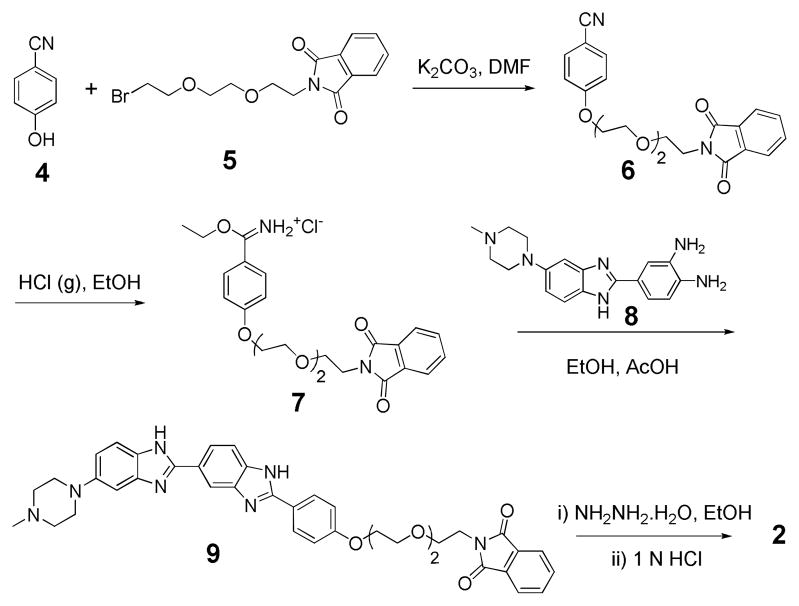
The retrosynthesis of 1 is shown in Scheme 1 and was accomplished by coupling the two synthetic building blocks 2 and 3 via a carbamate linkage. The compound 2 (Scheme 2) is a derivative of Hoechst with a terminal amine, and was synthesized using modified literature procedures. 7 We chose this route, after unsuccessfully trying to alkylate the commercially available Hoechst 33258 at its phenol group. The compound 2 was obtained in ten steps starting from commercially available 4-Hydroxy benzonitrile (4). Nucleophilic substitution of 4 with bromo-PEG-phthalimide 58 provided the ether 6 in 92% yield, which upon acidification generated the iminium ether 7. Condensation of 7 with piperazyl diamine 8 9 gave the phthalimide protected Hoechst 9 in a 38% yield. Finally, deprotection of 9 with hydrazine hydrate and acidification with aqueous HCl generated the Hoechst amine salt 2 in quantitative yield.
Scheme 2.
Synthesis of Hoechst amine (2)
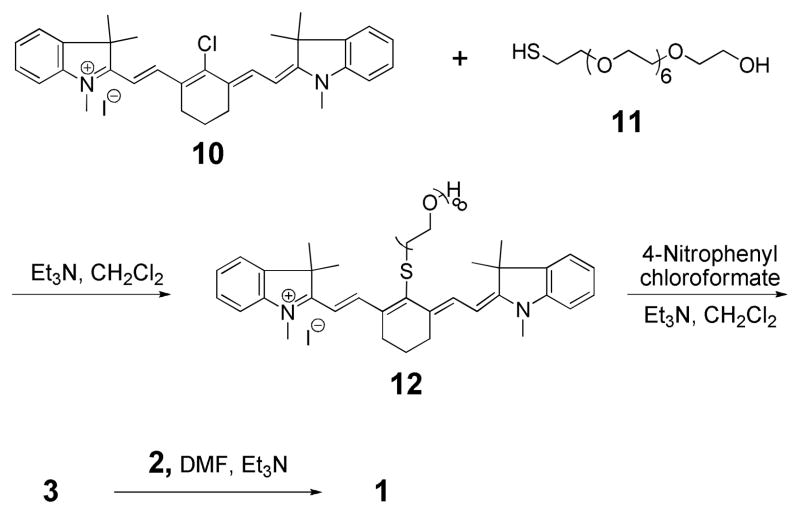
The second synthetic building block 3 (Scheme 3) is a derivative of the cyanine dye IR-786 iodide (10), and was synthesized in three steps. Treatment of 10 with PEG8-thiol 11 generated the thioether 12 in 60% yield via a nucleophilic addition/elimination reaction.10 Activation of the hydroxyl group in 12 with 4-nitrophenyl chloroformate generated the carbonate ester 3 in 78% yield, which upon coupling with 2 afforded the final conjugate 1 in 31% yield. We verified that Hoechst-IR binds DNA with high affinity by titrating it with a 22 bp double stranded oligonucleotide (sense strand: 5′-AGTTGAGGGGACTTTCCCAGGC-3′, complementary strand: 3′-TCAACTCCCCTGAAAGGG TCCG-5′) and determined that it has a dissociation constant (Kd) of 0.2 nM (see Supporting Information). 11 Interestingly, this binding affinity is a little higher than that of free Hoechst (Kd = (1–3) nM).3a We anticipate that this additional increase in binding affinity may be due to the affinity of cyanines dyes for DNA, which bind DNA with moderate affinity.12
Scheme 3.
Synthesis of Hoechst-IR (1)
A key issue in developing molecules that can image E-DNA is ensuring that they are membrane impermeable. Hoechst based compounds are potentially problematic for imaging E-DNA because commercially available Hoechst 33258 is membrane permeable. We therefore evaluated the membrane permeability of Hoechst-IR in vitro in live cells and cells that were fixed and permeabilized with methanol.
RAW 264.7 macrophages and methanol fixed macrophages were incubated with Hoechst-IR and imaged by fluorescence microscopy. Figure 1 demonstrates that Hoechst-IR has very low cell permeability. For example, live cells incubated with Hoechst-IR generate low levels of intracellular fluorescence, indicating that Hoechst-IR has very low cell permeability, presumably because of its 11 unit PEG chain (Figure 1A). In contrast, methanol treated permeabilized cells incubated with Hoechst-IR generate high levels of intracellular fluorescence (Figure 1B). This further confirms that the low levels of fluorescence seen in Figure 1A were due to the low membrane permeability of Hoechst-IR and not due to a reduction in its DNA binding capability. Additionally, live cells incubated with Hoechst 33258 generate high levels of intracellular fluorescence, demonstrating that Hoechst 33258 can easily cross cell membranes and bind intracellular DNA (Figure 1C).
Figure 1.
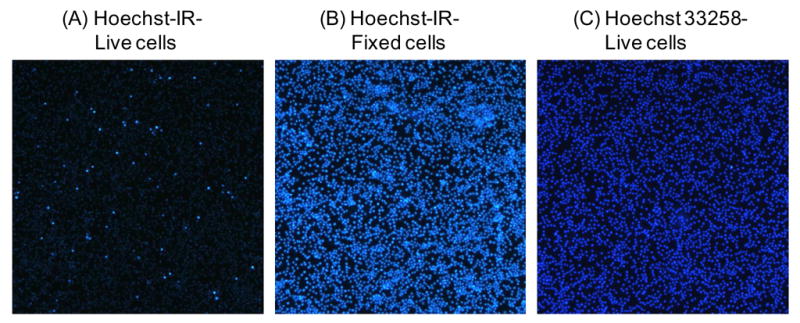
Hoechst-IR is membrane impermeable: A–C): RAW 264.7 macrophage cells were incubated for 30 min with either Hoechst-IR (20 μM) or Hoechst 33258 (20 μM) and imaged by fluorescence microscopy, using an excitation of 340 nm. (A) Live cells treated with Hoechst-IR have negligible fluorescence, demonstrating that Hoechst-IR is membrane impermeable. (B) Fixed cells treated with Hoechst-IR and (C) live cells treated with Hoechst 33258 have high fluorescence.
We examined the ability of Hoechst-IR to target E-DNA generated by tissue necrosis after a myocardial infarction (MI). Myocardial infarction generates large amounts of tissue necrosis because of reperfusion injury. There is great clinical interest in identifying necrotic tissue in patients suffering from MI because it helps identify infarcted tissue and also the development of congestive heart failure, which usually follows after an MI.13 A mouse ischemia-reperfusion model of MI was used to evaluate the efficacy of Hoechst-IR.
Briefly, the left anterior descending coronary artery was ligated with a suture, generating ischemia for 30 minutes and then opened to allow for reperfusion. Two hours after the suture was removed Hoechst-IR (10 nmol) was administered intravenously via a retro-orbital injection and allowed to circulate for one hour. The mice were then sacrificed, perfusion fixed with paraformaldehyde, and the organs were harvested and imaged using an in vivo imager. Figure 2, A–C demonstrate that Hoechst-IR can detect necrosis in the myocardium after an MI. For example, Figure 2A shows a representative image of the heart of a mouse that received Hoechst-IR, after ischemia-reperfusion surgery, and demonstrates that Hoechst-IR accumulates in the vicinity of the necrotic zone of the infarct. Figure 2B & 2C also demonstrate that Hoechst-IR accumulates to a higher level in infarcted hearts than in sham operated hearts, further suggesting again that Hoechst-IR can detect E-DNA in necrotic tissue.
Figure 2.
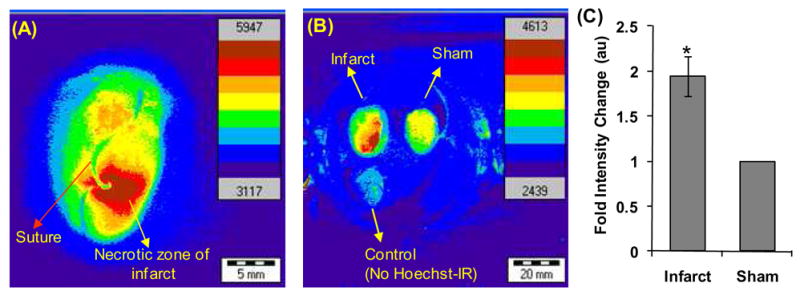
Hoechst-IR targets tissue necrosis in vivo after a myocardial infarction. (A) Micrograph of a heart isolated from a mouse subjected to ischemia-reperfusion injury and treated with Hoechst-IR. Hoechst-IR accumulates in the necrotic zone of the infarct. (B) Micrographs of mouse hearts subjected to ischemia-reperfusion surgery + Hoechst-IR, sham surgery + Hoechst-IR, or sham surgery + saline injection. (C) Mouse hearts subjected to ischemia-reperfusion surgery + Hoechst-IR have a 2 fold increase in IR-786 fluorescence than mouse subjected to sham surgery + Hoechst-IR (*P < 0.05, n = 3).
Finally, Hoechst-IR was evaluated for its ability to image necrosis in vivo during sepsis. Sepsis is a leading cause of death worldwide, and generates severe necrosis in many vital organs, such as in the lungs.14 Sepsis was induced in mice by an intraperitoneal (i.p.) injection of LPS-GalN and 24 hours later, Hoechst-IR was injected via the jugular vein and allowed to circulate for 24 hours. The mice were then imaged in an IVIS imaging system and the total fluorescence from the lung area was integrated.
Figures 3A and 3B demonstrate that mice treated with LPS-GalN and Hoechst-IR have a four fold increase in fluorescence over mice treated with saline and Hoechst-IR, demonstrating that Hoechst-IR can detect necrosis caused by sepsis. Surprisingly, the fluorescence intensity from the i.p. cavity of LPS-GalN treated and control mice were both very low, suggesting that the E-DNA generated from the organs of the i.p. cavity are either rapidly degraded or cleared by phagocytic cells.
Figure 3.
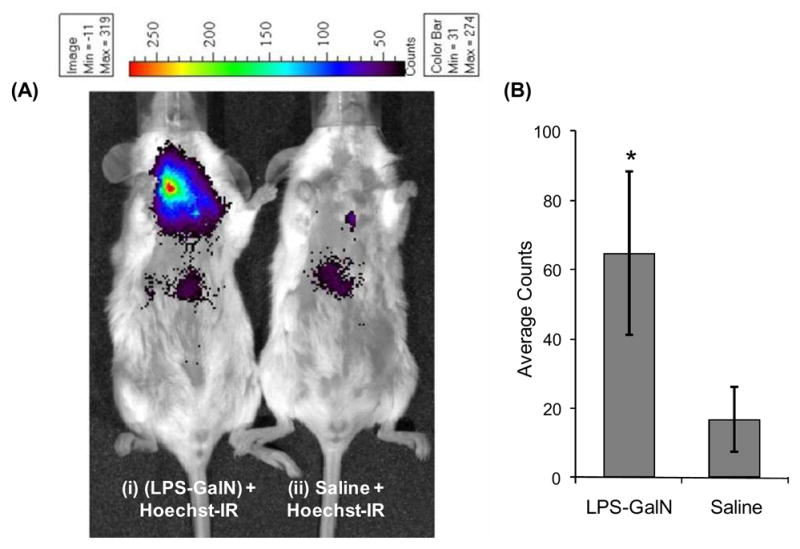
Hoechst-IR can image tissue necrosis in vivo caused by LPS-GalN induced sepsis. (A) (i) Representative fluorescent image of mouse treated with an i.p. injection of LPS (2.5 μg/kg) and GalN (700 mg/kg), followed by an i.v. injection of Hoechst-IR (5 mg/kg), (ii) representative fluorescent image of mouse treated with an intraperitoneal injection of saline, followed by an i.v. injection of Hoechst-IR (5 mg/kg). (B) Quantification of Hoechst-IR fluorescence intensities of mice treated with LPS-GalN and saline. Mice treated with LPS-GalN have a 3–4 fold increase in fluorescence over mice treated with saline (*P < 0.05, n = 5).
In summary, we demonstrate that Hoechst-IR can image necrotic tissue in vivo via fluorescence imaging. Hoechst-IR was synthesized by the coupling of hoechst and IR derivatives via a carbamate linkage. Hoechst-IR detects necrosis by binding E-DNA and was able to image necrosis generated from a myocardial infarction and LPS-GalN induced sepsis. We anticipate numerous applications of Hoechst-IR given the large number of diseases associated with tissue necrosis.
Supplementary Material
Acknowledgments
This work was supported by NSF-BES-0546962 Career Award (N.M.), NIH UO1HL80711-01 (N.M.), and NIH RO1 HL80711-01 (N.M.).
Footnotes
Supporting Information Available: Experimental procedures, characterization data for all compounds and DNA binding isotherm for Hoechst-IR. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Amaravadi RK, Thompson CB. Clin Cancer Res. 2007;13:7271. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]; (b) Artal-Sanz M, Tavernarakis N. FEBS Lett. 2005;579:3287. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]; (c) Smith MK, Mooney DJ. J Biomed Mater Res, Part A. 2007;80:520. doi: 10.1002/jbm.a.30930. [DOI] [PubMed] [Google Scholar]; (d) Tabas I. Curr Drug Targets. 2007;8:1288. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 2.(a) Vlassov VV, Laktionov PP, Rykova EY. Bioessays. 2007;29:654. doi: 10.1002/bies.20604. [DOI] [PubMed] [Google Scholar]; (b) Fleischhacker M, Schmidt B. Nat Med. 2008;14:914. doi: 10.1038/nm0908-914. [DOI] [PubMed] [Google Scholar]; (c) Pisetsky DS, Fairhurst AM. Autoimmunity. 2007;40:281. doi: 10.1080/08916930701358826. [DOI] [PubMed] [Google Scholar]; (d) Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. Cancer Res. 2001;61:1659. [PubMed] [Google Scholar]
- 3.(a) Strekowski L, Wilson B. Mutat Res. 2007;623:3. doi: 10.1016/j.mrfmmm.2007.03.008. [DOI] [PubMed] [Google Scholar]; (b) Telford WG, King LE, Fraker PJ. Cytometry. 1992;13:137. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]; (c) Garanger E, Hilderbrand SA, Blois JT, Sosnovik DE, Weissleder R, Josephson L. Chem Commun. 2009:4444. doi: 10.1039/b907375b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Kraut EH, Fleming T, Segal M, Neidhart JA, Behrens BC, MacDonald J. Invest New Drugs. 1991;9:95. doi: 10.1007/BF00194556. [DOI] [PubMed] [Google Scholar]; (b) Patel SR, Kvols LK, Rubin J, Oconnell MJ, Edmonson JH, Ames MM, Kovach JS. Invest New Drugs. 1991;9:53. doi: 10.1007/BF00194545. [DOI] [PubMed] [Google Scholar]
- 5.(a) Reddy PM, Bruice TC. J Am Chem Soc. 2004;126:3736. doi: 10.1021/ja031557s. [DOI] [PubMed] [Google Scholar]; (b) Telford WG, King LE, Fraker PJ. Cytometry. 1992;13:137. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]; (c) Neidle S. Nat Prod Rep. 2001;18:291. doi: 10.1039/a705982e. [DOI] [PubMed] [Google Scholar]
- 6.Delaey E, van Laar F, De Vos D, Kamuhabwa A, Jacobs P, de Witte P. J Photochem Photobiol, B. 2000;55:27. doi: 10.1016/s1011-1344(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 7.(a) Rajur SB, Robles J, Wiederholt K, Kuimelis RG, McLaughlin LW. J Org Chem. 1997;62:523. doi: 10.1021/jo9618536. [DOI] [PubMed] [Google Scholar]; (b) Arya DP, Willis B. J Am Chem Soc. 2003;125:12398. doi: 10.1021/ja036742k. [DOI] [PubMed] [Google Scholar]
- 8.Yeo WS, Min DH, Hsieh RW, Greene GL, Mrksich M. Angew Chem, Int Ed. 2005;44:5480. doi: 10.1002/anie.200501363. [DOI] [PubMed] [Google Scholar]
- 9.Kelly DP, Bateman SA, Martin RF, Reum ME, Rose M, Whittaker ARD. Aust J Chem. 1994;47:247. [Google Scholar]
- 10.Hilderbrand SA, Kelly KA, Weissleder R, Tung CH. Bioconjugate Chem. 2005;16:1275. doi: 10.1021/bc0501799. [DOI] [PubMed] [Google Scholar]
- 11.Loontiens FG, Regenfuss P, Zechel A, Dumortier L, Clegg RM. Biochemistry. 1990;29:9029. doi: 10.1021/bi00490a021. [DOI] [PubMed] [Google Scholar]
- 12.(a) Fang F, Zheng H, Li L, Wu Y, Chen J, Zhuo S, Zhu C. Spectrochim Acta, Part A. 2006;64:698. doi: 10.1016/j.saa.2005.07.071. [DOI] [PubMed] [Google Scholar]; (b) Davidson YY, Gunn BM, Soper SA. Appl Spectrosc. 1996;50:211. [Google Scholar]
- 13.(a) Baron N, Kachenoura N, Beygui F, Cluzel P, Grenier P, Herment A, Frouin F. Comput Cardiol. 2008;35:781. doi: 10.1016/j.ijcard.2012.03.056. [DOI] [PubMed] [Google Scholar]; (b) Liu Z, Zhao M, Zhu X, Furenlid LR, Chen YC, Barrett HH. Nucl Med Biol. 2007;34:907. doi: 10.1016/j.nucmedbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. N Engl J Med. 2001;345:1368. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]; (b) Huber-Lang M, Sarma VJ, Lu KT, McGuire SR, Padgaonkar VA, Guo RF, Younkin EM, Kunkel RG, Ding J, Erickson R, Curnutte JT, Ward PA. J Immunol. 2001;166:1193. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.